Abstract
Purpose:
To examine the relationship between lung radiation dose and survival outcomes in children undergoing total body irradiation (TBI)-based hematopoietic stem cell transplantation (HSCT) for acute lymphoblastic leukemia (ALL) on Children’s Oncology Group (COG) trial.
Patients and Methods:
TBI (1200 or 1320 cGy given twice daily in 6 or 8 fractions) was used as part of 3 HSCT preparative regimens; allowing institutional flexibility regarding TBI techniques, including lung shielding. Lung doses as reported by each participating institution were calculated for different patient setups, with and without shielding, with a variety of dose calculation techniques. The association between lung dose and transplant-related mortality (TRM), relapse-free (RFS) and overall-survival (OS) was examined using Cox proportional hazard regression model controlling for the following variables: TBI dose rate, TBI fields, patient position during TBI, donor type, and pre-HSCT minimal residual disease (MRD) level.
Results:
From a total of 143 eligible patients127 had lung doses available for this analysis. The TBI techniques were heterogeneous. The mean lung dose was reported as 904.5cGy (SD ±232.3). Patients treated with lateral fields were more likely to receive lung doses ≥800cGy (p<0.001). Lung dose ≥800cGy influence on TRM was not significant (HR 1.78; p=0.21). On univariate analysis, lung dose ≥800cGy was associated with inferior RFS (HR 1.76; p=0.04) and OS (HR 1.85; p=0.03); in the multivariate analysis, OS maintained statistical significance (HR 1.85; p=0.04).
Conclusion:
The variability in TBI techniques result in an uncertainty with reported lung doses. Lateral fields were associated with higher lung dose, hence better be avoided. Patients treated with lung dose <800 cGy in this study had better outcome. This approach is currently been investigated in COG AALL1331 study. Additionally, the Imaging and Radiation Oncology Core (IROC) Group is evaluating effects of TBI techniques on lung doses using a phantom.
Summary:
A secondary analysis of data from a phase III trial demonstrated no difference in relapse and inferior survival of patients receiving the lung dose ≥800cGy as part of their TBI regimen. TBI techniques, including the methods of lung dose calculation, were heterogeneous across the institutions participating in this study. These findings led to recommendations of lung-shielding above 800cGy for COG TBI protocols and triggered phantom-based dosimetry investigation of TBI techniques across the institutions.
Background:
Pulmonary toxicity is a common complication of hematopoietic stem cell transplantation (HSCT) and can be a significant contributor to transplant-related mortality (TRM).1, 2 While infections are a leading cause of early post-HSCT pulmonary toxicity, multiple factors can contribute to non-infectious lung injury.3–7Lung injury is a major dose-limiting toxicity for the total body irradiation (TBI). Because of the multiple confounding factors involved in HSCT treatment and institutional preferences, it has been challenging to define clear TBI parameters necessary to avoid pulmonary toxicity post HSCT.8–11 There is substantial heterogeneity in TBI techniques and dose/fractionation regimens utilized by transplant centers.
The Children’s Oncology Group (COG) trial was a phase III study that randomized children, adolescents, and young adults with acute lymphoblastic leukemia (ALL) in first (CR1) or second (CR2) complete remission to receive Graft Versus Host Disease (GVHD) prophylaxis with or without sirolimus.12 Patients enrolled on study received one of three myeloablative regimens prior to HSCT, which included chemotherapy plus 1200–1320 centiGray (cGy) TBI. Because this study allowed a range of institution-defined TBI techniques (specifically, the degree of lung dose attenuation) a study provided a unique opportunity to examine the relationship between lung dose, other TBI variables, and transplant-related outcomes.
Materials and Methods:
This phase III trial that compared a sirolimus-based GVHD prophylaxis regimen with a control regimen in children 1–22 years of age with ALL in CR1 or CR2 undergoing a TBI plus cyclophosphamide (Cy)-based myeloablative preparative regimen prior to allogeneic HSCT. The protocol was available to member institutions of the COG and Pediatric Bone & Marrow Transplant Consortium (PBMTC) from March 2007 to May 2011. Patients with high-risk ALL were eligible if they were aged 1 to 21 years and in a morphological complete remission (<5% bone marrow [BM] blasts, normal cerebrospinal fluid) tested within 14 days of initiating the preparative regimen. Three risk categories of patients were allowed: high-risk CR1 (Philadelphia chromosome positive [Ph1] ALL, extreme hypodiploidy [<44 chromosomes], or primary induction failure [>25% marrow blasts at induction day 29 or M2 (5–25% blasts) or >1% MRD at day 29 with persistence of M2 or >1% MRD at day 43); high-risk CR2 (B-cell BM relapse <36 months from diagnosis, T-cell or Ph1 BM relapse at any time, T-cell isolated extramedullary [IEM] relapse, <18 months from diagnosis); and intermediate risk CR2 (B-cell BM relapse >36 months from diagnosis, B-cell IEM, <18 months from diagnosis, intermediate risk patients only if matched sibling available). The CR1 was defined as no prior relapse; CR2: one prior relapse. Patients were required to have a stem cell donor who was an HLA-matched sibling (intermediate- and high-risk groups), or a 7–8/8 allele-level HLA-matched related or unrelated donor or a 4–6/6 matched single cord blood unit. Primary results of the trial have been published previously.12 Three preparative regimens utilized on study are described in Table 1. GVHD prophylaxis for the standard arm consisted of tacrolimus starting day −2 and methotrexate on days +1, +3, +6 for all stem cell sources and day +11 for unrelated BM or peripheral blood stem cells (PBSCs). Patients on the experimental arm received tacrolimus/methotrexate as in the standard arm with the addition of sirolimus by mouth starting on day 0.Patients enrolled on the study underwent an extensive pre-transplant evaluation to assess remission status, assure adequate organ system function, and document freedom from active viral, bacterial, and fungal infection. Patients were required to have good pulmonary function based on FEV1, FVC, and DLCO corrected for Hgb ≥ 60% by pulmonary function tests (PFTs). Children who were unable to cooperate for PFTs had to have no evidence of dyspnea at rest, no exercise intolerance, and no requirement for supplemental oxygen therapy. At study entry, patients were stratified by risk group and hematopoietic stem cell source.
