Abstract
Gasdermin D (GSDMD) cleavage by caspase-1 or caspase-11 inflammasomes triggers pyroptosis, a lytic form of cell death protective against intracellular bacteria. Here we examine the role of GSDMD in a mouse model of melioidosis. Gsdmd−/− mice were more susceptible than wild type mice to intranasal infection with Burkholderia thailandensis. Production of IL-18, but not IL-1β, was decreased in Gsdmd−/− infected mice. Despite lower IL-18, IFNγ was produced in similar amount in wild type and Gsdmd−/− mice. In vitro, secretion of both IL-1β and IL-18 by macrophages or dendritic cells infected with B. thailandensis was dependent on GSDMD. Surprisingly, wild type or GSDMD-deficient neutrophils secreted similar amount of IL-1β suggesting these cells may be the source of the GSDMD-independent IL-1β detected in vivo. Recombinant GSDMD was able to directly kill B. thailandensis in vitro upon processing by active caspase-1. Moreover, bacteria harvested from wild type, but not Gsdmd−/−, macrophages were more susceptible to the microbicidal effect of hydrogen peroxide or human β-defensin-3. Finally, we provide evidence that pyroptosis of in vitro infected macrophages is directly microbicidal. Taken together, these results indicate that the protective action of GSDMD in melioidosis is primarily due to induction of pyroptosis and direct killing of bacteria rather than production of cytokines.
INTRODUCTION
Pyroptosis is a lytic form of cell death that has been shown to be protective against infection with a variety of intracellular bacteria(1) (2) (3). Pyroptosis is triggered by activation of caspase-1 or caspase-11 in the context of canonical and non-canonical inflammasomes (4). Once activated, these caspases cleave the cellular protein gasdermin D (GSDMD) whose amino-terminal domain inserts into the cell membrane lipid bilayer forming pores that leads to osmotic lysis of the cell(5) (6) (7) (8). Several mechanisms may account for the protective action of pyroptosis. First, pyroptotic lysis of infected cells eliminates the replicative niche, a response highly effective against obligate intracellular bacteria. Moreover, disruption of cell membrane integrity allows various soluble antimicrobial molecules to gain access to the cell cytoplasm where bacteria replicate. It has also been proposed that during pyroptosis bacteria remain trapped inside the infected cells and eventually are killed by recruited neutrophils(9). Pyroptosis amplifies the inflammatory response by controlling secretion of IL-1β and IL-18, which depends on GSDMD in several, but not all, settings (10) (11) (12), and through the release of a cocktail of inflammatory cellular components. Finally, recent reports have shown that GSDMD is able to directly kill bacteria(7) (13) (14). This outside-in ability to damage bacterial membranes appears to contrast with the exclusively inside-out activity of GSDMD on eukaryotic membranes.
B. pseudomallei is a gram negative intracellular bacterium that infects various cell types and causes melioidosis, a disease endemic in South East Asia and other tropical regions(15). B. thailandensis is a related species not pathogenic for humans that causes a disease similar to melioidosis in mice(16). Although infection can be acquired through different routes, inhalation leads to the most severe form of melioidosis and is associated with high mortality. We and others have shown that the NLRP3 and NLRC4 canonical inflammasomes and Caspase-11 play critical protective roles during melioidosis(17) (18) (19) (20) (21). NLRP3 controls production of IL-18, a cytokine needed to survive melioidosis, but also IL-1β, a cytokine that is deleterious during infection with Burkholderia species. The role of NLRC4 in melioidosis is to trigger pyroptosis in myeloid cells while caspase-11 mediates this response in lung epithelial cells (19).
Here we examine the response to infection with B. thailandensis in mice lacking GSDMD. Our results show that Gsdmd−/ − mice were more susceptible to the infection not because of decreased production of protective IL-18/IFNγ cytokines but rather due to impaired pyroptosis and the microbicidal activity of GSDMD.
MATERIALS and METHODS
Ethics statement
All the animal experiments described in the present study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were conducted under protocols approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee (IACUC). All efforts were made to minimize suffering and ensure the highest ethical and humane standards.
