Abstract
Background
Most carotid revascularization studies define asymptomatic as symptom-free for >180 days; however, it is unknown if intervention carries similar risk among those currently asymptomatic but with previous symptoms (PS) versus those who were always asymptomatic (AA).
Methods
We compared peri-procedural and 4-year risk of PS versus AA patients in CREST randomized to endarterectomy (CEA) or stent/angioplasty (CAS). Proportional hazards models adjusting for age, sex and treatment were used to assess risk of peri-procedural stroke or death (S+D) (any stroke or death during peri-procedural period), stroke and death at 4 years (any S+D within peri-procedural period and ipsilateral stroke out to 4 years) and the primary endpoint at 4 years (any stroke, death and MI within peri-procedural period and ipsilateral stroke out to 4 years), of. Analysis was performed pooling the CEA-treated and CAS-treated patients, and separately for each treatment.
Results
Of 1181 asymptomatic patients randomized in CREST, 1104 (93%) were AA and 77 (7%) were PS. There was no difference in risk when comparing AA and PS in the pooled CAS+CEA population for peri-procedural S+D (2.0% vs 1.3%) S+D at 4 years (3.6% vs 3.2%) or for the primary endpoint (5.2% vs 5.8%). There were also no differences among those assigned to CEA (peri-procedural S+D, 1.5% vs 0%; S+D 4 year, 2.7% vs 0%; or primary endpoint (5.1% vs 2.4%)) or CAS (peri-procedural S+D, 2.5% vs 2.8%; S+D 4 year, 4.4% vs 6.9%; or primary endpoint (5.3% vs 9.8%)) when analyzed separately.
Conclusions
In CREST, only a small minority of asymptomatic patients had previous ipsilateral symptoms. The outcomes of peri-procedural S+D, peri-procedural S+D and ipsilateral stroke up to 4 years, and the primary endpoint did not differ for AA patients compared to PS patients.
Clinical Trial Registration
URL: http://clinicaltrials.gov. Unique Identifier:NCT00004732.
TOC summary:
Sub analysis of the CREST trial showed that carotid endarterectomy and carotid stenting in patients with symptoms >180 days before intervention had similar periprocedural and 4 year risk of stroke than patients who were never symptomatic. While symptomatic patients carry a higher procedural risk, that risk seem to revert to asymptomatic status after 6 months.
Introduction
Previous reports have documented that the peri-procedural complication rate following carotid endarterectomy (CEA) and carotid artery stenting (CAS) is influenced by the indication for operation.1-3 The highest complication rate (death and stroke) occurs in patients with acute stroke as indication for operation. The complication rate for patients with recent prior stroke, cerebral transient ischemic attack (TIA), and transient monocular blindness occurs in declining order with operations for asymptomatic stenosis resulting in the lowest complication rates.4 In the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), patients who had never had stroke symptoms or had had prior symptoms but had been asymptomatic for six months or more were categorized as being asymptomatic.5 The objective of this report was to determine whether or not there was a difference in outcome when comparing these two types of asymptomatic patients: those who were always asymptomatic (AA) versus those who were previously symptomatic (PS) but had been asymptomatic for more than six months.
Methods
CREST was a prospective, randomized, multi-centered trial with blinded endpoint adjudication comparing the safety and efficacy of CEA versus CAS in patients with symptomatic or asymptomatic extracranial carotid disease. The protocol was approved by the Institutional Review Boards or Ethics Committees at participating clinical centers, and all participants provided written informed consent. The study design and methods,6 and 4- and 10-year follow-up outcomes have been previously reported.5, 7
The primary focus of the paper is on whether there are differences in the safety of the procedure during the peri-procedural period and differences in the durability of the procedure, with a focus on stroke as the primary major risk of the procedure. Patients were followed by a combination of clinic visits and telephone contacts as previously described,6 and with medical records retrieved and suspected stroke events were adjudicated by a physician panel. In revascularization studies (such as CREST), these stroke events can be considered as both efficacy and safety outcomes. While for efficacy assessments the intention-to-treat analysis is the standard, with our focus on how safely the procedure we have focused on stroke event rates among those receiving the treatment (i.e., per protocol analysis). As such, for this study the CREST database was queried for patients who were categorized as asymptomatic and who received their assigned treatment within 30-days. These patients were divided into two groups, AA and PS. The demographic factors were compared between the two groups. Proportional hazards models adjusting for age and treatment were used to assess risk of 30-day stroke and/or death (S+D), and the risk of any 30-day S+D plus ipsilateral stroke at 4 years by asymptomatic status.