Table 1.
Preparative Regimens
| Preparative Regimen | Treatment | Route | Dose | Days | Important Notes |
|---|---|---|---|---|---|
| Desired preparative regiment administration | TBI | 200cGy BID | Day −8, −7, &−6 | May deliver 1200cGy over 3 days per center preference | |
| Thiotepa | IV | 5 mg/kg/day | Day −5&−4 | ||
| Cyclophosphamide | IV | 60 mg/kg/day | Day −3&−2 | ||
| Rest | Day −1 | ||||
| Infusion of allogeneic HSCT | Day 0 | ||||
| Allowed Preparative Regimen Administration Variant 1. TBI/Cy/VP-16 Administration |
TBI | 200cGy BID | Day −8, −7, &−5 | May deliver 1200cGy over 3 days per center preference | |
| Etoposide | IV | 1500 mg/m2 | Day −4 | ||
| Cyclophosphamide | IV | 60 mg/kg/day | Day −3&−2 | ||
| Rest | Day −1 | ||||
| Infusion of allogeneic HSCT | Day 0 | ||||
| Higher Dose TBI/Cy Administration | TBI | 165cGy BID | Day −7, −6, −5&−4 | Total dose must be1320cGy | |
| Cyclophosphamide | IV | 60 mg/kg/day | Day −3&−2 | ||
| Rest | Day −1 | ||||
| Infusion of allogeneic HSCT | Day 0 |
Fractionated TBI was administered on this study to all patients as part of the HSCT conditioning regimen. The participating centers had flexibility in choosing between the 3 TBI –based preparative regimens (Table 1). Local centers were allowed to use their previously adopted TBI techniques, which included choosing beam and patient orientation. High-energy photons with energy ≥ 6 MV photons were required. Most centers utilized one of several positions: upright, reclining, sitting, prone/supine or lateral decubitus. The dose at selected anatomical points was required to be calculated and/or measured and submitted as part of the dose reporting process. Lung dose was to be reported at a reference point located on the right chest wall under the lung block. The depth was required to be taken as midway between the entrance and exit points of the opposing radiation beams. Lung shielding was encouraged for all patients, but only mandatory for patients receiving a total TBI dose exceeding 1200 cGy. To reduce the lung dose using partial transmission blocks of thickness 2 HVL (HVL=half-value layer is the thickness of lead or cerrobend1 where 50% of the incident radiation intensity was attenuated), the protocol provided the following guidelines: lung blocks were to be used for the first 3 fractions for 200 cGy twice daily and first 4 fractions for 150 cGy or 165 cGy twice daily regimens. For institutions using lung shielding, an electron boost to the chest wall was not required.
A mid-plane dose rate of between 6 and 15 cGy per minute was required. The goal for dose uniformity was to deliver at least 90% of the prescription dose throughout the body, from the midpoint of the thickest part of the body to within 2 mm of the skin surface. Prior to transplant, designated patients with extramedullary relapse could receive cranial or testicular radiotherapy boosting in addition to the doses of TBI associated with the preparative regimen.
All institutions participating in this protocol were required to have an approved TBI benchmark on file with the Quality Assurance Review Center (currently the IROC Rhode Island QA Center). Within one week of the completion of radiotherapy, participating centers submitted a copy of the treatment chart and the TBI summary form, which contained the required information concerning the TBI treatment including the reported lung dose.
Adverse events (AE) were reported using the NCI system for reporting for commercial agents (AdEERS), with routine AEs reported on COG case report forms using the NCI CTCAE version 4.0. Study routine reporting included all Grade 4 and higher Adverse Events, including lung toxicity, with an attribution of possible, probable or definite.