Mice
C57BL/6J, Casp1−/−/Casp11−/−, and Gsdmd−/− mice (provided by Thirumala Kanneganti, StJude Children Hospital(22)), all on C57BL/6J genetic background, were bred under specific pathogen-free conditions in the RFUMS animal facility. Age-(8–12 weeks old) and sex-matched animals were used in all experiments. Experimental groups were composed of at least 5 mice, unless stated otherwise.
Bacteria strains, intranasal infections, and treatments
B. thailandensis E64 (obtained from ATCC) was grown in Luria broth to mid-logarithmic phase, the titer was determined by plating serial dilutions on LB agar, and stocks were maintained frozen at −80°C in 20% glycerol. For mouse infections, frozen stocks were diluted in sterile PBS to the desired titer. Mice were anesthetized using isoflurane and the infectious doses were applied to the nares in 50 μl total volume PBS.
Determination of bacterial growth in organs
Organs aseptically collected were weighed and homogenized in 1 ml PBS. Serial dilutions were plated on LB agar plates containing Streptomycin (100 μg/ml) using the Eddy Jet Spiral Plater (Neutec). Bacterial colonies were counted 24 hours later using the Flash & Grow Automated Bacterial Colony Counter (Neutec).
BALF collection, flow cytometry, and cytokine measurements
BALF were collected from euthanized mice by intratracheal injection and aspiration of 1 ml PBS. Cells obtained from BALF were counted and stained for 30 minuets with anti-CD11b (Biolegend, clone M1/70), anti-CD11c (Biolegend, clone N418), anti-F4/ 80 (Biolegend, clone BM8), and anti-Ly6G (BD Pharmingen, clone 1A8) and acquired with a LSRII BD flow cytometer. Data analyzed using FlowJo (TreeStar, OR, USA) software. Cytokine levels in tissue culture conditioned supernatants, BALF, and sera were measured by ELISA using the following kits: IL-1β, IFNγ, IL-12p70 (eBioscience), IL-18 (MBL Nagoya, Japan).
BMM, BMDC, and neutrophils, pyroptosis, and intracellular bacterial growth
BMM and BMDC were derived in RPMI1640–10% FCS from bone marrow in presence of conditioned media of L929 fibroblasts or HEK293T cells expressing GM-CSF. Neutrophils were purified from bone marrow using StemCells Technologies reagents (Mouse neutrophil enrichment kit). The purity of each cell preparations was determined by flow cytometry staining and routinely found to be of more than 90% for macrophages (defined as CD11b+/F4/80+) or neutrophils (CD11b+/Ly6G+) and of 70% for DC (CD11b+/CD11c+). Release of LDH in tissue culture media, a reflection of pyroptosis, was measured using the Roche Cytotoxicity Detection Kit (Roche Applied Science, 11644793001). Cells were plated in 48-well plates. Bacteria were added to the cell culture and the plates were centrifuged at 300 x g for 10 minutes to maximize and synchronize infection and incubated for 30 minutes at 37 °C. Cells were washed with PBS to remove extracellular bacteria and medium containing kanamycin and gentamicin (200 μg/ml each) was added to inhibit extracellular bacteria growth. Media were collected at different time points for LDH and cytokine measurement. Cells were lysed in PBS-2 % saponin-15 % BSA and serial dilutions of the lysates were plated on LB agar plates containing streptomycin (100 μg/ml).
Recombinant GSDMD expression and purification
A stationary phase culture of E. coli BL21 (DE3) transformed with the pDB.His.MBP vector encoding full length human GSDMD (kindly provided by Dr. Wu(7)) was diluted ten times in LB broth and protein expression was induced with 1mM IPTG for 4 hours. The bacteria pellet was lysed by four freeze-thaw and sonication cycles in 20 mM Tris-HCL, pH 7.4, 0.2 M NaCl, 1 mM EDTA, 1% NP40 and 0.1 mg/ml egg white lysozyme. Crude lysate was passed on an Amylose resin (NEB) column followed by wash with 10 column volumes of 20 mM Tris-HCL, pH 7.4, 0.2 M NaCl, 1 mM EDTA. His-MBP-GSDMD protein was eluted with 10 mM maltose and concentrated with Microcon centrifugal filters 30 kDa cut-off (Millipore). To obtain full length GSDMD the His-MBP tag was removed by digestion with AcTEV protease (100 U/ml, Invitrogen), 6 hours, 16 C. AcTEV protease, which carries a 6His tag at the N-terminus was removed from the cleavage reaction by Nickel resin chromatography.
