Abstract
High-density lipoprotein (HDL) is thought to be protective against cardiovascular disease (CVD), and HDL dysfunction is considered to be a risk factor for CVD. It is unclear whether there is an association between Human T lymphotropic virus type 1 (HTLV1) infection and CVD risk. We have assessed HDL lipid peroxidation (HDLox) as a marker of HDL dysfunction and CVD risk in a subgroup of the MASHAD cohort study. One hundred and sixty two individuals including 50 subjects positive for HTLV1 infection and 112 individuals negative for HTLV1 infection were recruited. Anthropometric and biochemical parameters including serum hs-CRP, fasted lipid profile (HDL-C, LDL, triglycerides, and cholesterol), and fasting blood glucose were determined. Serum HDLox was also measured in the study participants. Multivariate analyses were used to evaluate the association between serum HDLox and HTLV1 infection. None of the traditional CVD risk factors were associated with HTLV1 infection, including serum HDL-C. However, serum HDLox was independently associated with the presence of HTLV1 infection. Logistic regression analysis showed that subjects who were positive for HTLV1 infection were also significantly more likely than uninfected individuals to have higher HDLox (odds ratio 9.35, 95%CI: 3.5–24.7; P < 0.001). HDLox was increased approximately 20% (P < 0.001) in infected subjects compared to the uninfected group. Serum HDLox is a marker of CVD risk factor and increased in individuals affected by HTLV1 infection compared to healthy subjects.
Keywords: high-density lipoprotein, HDL lipid peroxidation, HDLox, HTLV1 infection, viral infectious disease
1. Introduction
Human T lymphotropic virus type 1 (HTLV1) is a deltavirus and a member of the Retroviridae family [1]. It predominantly infects CD4+ T cells, but CD8+ may also be infected [2]. HTLV1 is an oncogenic retrovirus comprised a single-stranded RNA that can cause adult T-cell leukemia lymphoma [2,3]. It is also the causative agent for a neurological disease, namely HTLV1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [4]. Fortunately, both diseases only develop in a small percentage of infected individuals; most (95%) remain asymptomatic carriers throughout their lives [3]. In parts of the world in which the prevalence rate of HTLV infection is >1%, it is assumed to be endemic [5]; these include: Japan, Africa, Caribbean Islands, South America [6], and northeastern of Iran [7]. The prevalence varies between 1 and 7.2% in the general population of the mentioned areas [7,8]. It is estimated that 10‑20 million individuals were infected with the virus globally [2]. The virus is transmitted via four principal routes: (1) Mother to child transmission which is mainly through breastfeeding, (2) Sexual transmission with a higher transmission rate from men to women, (3) through contaminated blood products, and (4) transmission between intravenous drug users [2,8]. Chronic infections induce a state of systemic inflammation and may increase the risk for comorbidities like cardiovascular disease (CVD) [9]. However, there are limited data about the contribution of chronic HTLV1 infection in CVD and the potential mechanisms. High-density lipoprotein (HDL) is involved in reverse cholesterol transport, and its levels have been shown to be inversely related to CVD risk [10,11]. The athero-protective effects of HDL, apart from its well described role in reverse cholesterol transport (RCT), include: antioxidant, antithrombotic, anti-inflammatory, regulating immune response, and endothelial protection [12]. The atheroprotective effects of HDL may be altered, and it may develop proatherogenic properties as a result of changes in lipid and protein content of HDL (abnormal HDL) in conditions such as acute inflammation. Niacin and the CETP inhibitors that have been used to increase HDL-C concentrations have no significant impact on CVD outcomes [13]. Although evaluating HDL-C and apoA1 (the principal HDL apolipoprotein) provide information about the number of circulating HDL particles, their composition and function remain unclear and the functionality of all HDL particles is not the same [14]. Therefore, the development of standard assays for HDL function appears to be necessary along with HDL-C levels to be clinically useful. Several studies have used novel techniques to determine HDL function and have shown that HDL function represents a more reliable and accurate predictor of CVD outcomes as compared to circulating HDL-C [14–16]. In the chronic inflammatory situations, the antioxidant function of HDL is impaired and individuals may then be exposed to enhanced risk of CVD as a consequence of the presence of abnormal HDL [17]. We have also shown that oxidized dysfunctional HDL (HDLox) has a major role in systemic inflammation and immune activation in chronic viral infections like chronic HIV-1 infection [18]. The impact of HTLV1 infection on the properties of HDL has not been studied previously. Therefore, the aim of the current study was to assess HDL lipid peroxidation as a CVD risk factor in HTLV1-infected individuals in a population recruited from the Mashhad-Stroke and Heart-Atherosclerotic-Disorders (MASHAD) cohort.