Results
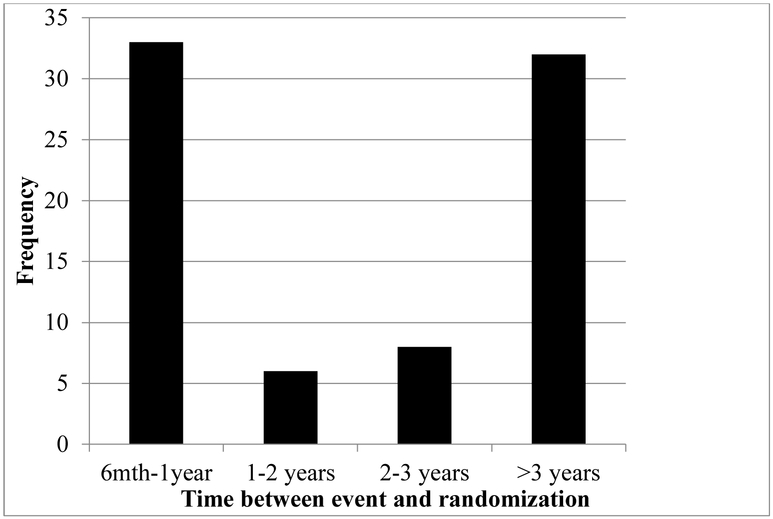
Of the 2502 patients in CREST, 1181 were asymptomatic of those, 1104 (93%) were AA and 77 (7%) were PS. The interval between symptoms and intervention is summarized in Figure 1. The characteristics of the AA and PS groups were generally similar (Table I). Five hundred ninety-four patients were CAS-treated, and 587 patients were CEA-treated. Of those randomized to CAS, 558 were AA and 36 were PS. Of those randomized to CEA, 546 were AA and 41 were PS.
Figure 1.
Time between event and randomziation for previously symptomatic subjects (n=79).
Table I.
Baseline characteristics of asymptomatic patients randomized to CAS or CEA who received procedure within 30 days of randomization.
| Asymptomatic Always (n=977) |
Previously Symptomatic (n=79) |
P-value | |
|---|---|---|---|
| Age (mean ± SD) | 69.1+8.0 | 70.1+7.1 | 0.26 |
| Female sex (%) | 32.9 | 31.7 | 0.83 |
| CAS treated (%) | 49.6 | 49.4 | 0.96 |
| Diabetes (%) | 34.2 | 29.1 | 0.36 |
| Hypertension (%) | 88.2 | 84.8 | 0.37 |
| Dyslipidemia (%) | 90.8 | 93.7 | 0.38 |
| Current smoker (%) | 24.6 | 21.8 | 0.58 |
CAS, carotid artery stenting; CEA, carotid endarterectomy; SD, standard deviation.
The number of events and event rates overall and by treatment are summarized in Table II The peri-procedural S+D rate for AA was 2.0% compared to 1.3% in the PS group, with a 57% higher hazard for stroke or death (HR = 1.57; 95% CI: 0.21 – 11.68) in the AA group; however, this difference was non-significant. The overall 4-year S+D event rate for the AA group was 3.6% and for the PS group was 3.2%, with a 27% higher hazard in the AA group (HR = 1.27; 95% CI: 0.31 – 5.28), also non-significant. The overall primary endpoint event rate for the AA group was 5.2% and for the PS group was 5.8% (HR = 0.95; 95% CI: 0.34 – 2.63), also non-significant.
Table II.