The reported lung dose received during TBI (1200 or1320 cGy given twice daily in 6 or 8 fractions) was analyzed in relation to the following variables: total TBI dose, TBI dose per fraction, TBI dose rate, TBI fields, patient position during TBI, pulmonary toxicity, acute graft versus host disease (GVHD), veno occlusive disease (VOD), transplant-related mortality (TRM), donor type, minimal residual disease (MRD) levels, relapse-free (RFS) and overall survival (OS).
Statistical Analysis
The Receiver Operating Characteristic (ROC) curves at various time points of OS were examined in order to determine the most reasonable cutoff of lung dose. The primary objective of this analyses was to assess the effect of lung dose level on RFS, TRM and OS, where RFS is defined as from enrollment to disease relapse or the last contact; the TRM is defined as the time from enrollment to death before relapse or the last contact, and OS is defined as the time from enrollment to death or the last contact. The cutoff of 800 cGy was selected based on the method of time-dependent ROC for censored survival data13. RFS and OS were estimated using the Kaplan-Meier method, and compared between groups using the Log rank test. The corresponding hazard ratio and its 95% confidence interval were estimated through the Cox proportional hazard regression model. Both univariate and multivariate analysis were performed to assess the association of clinical variables with TRM, RFS and OS. In a multivariate analysis, a backward selection method (with elimination probability of 0.10) was used to select prognostic factors from the following set of variables: dichotomized lung dose, field name, position, risk-donor group, treatment assignment and MRD at 0.10% level. All independent variables included in the multivariate model were categorical. The selected variables to be included in the final model were dichotomized lung dose and risk-donor group. All other variables were not significantly associated with the survival outcome when controlling for these two variables.
In addition, the association of lung dose level with each of the other clinical variables was tested using an exact Chi-square test. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results:
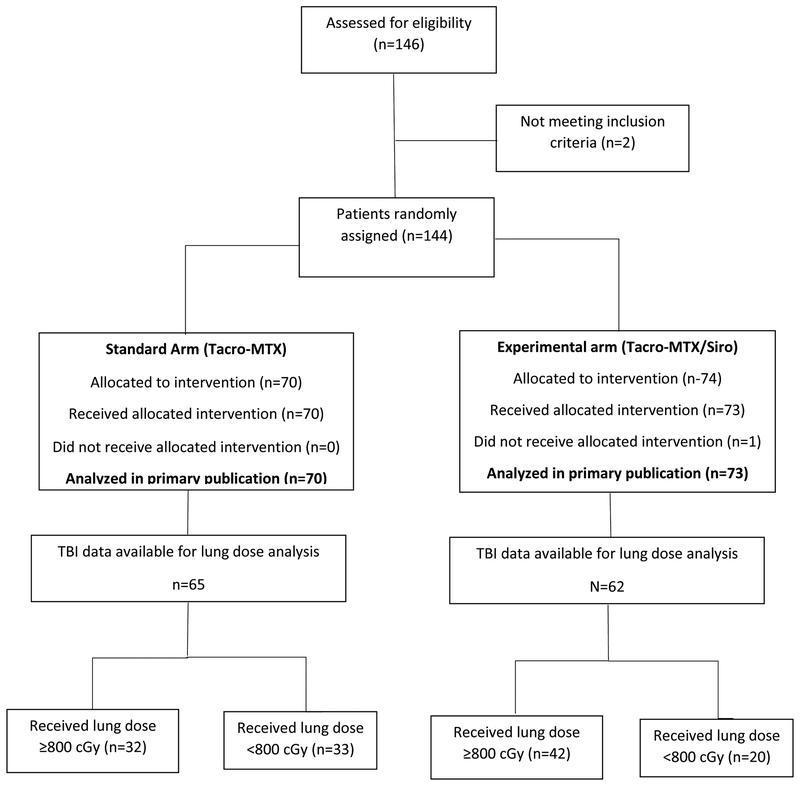
There were 146 patients enrolled on the study between 3/19/2007 and 5/10/2011, of which 143 were determined to be eligible and randomized and received either the standard (70) or the experimental (73) GVHD prophylaxis regimens. Treatment allocation is shown in the consort diagram (Figure 1). The median follow up was 2.97 years (range 0.04–6.93).
Figure 1.
Flowchart of study subject selection.
The large majority (85%) of patients received a preparative regimen of TBI at a dose of 1200 cGy given in 6 fractions over 3 or 4 days followed by thiotepa 5 mg/kg per day for 2 days and cyclophosphamide 60 mg/kg per day for 2 days. Etoposide1500 mg/m2 was substituted for thiotepa (allowed regimen variant 1) in 6% of patients. Nine percent of patients had an omission of thiotepa or etoposide and instead received a total of 1320 cGy TBI in 8 doses in addition to cyclophosphamide (allowed regimen variant 2). Patient, donor and disease characteristics were reported in a previous publication.12
The lung dose cutoff of 800 cGy was selected based on the method of time-dependent ROC for censored survival data. The 800 cGy is the operating point on the ROC curve with the largest True Positive and smallest False Positive rates. The AUC at 4-year RFS was 0.542, with a bootstrap 95% confidence interval [0.430, 0.654].