2. Experimental procedures
2.1. Study design
The MASHAD study is a cohort study of a representative sample population from north-eastern Iran; recruitment started in 2010, and participants will be followed up until 2020. Eligible participants of 35‑65 years, without prevalent CVD, were recruited [19]. The study was approved by Ethics Committee of Mashhad University of Medical Sciences and informed written consent was obtained from all participants.
Study subjects underwent physical examinations and medical interviews. 112 individuals without clinical HTLV1 infection and 50 infected subjects with HTLV1 (matched by sex and age) were assessed from the MASHAD cohort study. Demographic and anthropometric information of 162 participants included as age, sex, smoking status, history of diabetes, educational level, hypertension, and diabetes were applied to our study.
2.2. Reagents
The assay was conducted using reagents from Amplex Red Cholesterol Assay Kit (Invitrogen, Life Technologies, Grand Island, NY), phosphate buffered saline PBS (BioShop, Burlington, ON, Canada), polyethylene glycol (PEG) molecular weight (MW) 6000 Sigma-Aldrich (St. Louis, MO), deionized water, catalase enzyme Sigma-Aldrich (St. Louis, MO), and Black 96-well plates (SPL life science, Pocheon, South Korea). Serum HDL-C (mg/dL) was quantified by routine methods on an auto analyzer (Eppendorf, Germany).
2.3. HTLV1 infection assessment
All participants of MASHAD study were screened for HTLV1-specific antibodies using ELISA (Dia.Pro Diagnostic, Italy). A confirmatory test for HTLV1 antibody positive cases was undertaken using specific PCR primers as follows: TAX (5′ – AGGGTTTGG ACAGAGTCTT – 3′ and 5’-AAGGACCTTGAGGGTCTTA-3′) and LTR (5’-CATAAGCTCAGACCTCCGGG-3′ and 5′ -GGATGGCGGCCTCA GGTAGG-3′). If either of the two genes was present, the patient was confirmed to be infected with HTLV1 [20].
2.4. Determination of HDL lipid peroxidation
PEG precipitation was used to obtain ApoB depleted serum as follows: 40 μl PEG was added to 100 μl serum of each sample with proportion of 1:2.5 and incubated by 30 min at room temperature and centrifuged at 1000 rpm (4°C). A validated fluorometric assessment based on cell-free method was used to measure HDL lipid peroxidation (HDLox) [14]. In brief, 50 μl of ApoB depleted serum was added to the wells of a 96-well plates in duplicate. The negative control was 1X reaction buffer and positive control included 20 mM hydrogen peroxide (H2O2) working solution. The readout of conversion of Amplex Red to fluorescence resorufin was quantified for each sample every 5 min for an hour, at wavelengths of 530/590 nm using a plate reader (Biotek, Vermont). Pooled ApoB depleted serum of the healthy group was used as a control for each plate aiming at minimizing experimental variability. Using mean fluorescent readout of the pooled control and HDL-C level, mean fluorescence of each sample was normalized by the following calculation: “normalized” oxidized HDL (nHDLox) = [HDLox_sample × 40 (mg/dL)]/ [HDLox_control × HDLC sample (mg/dL)], where 40 mg/dL represents HDL-C of the pooled control [21].