Hazard ratio and 95% confidence interval for 30-day and 4-year stroke and death by asymptomatic category in the pooled CAS+CEA population.
| AA # events (% ± SE) |
PS # events (% ± SE) |
HR (95% CI) AA vs PS |
P-value | |
|---|---|---|---|---|
| Stroke and death endpoint (30-day) | 19 (1.9±0.4) |
1 (1.3±1.3) |
1.60 (0.21, 11.97) |
0.65 |
| Stroke and death endpoint (4-year) | 32 (3.7±0.7) |
2 (3.1±2.2) |
1.37 (0.33, 5.71) |
0.67 |
AA, always asymptomatic; CAS, carotid artery stenting; CEA, carotid endarterectomy; HR, hazard ratio; PS, previously symptomatic; SE, standard error.
In the CAS-treated group, the peri-procedural S+D event rate for the AA group was 2.5% and for the PS group was 2.8%, with a hazard non-significantly higher in the AA group (HR = 1.03; 95% CI: 0.14 – 7.82). The 4-year S+D event rate for the AA group in CAS was 4.4% compared to 6.9% in the PS group, (HR = 0.79; 95% CI: 0.19 – 43.38). The primary endpoint at 4-years for the AA group in CAS was 5.3% compared to 9.8% in the PS group, (HR = 0.65; 95% CI: 0.20 – 2.13). (Table III). For CEA, the peri-procedural S+D rate in the AA group was 1.5% versus 0% in the PS group. The 4-year S+D rate for AA in the CEA group was 2.9% versus 0% in the PS group. Finally the primary endpoint at 4-years, for the AA group in CEA was 5.1% compared to 2.4% in the PS group, with a hazard non-significantly higher in the AA group (HR = 1.94; 95% CI: 0.26 – 14.34). (Table III).
Table III.
Hazard ratio and 95% confidence interval 30-day and 4-year stroke and death by asymptomatic category and treatment group.
| CAS only | CEA only | |||||||
|---|---|---|---|---|---|---|---|---|
| AA # events (% ± SE) |
PS # events (% ± SE) |
HR (95% CI) AA vs PS |
P-value | AA # events (% ± SE) |
PS # events (% ± SE) |
HR (95% CI) AA vs PS |
P-value | |
| Stroke and death endpoint (30-day) | 13 (2.7±0.7) |
1 (2.6±2.5) |
1.20 (0.15, 9.01) |
0.88 | 6 (1.2±0.5) |
0 (0.0±0.0) |
NA | NA |
| Stroke and death endpoint (4-year) | 21 (4.7±1.0) |
2 (6.3±4.4) |
0.95 (0.22, 4.05) |
0.94 | 11 (2.7±0.9) |
0 (0.0±0.0) |
NA | NA |
AA, always asymptomatic; CAS, carotid artery stenting; CEA, carotid endarterectomy; HR, hazard ratio; PS, previously symptomatic; SE, standard error.
Discussion
Early in the experience with CEA, it was recognized that the complication rate in symptomatic patients was higher than asymptomatic patients. However, it was not until 1989 when a special report requested and published by the Stroke Council of the American Heart Association actually codified this observation. This report evaluates the risk of a “gray zone” of previously symptomatic patients who have traditionally been included in the asymptomatic strata of revascularization trials, with the goal of assessing if their risk is similar to those who have been always asymptomatic. These data fail to demonstrate that these previously symptomatic patients should be treated differently than those always symptomatic.
There have been no publications to date suggesting that as the interval since the last symptom extends beyond a certain limit, the risk of CEA for a symptomatic indication may change. It is well recognized that a patient who experiences a TIA or a mild stroke is at greatest risk of a new stroke within a week of that event. However, as time from an event expands, that risk declines. In the medical arm of NASCET, the declining risk of stroke in a previously symptomatic patient approached that of the always asymptomatic patient when the interval from the last event was approximately two years.8 In 2009, Halm et al published their experience showing that the risk of complications after CEA was 2.7% in patients who were always asymptomatic, 4.1% in currently asymptomatic patients but with a past history of cerebrovascular disease, 5.6% in patients with TIA, 7.9% in patients with prior stroke, and 13.3% in patients with acute stroke.2 Geraghty et al, in 2014 reported the results of both CEA and CAS as a function of presenting symptom type from the Society for Vascular Surgery Registry. For CEA, the incidence of death and stroke in patients with prior ipsilateral stroke was 6.2%, 4% for TIA, and 2.2% in always asymptomatic patients. In contrast, CAS carried an 11% death/stroke rate in patients with prior stroke, 8.4% for TIA patients, and 4.3% for always asymptomatic patients.3
Our current study is unique. We found that in CREST only a small minority of the asymptomatic patients had had previous ipsilateral symptoms beyond 180 days prior to randomization. In addition, we report, for the first time, that the peri-procedural complication rate in patients who were previously symptomatic but had been asymptomatic for at least six months did not differ from those who had always been asymptomatic. This was true for both CAS and CEA patients although the CAS patients carried twice the risk of peri-procedural stroke and death than did the CEA patients, as has been shown in other studies.