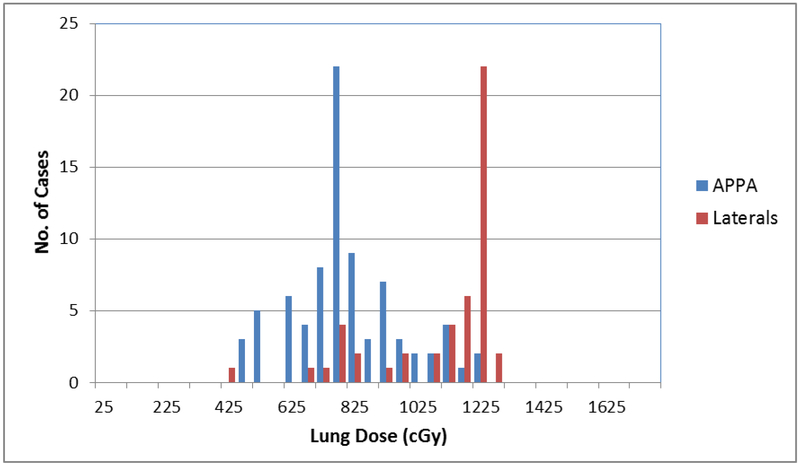
The dichotomized lung dose distribution by variables is shown in Table 2. The TBI position was supine for 51 patients, seated for 30 patients, standing for 27 patients, decubitus for 18 patients, and one patient with an unknown position. Eighty patients were treated with AP-PA fields, and 47 with opposed lateral fields. The reported mean lung dose was 904.5cGy (SD ±232.3). Patients treated with lateral fields were significantly more likely to receive reported lung dose estimates ≥800cGy (p<0.001). Patients treated with seated or supine position also were more likely to receive a higher lung dose (<0.001), but did not affect survival outcome. Figure 2 shows the range of lung doses reported for all the cases included in this analysis, with lung dose plotted in 50 cGy dose bins. The figure shows the difference in lung dose received by patients treated with APPA fields vs. those treated with lateral fields. The mean reported lung dose was 818 cGy (SD +/− 220 cGy) for patients treated APPA and 1139 cGy (SD +/− 103 cGy) for patients treated with lateral fields.
Table 2.
Variables Dichotomized by Lung Dose Level Groups
| Variable | Level | Lung dose level | Exact Chi-squre Test | |
|---|---|---|---|---|
| >=800 | < 800 | |||
| MRD levels | ||||
| MRD < 0.01% | 37 (61.67%) | 23 (38.33%) | 1.0000 | |
| MRD >= 0.01% | 13 (65.00%) | 7 (35.00%) | ||
| MRD levels | ||||
| MRD < 0.1% | 49 (59.76%) | 33 (40.24%) | 0.5557 | |
| MRD >= 0.1% | 10 (71.43%) | 4 (28.57%) | ||
| MRD levels | ||||
| Negative | 37 (61.67%) | 23 (38.33%) | 0.5016 | |
| Positive, 0.1%+ | 10 (71.43%) | 4 (28.57%) | ||
| Positive, <0.1% | 2 (40.00%) | 3 (60.00%) | ||
| Risk groups | ||||
| HR_CR2 | 39 (61.90%) | 24 (38.10%) | 0.3059 | |
| IR_CR2 | 8 (42.11%) | 11 (57.89%) | ||
| VHR_CR1 | 27 (60.00%) | 18 (40.00%) | ||
| Donor | ||||
| Matched | 40 (58.82%) | 28 (41.18%) | 1.0000 | |
| Other | 34 (57.63%) | 25 (42.37%) | ||
| Risk and Donor | ||||
| HR_CR2 Cord | 9 (42.86%) | 12 (57.14%) | 0.1966 | |
| HR_CR2 Matched | 17 (73.91%) | 6 (26.09%) | ||
| HR_CR2 Other | 13 (68.42%) | 6 (31.58%) | ||
| IR_CR2 Matched | 8 (42.11%) | 11 (57.89%) | ||
| VHR_CR1 Matched | 15 (57.69%) | 11 (42.31%) | ||
| VHR_CR1 Other | 12 (63.16%) | 7 (36.84%) | ||
| Treatment | ||||
| TacroMTX | 32 (49.23%) | 33 (50.77%) | 0.0475 | |
| TacroMTX Siroli | 42 (67.74%) | 20 (32.26%) | ||
| FieldName | ||||
| AP | 33 (41.25%) | 47 (58.75%) | 0.0000 | |
| RLAT | 41 (87.23%) | 6 (12.77%) | ||
| Position | ||||
| Decubitus | 14 (77.78%) | 4 (22.22%) | 0.0005 | |
| Seated | 22 (73.33%) | 8 (26.67%) | ||
| Standing | 7 (25.93%) | 20 (74.07%) | ||
| Supine | 30 (58.82%) | 21 (41.18%) | ||
Abbreviations: HR- high risk; CR-complete remission; VHR-very high risk; aGVHD-acute graft versus host disease; VOD-veno-occlusive disease; MRD-minimal residual disease; TRM-transplant-related mortality, TBI-total body irradiation;
Exact Chi-square Test.