2.5. Statistical analysis
All statistical analyses were performed using SPSS software, version 22. Results for normal and non-normally distributed data were reported as mean ± SD and median (interquartile range), respectively. Baseline characteristics of participants with and without HTLV1 were compared by Student’s t test for normal distributed parameters, chi-square for categorical ones, and Mann–Whitney test for variables with skewed distribution. The association between HDLox and HTLV1 infection was assessed by logistic regression model after adjustment for potential confounders including age, sex, BMI, smoking status, total cholesterol, diabetes, and hypertension.
3. Results
3.1. General characteristics of studied population across HTLV1 infection
Twenty nine percent of HTLV1 carriers were males and 71% were females. Our findings revealed that the mean age of the total population was 50 ± 8.4 years. As expected, HTLV1 infection was significantly higher in women than in men. We assessed demographic, anthropometric, and biochemical variables in HTLV1 and non-HTLV1 studied population (n = 161) (Table 1). There were no significant differences in weight, waist circumference, physical activity, diastolic blood pressure, fasting blood glucose among the two groups of individuals with and without HTLV1 infection (P > 0.1). The uninfected group had-higher systolic blood pressure (126.2 ± 19.9 mm Hg vs. 120.5 ± 19.8 mm Hg) than HTLV1 carriers.
TABLE 1.
General characteristics of studied population by HTLV1 serostatus (independent of CVD risk) (n = 162)
| Variable | Without HTLVI | With HTLVI | P-value |
|---|---|---|---|
| Anthropometrics | |||
| Age (year) | 50.8 ± 8.6 | 50.9 ± 8.2 | 0.9 |
| Gender (female) | 70.8% (N = 80) | 71.4% (N = 35) | 0.9 |
| Weight (kg) | 68.52 ± 13.9 | 68.4 ± 11.75 | 0.9 |
| BMI (kg/m2) | 27.07 ± 4.84 | 27.37 ± 4.96 | 0.7 |
| WC (cm) | 94.49 ± 12.07 | 94.42 ± 11.5 | 0.9 |
| PAL | 1.63 ± 0.28 | 1.66 ± 0.3 | 0.5 |
| Blood pressure | |||
| SBP (mm Hg) | 126.2 ± 19.9 | 120.5 ± 19.8 | 0.1 |
| DBP (mm Hg) | 81 ± 11.89 | 80.25 ± 14 | 0.7 |
| Lipid profile | |||
| Serum total cholesterol (mg/dl) | 198.3 ± 34 | 194.4 ± 38 | 0.5 |
| TG (mg/dl) | 141.5 ± 107.7 | 146.43 ± 140.7 | 0.8 |
| HDL-C (mg/dl) | 51.31 ± 18.03 | 47.9 ± 10.5 | 0.06 |
| LDL (mg/dl) | 116.15 ± 33.22 | 115 ± 34.23 | 0.8 |
| Blood glucose | |||
| FBG (mg/dl) | 93.26 ± 36.78 | 90.2 ± 24.7 | 0.6 |
| Inflammation | |||
| SerumUric Acid (mg/dl) | 4.43 ± 1.36 | 4.85 ± 1.36 | 0.1 |
| Serum Hs-CRP (mg/dl) | 1.6(1.14–3.37) | 1.96(1.07–4.9) | 0.6 |
| Serum HDLox | 0.83 ± 0.24 | 1.05 ± 0.3 | <0.001 |
Abbreviations: BMI, body mass index; WC, waist circumference; PAL, physical activity level; BSP, blood systolic pressure; BDP, blood diastolic pressure; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; FBG, fasting blood glucose; Hs-CRP, high sensitive C-reactive protein; HDLox, HDL lipid peroxidation.
aData reported as mean ± STD except for Hs-CRP.