In vitro Gasdermin D cleavage and bactericidal assay
B. thailandensis (104 CFU) were incubated for 2 hours at 37° C with full length GSDMD (5 μg/ml) in presence or absence of 0.5 Unit active caspase-1 (Enzo) in a final volume of 10 μl. Bacteria dilutions were then plated on LB agar/ streptomycin.
Bacteria harvest from infected BMM and treatment with H202 or β-defensin-3
Wild type or Gsdmd−/− BMM were infected with B. thailandensis (MOI 50) for 4 hours in presence of glycine 5 mM to prevent cell lysis. BMM were washed in PBS twice and lysed in PBS-2 % saponin-15 % BSA to harvest intracellular bacteria. Lysates were treated for 30 minutes with PBS, H2O2 (2 mM), or human β-defensin-3 (50 μg/ml)(Preprotech) before plating on LB agar/ streptomycin.
Statistical analysis
All data were expressed as mean ± S.D. Survival curves were compared using the log rank Kaplan-Meier test. One-way ANOVA & Tukey Post-test or unpaired t-test were used for analysis of data as specified in the figure legends. Significance was set at p<0.05.
RESULTS
Gasdermin D is protective in melioidosis
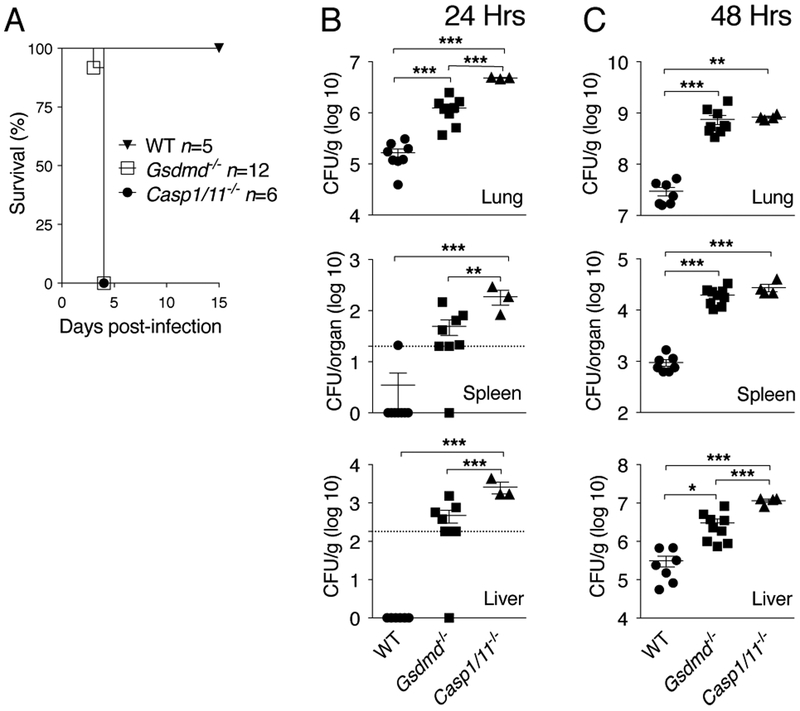
Previous works from our group and others have shown that canonical and non-canonical inflammasomes play a protective role during lung infection with B. thailandensis indicating a critical role for pyroptosis(17) (18) (19) (20). As shown in figure 1A, mice deficient in GSDMD, whose cleavage by caspase-1 or caspase-11 triggers pyroptosis, are more susceptible to B. thailandensis intranasal infection than wild type mice and succumb synchronously 4 days after infection, similarly to Casp1−/−/Casp11−/− mice. Compared to wild type mice, Gsdmd−/ −mice had higher bacterial burdens in organs 24 and 48 hours p.i. (figure 1B, C). Interestingly, bacterial burden in Casp1−/−/Casp11−/− mice was significantly higher than Gsdmd−/− mice suggesting the existence of GSDMD-independent protective mechanisms downstream of caspase-1/11.