Figure 2.
Distribution of lung dose by beam arrangement.
The trial required toxicity reporting of unexpected grade IV and all grade V toxicities along with specific transplant related toxicities: venoocclusive disease (VOD), thrombotic microangiopathy (TMA), and acute and chronic GVHD. Pulmonary toxicities were only reported as unexpected grade IV or V or part of a severe toxicity episode. In that context, definite pulmonary adverse events on this study were reported in 6% of patients, with possible pulmonary adverse events reported in 11% of enrolled subjects. The incidence of reported pulmonary adverse events was not affected by lung dose.
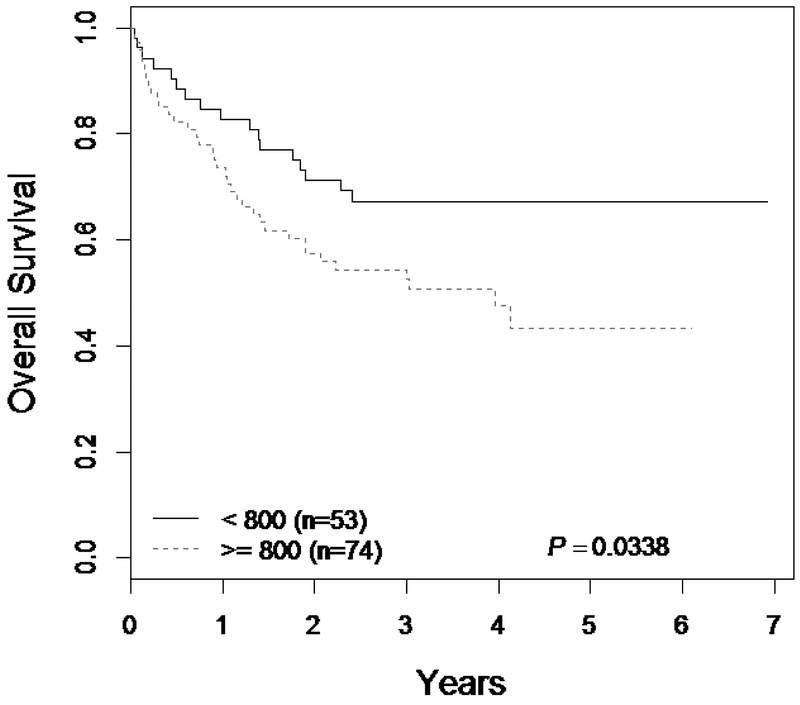
Univariate analysis showed that higher reported lung doses resulted in inferior OS (HR 1.85; p=0.03) and RFS (HR 1.76; p=0.04). TRM was strongly associated with the presence of pulmonary toxicity (HR 24; p<0.001), but the association lung dose ≥800cGy with TRM did not reach statistical significance (HR 1.6; p=0.29). Multivariable analysis identified reported lung doses ≥800cGy to be significantly associated with inferior OS (HR 1.85; p=0.043) along with a high-risk disease group and donor type (HR 1.34; p=0.007) (Table 4). Figure 2 shows Kaplan-Meier analysis of patients based on lung dose showing inferior OS for patients receiving lung doses ≥800cGy (p=0.03).
Table 4.
Multiviariable Analysis of Survival Outcome
| Parameter | Level | Reference level | Hazard Ratio | 95%CI for HR | P-value (compare to the reference level) | P-value (variable effect) | |
|---|---|---|---|---|---|---|---|
| Dose | >=800 | <800 | 1.855 | 1.021 | 3.372 | 0.0426 | 0.0426 |
| risk_donor | HR_CR2 Cord | VHR_CR1 Other | 1.342 | 0.562 | 3.204 | 0.5076 | 0.0074 |
| HR_CR2 Matched | VHR_CR1 Other | 1.069 | 0.455 | 2.508 | 0.8787 | ||
| HR_CR2 Other | VHR_CR1 Other | 0.955 | 0.393 | 2.317 | 0.9186 | ||
| IR_CR2 Matched | VHR_CR1 Other | 0.240 | 0.065 | 0.892 | 0.0331 | ||
| VHR_CR1 Matched | VHR_CR1 Other | 0.247 | 0.082 | 0.740 | 0.0125 | ||
Multivariate survival model include dose level, fieldname, position, disease risk group and donor type,
TacroMTX versus MacroMTX and Sirolimus treatment, MRD at 0.10% level.
Abbreviations: PFS-Progression-free survival, OS-overall survival; HR-high risk; IR-intermediate risk; CR-complete remission; VHR-very high risk;
Discussion:
The main objective of this analysis was to examine the impact of TBI variables on outcome of patients enrolled on this study that provided a large pediatric dataset with prospective clinical data. To the best of our knowledge, this is the first study to assess the impact of the range of TBI practices with a common target dose of 1,200 or 1,320 cGy in 6–8 fractions where the rest of the transplant variables were controlled. We focused specifically on the level of reported lung dosing and transplant-related outcomes in a pediatric population.