3.2. Lipid alterations
Based on our data, triglyceride, LDL, total cholesterol, and HDL-C level were not associated with HTLV1 status. There was no difference in mean serum HDL-C in subjects without HTLV1 infection (51.31 ± 18.03 mg/dl) and HTLV1 carriers (47.9 ± 10.5 mg/dl) (P = 0.6). Our results showed that although the fasted lipid profile was not affected by HTLV1 status, HDLox was significantly different between the groups (P < 0.001). Serum HDLox was 0.90 ± 0.27 for the total population (n = 162) and was significantly higher in the HTLV1 group (1.05 ± 0.3) compared to the healthy subjects (0.83 ± 0.24) (P < 0.001) (Figure 1 and Table 1).
FIG 1.
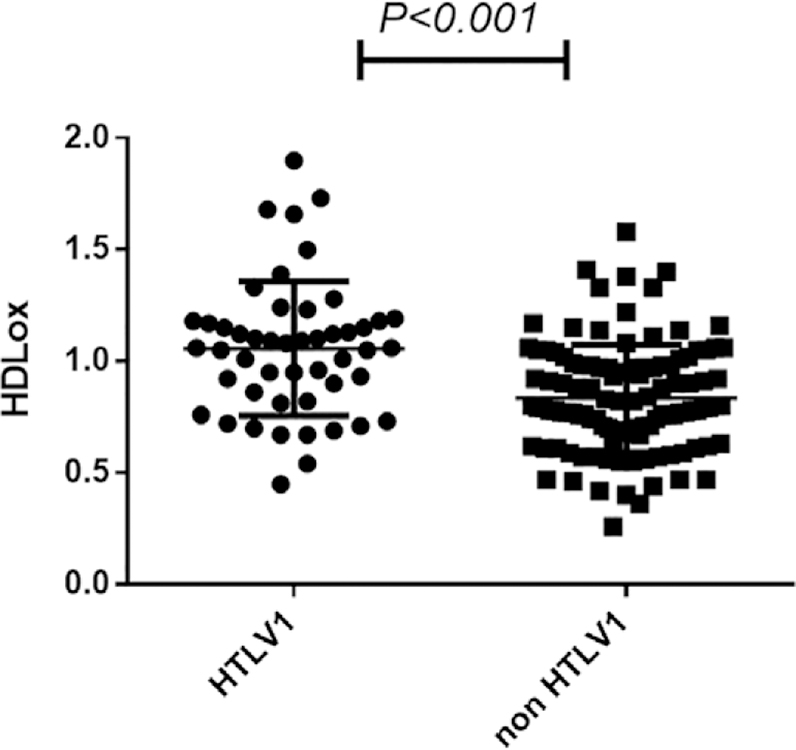
The Amplex red assay was used to assess serum HDLox in subjects with HTLV1 infection. ApoB depleted sera were prepared by PEG precipitation from 50 HTLV1 infected individuals and 112 uninfected subjects. The HDL lipid peroxidation (HDLox) was quantified as a marker of cardiovascular events in study participants, so that subjects with higher serum HDLox are exposed to an increased risk of cardiovascular outcomes. The HTLV1 infected individuals have significantly higher HDLox (1.05 ± 0.3) compared to the healthy subjects (0.83 ± 0.24) (P < 0.001).
We assessed the association between HDLox and HTLV1 infection in studied population and found a significant difference between infected subjects and healthy individuals. Univariate logistic regression analysis confirmed the association between HDLox and HTLV1 infection (odds ratio 6.38, CI: 3–13.6; P < 0.001). In a multivariate model, adjusted for age, sex, BMI, smoking, cholesterol, diabetes and hypertension, and HTLV1 infection were associated with increased HDLox (odds ratio 9.35, CI: 3.5–24.7; P < 0.001) (Table 2).
TABLE 2.