Figure 1. Gasdermin D is protective in melioidosis.
(A) Mice were infected intranasaly with B. thailandensis (105 CFU) and their survival was monitored. (B, C) Mice infected with B. thailandensis (5 × 105 CFU) were sacrificed at the indicated time points p.i. and the bacterial burden in organs were measured. For B and C data were pooled from two independent experiments. Dotted line indicates the bacterial titer detection limit. Data are expressed as mean ± S.D. *p<0.05, **p<0.01, ***p<0.001. (A) log rank Kaplan-Meier test, (B, C) One-way ANOVA.
Gasdermin D controls in vivo production of IL-18 but not IL-1β
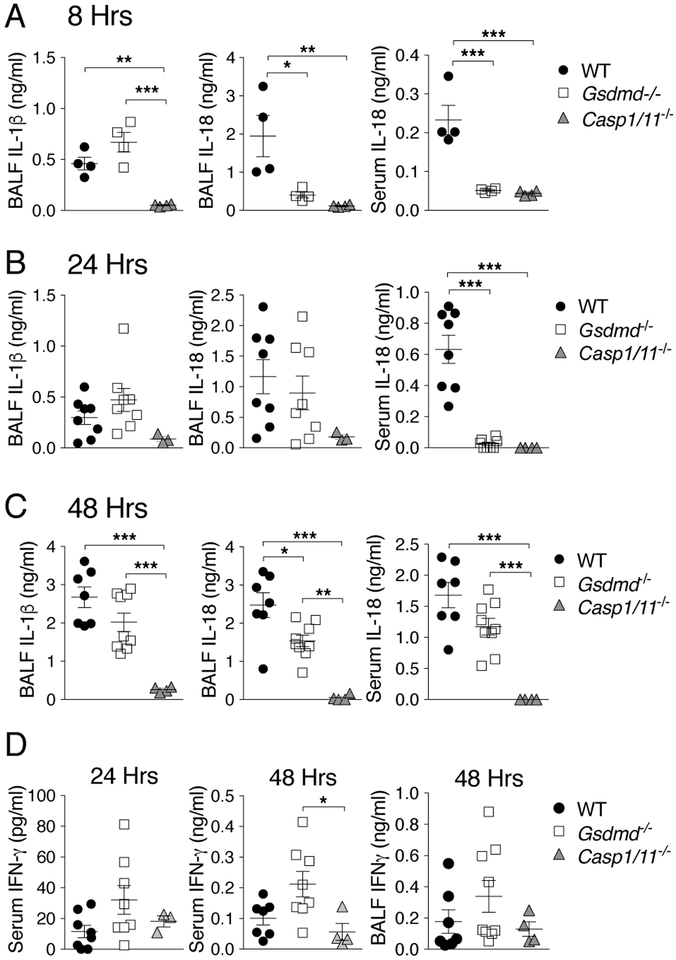
Levels of IL-1β and IL-18 were measured in wild type and Gsdmd−/− mice 8, 24, and 48 hours p.i. (figure 2). While IL-1β levels in BALF were not significantly different between these mouse strains at any time point, IL-18 was detected in reduced amount in BALF or serum of Gsdmd−/− mice. Despite reduced IL-18 production in Gsdmd−/− mice, the level of IFNγ, the cytokine that mediates the protective action of IL-18 in melioidosis(19), were comparable in sera or BALF of wild type or Gsdmd−/− mice (figure 2D). IL-12p70 was also decreased in Gsdmd−/− mice (Supplementary figure 1) indicating that it does not compensate the reduced level of IL-18 for induction of IFNγ. As expected, the levels of IL-1β and IL-18 were drastically reduced in Casp1−/−/Casp11−/− mice. Taken together, these results suggest that GSDMD protects mice from B. thailandensis infection and that it partially contributes to secretion of IL-18. Surprisingly, secretion of IL-1β in vivo appeared to be only modestly affected by absence of GSDMD.