The major limitations of this report result from the very low event rate, with only twenty 30-day events in the pooled analysis of CEA and CAS, and from the uneven distribution of patients in the AA versus PS groups (93% and 7% respectively). Combined, these two factors imply that hazards would be required to be larger than 11.65 or smaller than 0.09 to have 80% power for detection. For the 4-year outcome (where there are 34 events), hazards larger than 6.57 or smaller than 0.15 would be required to provide 80% power. While differences this large are unlikely, that our results failed to detect associations remains of importance as they provide estimates for potential subsequent meta-analyses. In addition, inability to detect differences in outcomes comparing 1056 PS and AA patients provides support for inclusion of both cohorts in subsequent treatment trials of asymptomatic carotid stenosis.9
The reasons for equal peri-procedural risk in the AA and PS patients are not clear. However, it suggests that with an increasing time interval following a cerebral ischemic event, the plaque appears to stabilize. The mechanisms are no doubt multi-factorial and perhaps reflect better medical management of the PS patient with the use of statins, antiplatelet drugs, and better risk factor management.
Conclusion
Carotid intervention in patients with asymptomatic carotid stenosis carries the lowest peri-procedural risk compared to other indications. Patients who have previously been symptomatic but are now asymptomatic for six months or longer carried the same peri-procedural risk as patients who have always been asymptomatic in CREST.
JVS-D-18-00126R1, Duration of Asymptomatic Status and Outcomes Following Carotid Endarterectomy and Carotid Artery Stenting in CREST
Type of Research: Subgroup analysis of the multicenter, prospective randomized CREST trial.
Key Findings: CEA and CAS in patients with symptoms >180 days prior to the carotid intervention had similar results compared to patients who were never symptomatic, with respect to periprocedural and 4 year risk of stroke.
Take Home Message: Patients with carotid stenosis and symptoms that occurred >180 days previously should be viewed as asymptomatic during the decision making process.
ACKNOWLEDGEMENTS
All those who meaningfully contributed to the manuscript are listed as an author.
SOURCES OF FUNDING
The study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (U01 NS 038384). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Supplemental funding was provided by Abbott Vascular, Inc.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
G. Roubin - Royalties: Cook Inc.; Ownership interest: Essential Medical.
All other authors - None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004;40:1126–1135 [DOI] [PubMed] [Google Scholar]
- 2.Halm EA, Tuhrim S, Wang JJ, Rockman C, Riles TS, Chassin MR. Risk factors for perioperative death and stroke after carotid endarterectomy: Results of the new york carotid artery surgery study. Stroke. 2009;40:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geraghty PJ, Brothers TE, Gillespie DL, Upchurch GR, Stoner MC, Siami FS, et al. Preoperative symptom type influences the 30-day perioperative outcomes of carotid endarterectomy and carotid stenting in the society for vascular surgery vascular registry. J Vasc Surg. 2014;60:639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery. Developed in collaboration with the american academy of neurology and society of cardiovascular computed tomography. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013;81:E76–123 [DOI] [PubMed] [Google Scholar]
- 5.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. New England Journal of Medicine. 2016;374:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the carotid revascularization endarterectomy vs. Stenting trial (crest). International journal of stroke : official journal of the International Stroke Society. 2010;5:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. The New England journal of medicine. 2010;363:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North american symptomatic carotid endarterectomy trial collaborators. The New England journal of medicine. 1998;339:1415–1425 [DOI] [PubMed] [Google Scholar]
- 9.Brajesh K Lal JFM, Brott Thomas G. Crest 2: Guiding treatments for asymptomatic carotid disease. Endovascular Today. 2013;12:73–76 [Google Scholar]