The association of serum HDL-C and HDLox with HTLV-1
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Serum variable | OR (95% CI) | P-value | OR (95% CI) | P-value |
| HDL-C | 0.97 (0.4–2) | 0.9 | 1.10 (0.5–2.45) | 0.8 |
| HDLox | 6.38 (2.97–13.67) | <0.001 | 9.35 (3.5–24.7) | <0.001 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HDLox, HDL lipid peroxidation.
The odds ratio was adjusted for age, sex, BMI, smoking, HDL-C, LDL, triglyceride, and total cholesterol level.
4. Discussion
There are limited data evaluating cardiovascular risk in individuals with HTLV1 infection [22]. Herein, we assessed HDL lipid peroxidation as a surrogate measure of HDL dysfunction and a predictor of CVD in the MASHAD cohort study. Subjects who were positive for HTLV1 infection had an increased level of HDL lipid peroxidation, compared to those who were negative. Serum HDLox was approximately 20% higher in HTLV1-infected subjects than the uninfected group. In univariate logistic regression, HDLox but not serum HDL-C was associated with HTLV1 infection. After adjustment for potential confounders, the association remained significant. To our knowledge, this is the first demonstration that patients with HTLV have impaired HDL function compared to uninfected subjects. HDL dysfunction may be a contributor to CVD in chronic HTLV infection.
A growing body of genetic and clinical evidence suggests that chronic viral infections such as HIV and hepatitis virus infections are related to heightened inflammation and CVD prevalence [23,24]. It has also been demonstrated that subjects with HTLV1 infection have increased inflammation in various tissues [25]. HIV-1 is a retrovirus that contributes to increased inflammation, endothelial dysfunction, and accelerated atherosclerosis [26], and HTLV1 has similar replication enzymes including retroviral proteases [27] and may also contribute to CVD. Lipoproteins like HDL and LDL are important for pathogenesis of atherosclerotic CVD [28]. In states of systemic inflammation like atherosclerosis and chronic infections, lipoproteins can be modified and get oxidized. Although many studies described the relation of LDLox and atherosclerosis, the impact of LDLox on atherogenesis remains unclear [29]. Unlike LDL, HDL is part of the innate immunity and also has the ability to influence cholesterol availability in lipid rafts in immune cells resulting in the modulation of toll-like receptors, MHC-II complex, as well as B-and T-cell receptors, while specific molecules shuttled by HDL such as sphingosine-1-phosphate (S1P) contribute to immune cells trafficking. Thus, as a platform integrating innate and adaptive immunity, HDL may have a major role in pathogenesis of chronic viral infections [12]. We confirmed this hypothesis in chronic HIV infection, where we found that HDLox rather than LDLox was consistently and independently associated with several biomarkers of systemic inflammation and immune dysfunction that predict morbidity, CVD, and mortality in chronictreated HIV infection [18]. In addition, emerging evidence suggests that HDLox may also be important for pathogenesis of atherosclerotic CVD [14]. Thus, HDLox rather than LDLox may be important for pathogenesis of chronic viral infections (like HIV and HTLV) and atherosclerosis. Our data in this study provide further evidence for this hypothesis that need to be validated in further models of chronic viral infections (e.g., chronic hepatitis and other chronic viral infections).
HTLV1 carriers showed a higher carotid intima-media thickness compared to the control group, and HTLV1 infection may consider to be an independent CVD risk factor [30]. Consistent with these findings, Shabestari et al. showed that the rate of HTLV1 sero-positivity in individuals who suffered from CVD is about three times higher than the general population [31]. However, the mechanism of HTLV-related CVD remains unclear.
Chronic inflammation present in chronic viral infections may contribute to CVD. In a cohort study in the HTLV1 endemic region of Japan, it has been shown that the interaction between HTLV1 infection and TNFα 1031 T/C as a polymorphism of inflammation may be a risk factor for CVD. Of note, several studies have reported changes of the immune-inflammatory status in subjects infected by HTLV1. TNFα plays a key role in the metabolism of lipid, insulin resistance, endothelial function, and coagulation, and particularly TNFα 1031 allele C was correlated with CVD risk and dyslipidemia [32,33]. However, consistent to our findings in other studies, serum hs-CRP and IL-6 were not significantly associated with the risk of CVD in the HAM/TSP patients [22]. Thus, other factors such as lipid alter ations may contribute to pathogenesis of CVD in chronic HTLV infection. We found no association between lipid parameters, including HDL-C, LDL, triglyceride, and total cholesterol, nor serum hsCRP with HTLV1 infection, as compared to the healthy subjects.