Figure 2. Gasdermin D controls in vivo production of IL-18 but not IL-1β.
Mice infected with B. thailandensis (A, 5 × 106 CFU; B-D, 5 × 105 CFU) were sacrificed at the indicated time points p.i. and cytokines in BALF and serum were measured. For B-D data were pooled from two independent experiments. Data are expressed as mean ± S.D. *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA.
Gasdermin D controls in vitro production of IL-1β and IL-18 in macrophages and dendritic cells but not in neutrophils
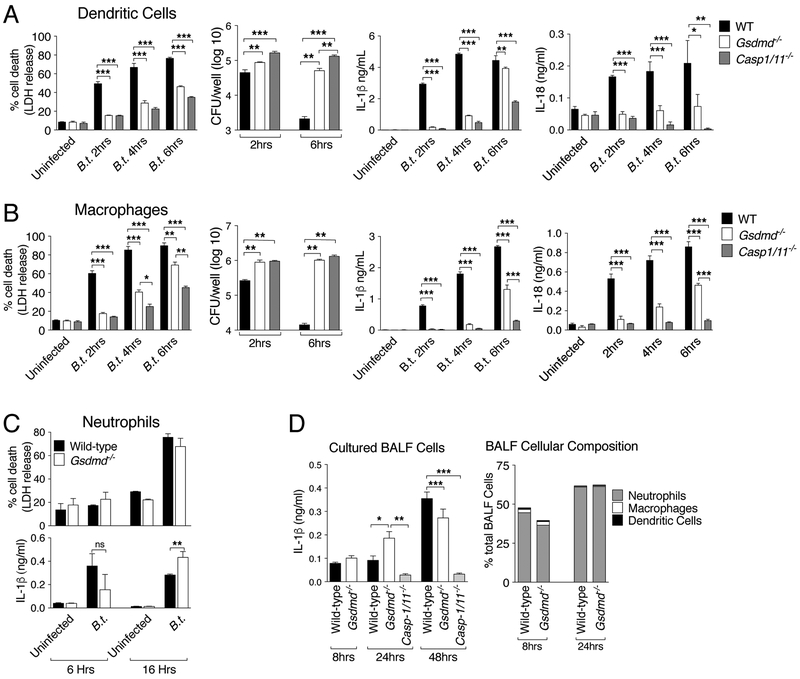
The observation that IL-18, but not IL-1β, production in vivo requires GSDMD is quite surprising as secretion of both cytokines is primarily dependent on processing by caspase-1. To better understand this phenomenon, we examined IL-1β/IL-18 secretion and pyroptosis in wild type and GSDMD-deficient bone marrow-derived dendritic cells (BMDC) or macrophages (BMM) infected in vitro with B. thailandensis. As shown in figures 3A, B, LDH release, a measure of pyroptosis, was drastically decreased in BMDC or BMM lacking GSDMD as previously shown(5, 6). Concomitantly, intracellular replication of B. thailandensis was more robust in GSDMD-deficient cells than wild type cells. Secretion of IL-1β and IL-18 was also significantly impaired in BMDC or BMM lacking GSDMD. This result contrasts with the secretion pattern observed in vivo and suggests that a cell type other than macrophages or DC may be responsible for the GSDMD-independent production of IL-1β observed in vivo. For this reason we focused our attention on neutrophils, the most abundant leukocytes that are capable of secreting IL-1β. As shown in Figure 3C, secretion of IL-1β by neutrophils was not affected by absence of GSDMD and was caspase-1-dependent (supplementary figure 2). IL-18 was not detected in neutrophil culture supernatants (not shown). Cell death of infected neutrophils was GSDMD-independent. Moreover, cells purified from the BALF of wild type or Gsdmd−/− mice 8, 24, or 48 hours p.i. secreted similar amounts of IL-1β when cultured in vitro for 6 hours (figure 3D). Flow cytometry analysis confirmed that the composition of the BALF cells was comparable in wild type and Gsdmd−/− mice and was found to be constituted primarily by neutrophils. Taken together, these results indicate that GSDMD controls production of IL-1β and IL-18 in macrophages and DC while neutrophils secrete IL-1β in a GSDMD-independent fashion and may be the cellular source of the GSDMD-independent IL-1β detected in vivo. These results also indicate that the protective action of GSDMD in melioidosis is not due to impaired IL-18/IFNγ production but rather may be related to GSDMD induction of pyroptosis.