The incidence and etiology of acute non-infectious pulmonary toxicity has been studied extensively and risk factors have been found to be older age, lower pre-transplantation performance status, transplantation for a malignancy other than leukemia, high-intensity conditioning regimens, total body irradiation, high-grade acute GVHD, and methotrexate based GVHD prophylaxis.5–7 Historical data based on single fraction TBI found a correlation between radiation dose and lung toxicities and led to reduction of lung doses to 500–800 cGy, achieved with various attenuation techniques8,9,15–20 In the current era of widely adopted fractionated TBI regimens lung dose is typically kept in a relatively narrow range (700–1200 cGy). It has been challenging to detect direct correlation between lung dose received during fractionated TBI and pulmonary toxicity.21–28
Institutions put considerable effort into developing the TBI technique they use in clinical practice based on available machines, and modifying their technique for a particular protocol was deemed to be challenging. As a result, the method of calculating and/or measuring lung dose and the point in the lung where it was specified cannot be assumed to have been uniform for all patients. In past COG protocols an attempt was made to accommodate the variety of TBI techniques in common use. In our study the centers were allowed to choose any technique for TBI delivery in regard to patient positioning, beam orientation, beam energy and dose rate ranging from 6 to 15 cGy/min. Lung shielding was encouraged but was not mandated. This resulted in heterogeneous TBI variables and therefore provided an opportunity to study their impact on HSCT outcome.
Our analysis failed to detect a direct association between lung radiation dose and pulmonary toxicity, which was measured as adverse outcome in acute period. Definite pulmonary adverse events on this study were reported in only 6% of patients, with possible pulmonary adverse events reported in 11% of enrolled subjects. Because the study was not designed to collect pulmonary toxicities specifically, reported pulmonary issues were either associated with unexpected grade IV/V events or were reported sporadically by centers that entered more than the data required. With this in mind along with known concerns about the accuracy of this type of adverse event ascertainment in clinical trials,4 it is likely we did not collect sufficient data on this trial to give an accurate reflection of the true effect of reported higher lung TBI dosing on pulmonary outcomes. Additionally, study was not initially designed to answer this question and most likely statistically underpowered to detect such correlation.
Reasons for the lung dose differences include shielding positioning, lack of accounting for lung heterogeneities in reported doses, varying percent transmission of lung shields, etc. In addition, most institutional TBI delivery technique dose calculations are not based on patient CT data, but rather on slab or anthropomorphic phantom measurements that were used to benchmark the calculation technique. This benchmarking did not often include robust agreement for lung doses. The incorporation of computer tomography data in lung dose calculation resulted in a 10–24% dose increase compared to patients with uncorrected lung density and has been adopted by a majority of centers.30, 31 Recently, there has been efforts to more accurately measure TBI doses to organs demonstrating overall good correlation between wide-spread 2-dimensional methods of dose calculations and more modern 3-dimensional treatment planning systems with accuracy ranging from 1–3%.29,32–35. “While we acknowledge the uncertainty in reported lung dose for individual cases, the results of the statistical analysis presented here demonstrate an association of increased lung doses with patients treated with lateral fields.”36
In summary, methods of TBI delivery of a planned dose vary among institutions performing HSCT in pediatric patients and result in a higher uncertainty associated with the reported lung doses. Our results show that reduction of lung dose exposure during TBI to <800 cGy was safe and did not appear to cause higher disease relapse. Furthermore, survival outcomes were favorable for this group. This approach is currently being investigated in COG AALL1331 protocol. Additionally, the IROC is currently evaluating effects of TBI techniques on lung doses across the COG institution using a phantom.
Figure 3.
Overall survival for patients with total body irradiation (TBI) data by lung doses.
Table 3.