These data are in consistent with prior cross-sectional studies that did not demonstrate consistent associations between traditional cardiovascular risk factors [34], lipid levels, and HTLV serostatus [22]. In a study that evaluated lipid alterations in women infected with HTLV1, increased levels of very low-density lipoproteins and triglyceride were found in the women with HTLV1 and HAM/TSP groups as compared with healthy subjects [35]. Thus, changes in function of HDL rather than alterations in the levels of serum lipids and HDL-C may be a better predictor of CVD in chronic HTLV infection.
Alterations in HDL function may contribute to both increased CVD risk and impaired host immune responses to pathogens such as viruses [36]. Multiple cohort studies using new techniques to evaluate HDL functionality rather than HDL-C level have consistently demonstrated HDL function represents a more accurate biomarker for predicting CVD compared to serum HDL-C. The inverse association between serum HDL and CVD risk is likely to be related to the multiple characteristics of HDL that include reverse cholesterol transport and its antioxidant properties [37,38]. Serum HDLox levels may reflect HDL dysfunction and is an independent predictor of CVD risk. Lipid hydroperoxides are produced during the oxidative modification of LDL, and this process in turn is involved in the formation of fatty streak. The role of lipid oxidation is well documented in the inflammation of arterial wall. The increased amount of lipid peroxide and oxidation of low-density lipoprotein have been demonstrated in systemic inflammation as well as atherosclerosis conditions [39]. Clinical studies have previously demonstrated the association between high-serum HDLox with cardiovascular events and obesity [40]. High-serum HDLox is found in oxidative stress such as Human Immunodeficiency Virus infection (HIV) [14]. Thus, similar to chronic HIV-1 infection, chronic HTLV infection is also associated with impaired HDL function that may contribute to increased CVD risk.
5. Conclusion
HDL lipid peroxidation is related to HDL dysfunction, and we have found higher serum levels of HDLox among subjects with HTLV1 infection than uninfected individuals, because HDLox is a marker of CVD risk, HDL lipid peroxidation may be associated with CVD outcomes in subjects with HTLV1 infection.
HDL dysfunction rather than traditional CVD risk factors and changes in lipid levels may be a predictor of CVD in subjects with HTLV1 infection. To our knowledge, these data are among the first to demonstrate that HDL dysfunction is present in chronic HTLV infection.
ACKNOWLEDGMENTS
This study was supported by the Mashhad University of Medical Sciences by a grant number 941684 as a part of PhD thesis of Mrs Sara Samadi.
Abbreviations:
- apoA1
Apolipoprotein A1
- CETP
Cholesteryl ester transfer protein
- CVD
cardiovascular disease
- FBG
Fasting blood glucose
- HAM/TSP
myelopathy/tropical spastic paraparesis
- HDL
High-density lipoprotein
- HDLox
HDL lipid peroxidation
- hs-CRP
High sensitive C-reactive protein
- HTLV1
Human T lymphotropic virus type
- LDL
Low density lipoprotein
- MASHAD
Mashhad-Stroke and Heart-Atherosclerotic-Disorders
- PEG
polyethylene glycol
- S1P
sphingosine-1-phosphate
Footnotes
CONFLICTS OF INTEREST
The authors have no conflict of interest to disclose.