Figure 3. Gasdermin D controls in vitro production of IL-1β and IL-18 in macrophages and dendritic cells but not in neutrophils.
BMM (A), BMDC (B), and neutrophils (C) were infected with B. thailandensis (MOI 50). LDH release, intracellular bacteria replication, and cytokine secretion were measured at the indicated time points p.i. (D) Cells obtained from BALF of mice (n=3) infected (5 × 105 CFU) for the shown amount of time were cultured in vitro for 6 hours and IL-1β was measured in conditioned supernatants. Right graph shows cellular composition of BALF. One representative experiment of two (D), three (C), four (A, B) is shown. Data are expressed as mean ± S.D. *p<0.05, **p<0.01, ***p<0.001. Unpaired t-test, One-way ANOVA.
Gasdermin D directly kills bacteria and renders them more susceptible to attack by H2O2 and β-defensin-3
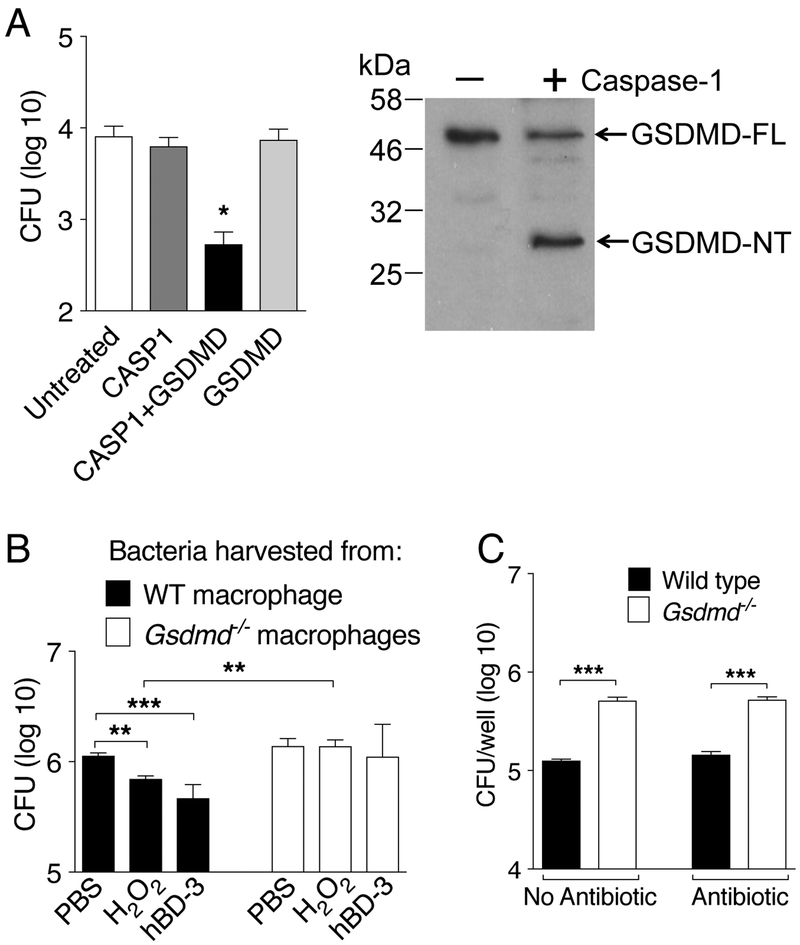
GSDMD has been proposed to be directly bactericidal(7) (13) (14). To test whether B. thailandensis is susceptible to this activity, bacteria were incubated with recombinant full length GSDMD in presence or absence of purified active caspase-1. As shown in figure 4A, bacteria viability was not affected by incubation with caspase-1 or full length GSDMD. However, when both proteins were present, bacteria viability was severely impaired. Importantly, recombinant caspase-1 was found to cleave full length GSDMD generating the N-terminal fragment responsible for executing pyroptosis(14) (7). Although it is still unclear how GSDMD may damage eukaryotic or bacterial membranes, it has been shown that bacteria recovered from wild type macrophages are more susceptible to attack by various damaging agents than bacteria recovered from GSDMD-deficient macrophages, likely due to destabilization of bacterial membrane by GSDMD(9). In agreement with this notion, B. thailandensis obtained from wild type BMM were significantly more susceptible to the microbicidal activity of hydrogen peroxide or human β-defensin-3 than bacteria obtained from GSDMD-deficient BMM (figure 4B). Finally, supporting a direct bactericidal activity of GSDMD, significantly more bacteria could be recovered from GSDMD-deficient than wild type BMM even when the infection occurred in presence of glycine, to prevent cell lysis, but in absence of gentamicin/kanamycin (figure 4C). These results are consistent with the notion that pyroptosis is protective against intracellular bacteria not just because cell lysis allows penetration of various extracellular anti-microbial agents but also because GSDMD directly damages bacterial membranes.