Univariate Analysis for patient with the lung dose data
| Variable | Level | TRM | RFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95%CI for HR | P-value | Hazard Ratio | 95%CI for HR | P-value | Hazard Ratio | 95%CI for HR | P-value | |||||
| Lung dose (Continuous) | 1.001 | 0.999 | 1.003 | 0.4635 | 1.001 | 1.000 | 1.002 | 0.1182 | 1.001 | 1.000 | 1.002 | 0.1026 | |
| Lung dose (dichotomized) | < 800 (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| >=800 | 1.781 | 0.721 | 4.398 | 0.2111 | 1.758 | 1.030 | 3.000 | 0.0384 | 1.855 | 1.039 | 3.312 | 0.0367 | |
| Dose rate (Continuous) | 0.963 | 0.858 | 1.081 | 0.5261 | 1.018 | 0.965 | 1.073 | 0.5227 | 1.008 | 0.947 | 1.073 | 0.7938 | |
| FieldName | AP (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| RIAT | 1.037 | 0.435 | 2.475 | 0.9341 | 1.337 | 0.807 | 2.214 | 0.2601 | 1.342 | 0.776 | 2.320 | 0.2919 | |
| Position | Standing (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| Decubitus | 1.540 | 0.446 | 5.325 | 0.4948 | 1.130 | 0.430 | 2.971 | 0.8037 | 1.242 | 0.462 | 3.336 | 0.6671 | |
| Seated | 1.039 | 0.300 | 3.600 | 0.9524 | 2.178 | 1.011 | 4.693 | 0.0467 | 1.669 | 0.722 | 3.859 | 0.2310 | |
| Supine | 0.848 | 0.268 | 2.683 | 0.7796 | 1.797 | 0.865 | 3.733 | 0.1161 | 1.612 | 0.745 | 3.488 | 0.2251 | |
| Risk groups | IR_CR2(Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| HR_CR2 | 5.966 | 0.784 | 45.411 | 0.0846 | 5.498 | 1.694 | 17.841 | 0.0045 | 5.066 | 1.557 | 16.484 | 0.0070 | |
| VHR_CR1 | 3.044 | 0.365 | 25.362 | 0.3033 | 3.655 | 1.089 | 12.263 | 0.0359 | 2.219 | 0.638 | 7.725 | 0.2103 | |
| Donor | Other (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| Matched | 0.221 | 0.081 | 0.601 | 0.0031 | 0.456 | 0.274 | 0.758 | 0.0025 | 0.440 | 0.253 | 0.764 | 0.0035 | |
| MRD levels | MRD < 0.1% (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| MRD >= 0.1% | 0.981 | 0.222 | 4.332 | 0.9795 | 2.818 | 1.417 | 5.605 | 0.0031 | 2.495 | 1.178 | 5.284 | 0.0170 | |
| Treatment | TacroMTX (Reference) | 1.000 | 1.000 | 1.000 | |||||||||
| TacroMTX_Sirolimus | 1.405 | 0.606 | 3.259 | 0.4277 | 1.286 | 0.781 | 2.117 | 0.3228 | 1.223 | 0.713 | 2.096 | 0.4653 | |
Abbreviations: TRM-treatment related mortality, PFS-Progression-free survival, OS-overall survival; HR-high risk; IR-intermediate risk; CR-complete remission; VHR-very high risk; aGVHD-acute graft versus host disease; VOD-veno-occlusive disease; MRD-minimal residual disease; TRM-transplant-related mortality, TBI-total body irradiation;
Logrank test
Acknowledgements:
This work was supported in part by National Institutes of Health grants (National Heart, Lung, and Blood Institute) N01 HC-45220/HHSN268200425220C, COG Chair’s grant U10 CA098543, and (National Cancer Institute) R01CA1116660. PBMTC activities were supported by (National Heart, Lung and Blood Institute) 2U01HL069254 and the St. Baldrick’s Foundation. IROC Rhode Island (QARC) activities is supported by grant U10CA29511 and IROC Houston activities by grant CA180803.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Authors have no relevant disclosures to this study; the ICMJE Form for Disclosure of Potential Conflicts of Interest will submitted by each author.
Cerrobend is an alloy composed of 50% bismuth, 26.7% lead, 13.3% tin, and 10% cadmium by weight.
References:
- 1.Krowka MJ, Rosenow EC 3rd, Hoagland HC: Pulmonary complications of bone marrow transplantation. Chest 87:237–246, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Quabeck K: The lung as a critical organ in marrow transplantation. Bone Marrow Transplant 14 Suppl 4:S19–28, 1994 [PubMed] [Google Scholar]
- 3.Spitzer TR: Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 27:893–898, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Afessa B, Tefferi A, Litzow MR, et al. : Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 166:641–645, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Afessa B, Litzow MR, Tefferi A: Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant 28:425–434, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Crawford SW, Longton G, Storb R: Acute graft-versus-host disease and the risks for idiopathic pneumonia after marrow transplantation for severe aplastic anemia. Bone Marrow Transplant 12:225–231, 1993 [PubMed] [Google Scholar]
- 7.Clark JG, Hansen JA, Hertz MI, et al. : NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis 147:1601–1606, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Fryer CJ, Fitzpatrick PJ, Rider WD, et al. : Radiation pneumonitis: experience following a large single dose of radiation. Int J Radiat Oncol Biol Phys 4:931–936, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Keane TJ, Van Dyk J, Rider WD: Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int J Radiat Oncol Biol Phys 7:1365–1370, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Barrett A, Depledge MH, Powles RL: Interstitial pneumonitis following bone marrow transplantation after low dose rate total body irradiation. Int J Radiat Oncol Biol Phys 9:1029–1033, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Morgan TL, Falk PM, Kogut N, et al. : A comparison of single-dose and fractionated total-body irradiation on the development of pneumonitis following bone marrow transplantation. Int J Radiat Oncol Biol Phys 36:61–66, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Pulsipher MA, Langholz B, Wall DA, et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: For Whom and When should Interventions be Tested? Bone Marrow Transplant, 50(9) (2015. September), pp. 