References
- [1].Bangham CR, Cook LB, and Melamed A (2014) HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection. Semin Cancer Biol 26, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Verdonck K, González E, Van Dooren S, Vandamme A-M, Vanham G, et al. (2007) Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect. Dis 7, 266–281. [DOI] [PubMed] [Google Scholar]
- [3].Ghezeldasht SA, Shirdel A, Assarehzadegan MA, Hassannia T, Rahimi H, et al. (2013) Human T lymphotropic virus type I (HTLV-I) oncogenesis: molecular aspects of virus and host interactions in pathogenesis of adult T cell leukemia/lymphoma (ATL). Int. J. Public Health 16, 179. [PMC free article] [PubMed] [Google Scholar]
- [4].Shoeibi A, Etemadi M, Ahmadi AM, Amini M, and Boostani R (2013) “HTLV-I infection” twenty-year research in neurology department of Mashhad University of medical sciences. Iran J. Basic Med. Sci 16, 202–207. [PMC free article] [PubMed] [Google Scholar]
- [5].Vrielink H, and Reesink HW (2004) HTLV-I/II prevalence in different geographic locations. Transfus. Med. Rev 18, 46–57. [DOI] [PubMed] [Google Scholar]
- [6].Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, and Murphy EL (2005) Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24, 6058–6068. [DOI] [PubMed] [Google Scholar]
- [7].Hedayati-Moghaddam M, Fathimoghadam F, Mashhadi IE, Soghandi L, and Bidkhori H (2011) Epidemiology of HTLV-1 in Neyshabour, northeast of Iran. Iran Red Crescent Med. J 13, 424–427. [PMC free article] [PubMed] [Google Scholar]
- [8].Gessain A, and Cassar O (2012) Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol 3, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Leuven SI, Franssen R, Kastelein J, Levi M, Stroes ES, et al. (2007) Systemic inflammation as a risk factor for atherothrombosis. Rheumatology 47, 3–7. [DOI] [PubMed] [Google Scholar]
- [10].Gordon DJ, and Rifkind BM (1989) High-density lipoprotein—the clinical implications of recent studies. N. Engl. J. Med 321, 1311–1316. [DOI] [PubMed] [Google Scholar]
- [11].Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, et al. (1999) Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med 341, 410–418. [DOI] [PubMed] [Google Scholar]
- [12].Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, et al. (2013) Aging affects high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1831, 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].H.-T. C. Group (2014) Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med 371, 203–212. [DOI] [PubMed] [Google Scholar]
- [14].Kelesidis T, Roberts CK, Huynh D, Martínez-Maza O, Currier JS, et al. (2014) A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PloS One 9, e111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Navab M, Ananthramaiah G, Reddy ST, Van Lenten BJ, Ansell BJ, et al. (2005) The double jeopardy of HDL. Ann. Med 37, 173–178. [DOI] [PubMed] [Google Scholar]
- [16].Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah G, and Fogelman AM (2009) The role of dysfunctional HDL in atherosclerosis. J. Lipid Res 50, S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McMahon M, Grossman J, Skaggs B, FitzGerald J, Sahakian L, et al. (2009) Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol 60, 2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kelesidis T, Jackson N, McComsey GA, Wang X, Elashoff D, et al. (2016) Oxidized lipoproteins are associated with markers of inflammation and immune activation in HIV-1 infection. AIDS 30, 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ghayour-Mobarhan M, Moohebati M, Esmaily H, Ebrahimi M, Parizadeh SMR, et al. (2015) Mashhad stroke and heart atherosclerotic disorder (MASHAD) study: design, baseline characteristics and 10-year cardiovascular risk estimation. Int. J. Public Health 60, 561–572. [DOI] [PubMed] [Google Scholar]