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heagerty PJ, Zheng Y Survival Model Predictive Accuracy and ROC Curves Biometrics, 61, 92–105, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Miller TP, Li Y, Kavcic M, et al. : Accuracy of Adverse Event Ascertainment in Clinical Trials for Pediatric Acute Myeloid Leukemia. J Clin Oncol 34:1537–1543, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RJ, Langdon EA, Tesler AS: Total body irradiation utilizing a single 60Co source. Int J Radiat Oncol Biol Phys 1:549–552, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Lam WC, Order SE, Thomas ED: Uniformity and standardization of single and opposing cobalt 60 sources for total body irradiation. Int J Radiat Oncol Biol Phys 6:245–250, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Schmitt G, Schaefer UW, Nowrousian MR, et al. : Total body irradiation in conditioning patients for bone marrow transplantation. Irradiation technique and preliminary results at the West German Tumour Centre, Universitatsklinikum Essen. Pathol Biol (Paris) 27:363–364, 1979 [PubMed] [Google Scholar]
- 18.Barrett A, Barrett AJ, Powles RL: Total body irradiation and marrow transplantation for acute leukaemia. The Royal Marsden Hospital experience. Pathol Biol (Paris) 27:357–359, 1979 [PubMed] [Google Scholar]
- 19.Speck B, Cornu P, et al. (1979). “The Basel experience with total body irradiation for conditioning patients with acute leukemia for allogeneic bone marrow transplantation.” Pathol Biol (Paris) 27(6): 353–355. [PubMed] [Google Scholar]
- 20.Thomas ED, Buckner CD, Banaji M, et al. : One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 49:511–533, 1977 [PubMed] [Google Scholar]
- 21.Beyzadeoglu M, Oysul K, Dirican B, et al. : Effect of dose-rate and lung dose in total body irradiation on interstitial pneumonitis after bone marrow transplantation. Tohoku J Exp Med 202:255–263, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Oya N, Sasai K, et al. (2006). “Influence of radiation dose rate and lung dose on interstitial pneumonitis after fractionated total body irradiation: acute parotitis may predict interstitial pneumonitis.” Int J Hematol 83(1): 86–91. [DOI] [PubMed] [Google Scholar]
- 23.Abugideiri M, Nanda RH, Butker C, et al. : Factors Influencing Pulmonary Toxicity in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation in the Setting of Total Body Irradiation-Based Myeloablative Conditioning. Int J Radiat Oncol Biol Phys 94:349–359, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Kelsey CR, Horwitz ME, Chino JP, et al. : Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors. Int J Radiat Oncol Biol Phys 81:812–818, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Miralbell R, Rouzaud M, Grob E, et al. : Can a total body irradiation technique be fast and reproducible? Int J Radiat Oncol Biol Phys 29:1167–1173, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Ekstrand K, Greven K, Wu Q: The influence of x-ray energy on lung dose uniformity in total-body irradiation. Int J Radiat Oncol Biol Phys 38:1131–1136, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Ho A, Kishel S, Proulx G: Partial lung shield for TBI. Med Dosim 23:299–301, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Sampath S, Schultheiss TE, Wong J: Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys 63:876–884, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Stein A, Palmer J, Tsai NC et al. Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia.Biol Blood Marrow Transplant. 2017. April;23(4):618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangili P, Fiorino C, Rosso A, et al. : In-vivo dosimetry by diode semiconductors in combination with portal films during TBI: reporting a 5-year clinical experience. Radiotherapy and Oncology 52:269–276, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Battista JJ, Rider WD, Van Dyk J: Computed tomography for radiotherapy planning. Int J Radiat Oncol Biol Phys 6:99–107, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Van Dyk J, Battista JJ, Rider WD: Half body radiotherapy: the use of computed tomography to determine the dose to lung. Int J Radiat Oncol Biol Phys 6:463–470, 1980 [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Nieto B, Sanchez-Doblado F, Terron JA: A CT-aided PC-based physical treatment planning of TBI: a method for dose calculation. Radiotherapy and Oncology 42:77–85, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Zabatis C, Koligliatis T, Xenofos S, et al. : Dosimetry in translation total body irradiation technique: a computer treatment planning approach and an experimental study concerning lung sparing. Journal of Buon 13:253–262, 2008 [PubMed] [Google Scholar]
- 35.Bloemen-van Gurp EJ, Mijnheer BJ, Verschueren TA, et al. : Total body irradiation, toward optimal individual delivery: dose evaluation with metal oxide field effect transistors, thermoluminescence detectors, and a treatment planning system. Int J Radiat Oncol Biol Phys 69:1297–1304, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Bailey DW, Wang IZ, Lakeman T, et al. :TBI lung dose comparisons using bilateral and anteroposterior delivery techniques and tissueden sity corrections. J Appl Clin Med Phys. 2015. March 8;16(2):5293. [DOI] [PMC free article] [PubMed] [Google Scholar]