- [20].Hamedi A, Akhlaghi F, Meshkat Z, Sezavar M, Nomani H, et al. (2012) The prevalence of human T-cell lymphotropic virus type 1 in pregnant women and their newborns. ISRN Obstet Gynecol 2012, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kelesidis T, Oda MN, Borja MS, Yee Y, Ng KF, et al. (2017) Predictors of impaired HDL function in HIV-1 infected compared to uninfected individuals. J. Acquir. Immune Defic. Syndr 75, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].do Prado FLS, Prado R, and Ladeia AMT (2017) Cardiovascular risk profile in patients with myelopathy associated with HTLV-1. Braz. J. Infect. Dis 21, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van Leuven SI, Sankatsing RR, Vermeulen JN, Kastelein JJ, Reiss P, et al. (2007) Atherosclerotic vascular disease in HIV: it is not just antiretroviral therapy that hurts the heart! Curr. Opin. HIV AIDS 2, 324–331. [DOI] [PubMed] [Google Scholar]
- [24].Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, et al. (2017) Risk of cardiovascular disease due to chronic hepatitis c infection: a review. J. Clin. Transl. Hepatol 5, 343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bangham CR (2000) HTLV-1 infections. J. Clin. Pathol 53, 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, et al. (2013) HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med 173, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rücker P, Horn AH, Meiselbach H, and Sticht H (2011) A comparative study of HIV-1 and HTLV-I protease structure and dynamics reveals a conserved residue interaction network. J. Mol. Model 17, 2693–2705. [DOI] [PubMed] [Google Scholar]
- [28].Upadhyay RK (2015) Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gao S, and Liu J (2017) Association between circulating oxidized lowdensity lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis. Translational Med 3, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Layegh P, Shoeibi A, Nikkhah K, Juibary AG, Raftari S, et al. (2014) Can HTLV-1 infection be a potential risk factor for atherosclerosis? Intervirology 57, 365–368. [DOI] [PubMed] [Google Scholar]
- [31].Shabestari M, Jabbari F, Farid Hosseni R, Rezaee S, Gharivani Y, et al. (2013) Human T lymphotropic virus type I (HTLV-I) is a risk factor for coronary artery disease. Iran. J. Basic Med. Sci 16, 224–227. [PMC free article] [PubMed] [Google Scholar]
- [32].Khovidhunkit W, Kim M-S, Memon RA, Shigenaga JK, Moser AH, et al. (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res 45, 1169–1196. [DOI] [PubMed] [Google Scholar]
- [33].Kairupan TS, Ibusuki R, Kheradmand M, Sagara Y, Mantjoro EM, et al. (2017) Interactions between inflammatory gene polymorphisms and HTLV-I infection for total death, incidence of cancer, and atherosclerosisrelated diseases among the Japanese population. J. Epidemiol 27, 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].de Aragão Dória GM, Gallazzi VO, Boa-Sorte N, Grassi MFR, and Galvão-Castro B (2015) No evidence of association between atherosclerosis, risk factors for cardiovascular disease and human T-cell lymphotropic virus type 1 (HTLV-1) infection. Retrovirology 12, P30. [Google Scholar]
- [35].Carvalho L. D. d., Gadelha SR, Marin LJ, Brito-Melo GEA, Martins CPS, et al. (2015) Are lipid disorders involved in the predominance of human T-lymphotropic virus-1 infections in women? Rev. Soc. Bras. Med. Trop 48, 759–761. [DOI] [PubMed] [Google Scholar]
- [36].Catapano AL, Pirillo A, Bonacina F, and Norata GD (2014) HDL in innate and adaptive immunity. Cardiovasc. Res 103, 372–383. [DOI] [PubMed] [Google Scholar]
- [37].Kelesidis T, Currier JS, Huynh D, Meriwether D, Charles-Schoeman C, et al. (2011) A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res 52, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, et al. (2014) HDL cholesterol efflux capacity and incident cardiovascular events. New England J. Med 371, 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Girotti AW (1998) Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res 39, 1529–1542. [PubMed] [Google Scholar]
- [40].Sen SR, Nguyen HCX, Angelovich TA, Hearps AC, Huynh D, et al. (2017) Cell-free biochemical fluorometric enzymatic assay for high-throughput measurement of lipid peroxidation in high density lipoprotein. J Vis Exp 2017, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]