Figure 4. Gasdermin D directly kills bacteria and renders them more susceptible to attack by H2O2 and β-defensin-3.
(A) B. thailandensis (104 cfu) was incubated with recombinant GSDMD in presence or absence of active caspase-1 for 2 hrs. Bacteria were then plated on LB agar/streptomycin. Left panel shows cleavage of full length GSDMD to generate the N-terminal domain. (B) Wild type or Gsdmd−/− BMM were infected with B. thailandensis (MOI 50) for 4 hours in presence of glycine (5 mM). Intracellular bacteria were harvested from lysed BMM and treated for 30 minutes with H2O2 (2mM) or human β-defensin-3 (50 μg/ml) before plating dilutions on LB agar/streptomycin. (C) BMM were infected with B. thailandensis (MOI 50) as in B in presence or absence of gentamicin/kanamycin (400 μg/ml) for 4 hours. Intracellular bacteria replication was measured. One representative experiment of two (A, B) or three (C) is shown. Data are expressed as mean ± S.D. *p<0.05, **p<0.01, ***p<0.001. (A,B) One-way ANOVA, (C) t-test.
DISCUSSION
Previous works from our and others lab have shown the critical role of inflammasome and pyroptosis during infection with Burkholderia species(21). In agreement with those results and a recent paper, we report here that Gsdmd−/− mice are more susceptible to melioidosis and succumb to infection with significantly higher bacterial burden than wild type mice. Surprisingly, while GSDMD-deficient BMM or BMDC secreted drastically lower amount of IL-1β or IL-18, absence of GSDMD in vivo significantly decreased production of IL-18 but not IL-1β. This contrasts with the nearly complete inability of Casp1−/−/Casp11−/− mice to produce mature IL-1β or IL-18 and suggests the existence of caspase-1-dependent but GSDMD-independent pathways for IL-1β secretion, as previously proposed(11) (23). Our data indicate that neutrophils are able to release IL-1β in a GSDMD-independent fashion and may be the cellular source of the IL-1β detected in vivo. It should be noted that in our experiments with neutrophils both pyroptosis and IL-1β secretion depended on caspase-1. Interestingly, IFNγ, the cytokine that mediates the protective action of IL-18 in melioidosis, was detected in similar amount in wild type or Gsdmd−/− mice suggesting that the amount of IL-18 produced by Gsdmd−/− mice, although lower than that of wild type mice, is sufficient to stimulate protective amount of IFNγ. Thus, the susceptibility of Gsdmd−/− mice to B. thailandensis does not appear to be due to inability to produce IL-18/IFNγ. Rather, the protective response absent in Gsdmd−/− mice may be related to pyroptosis of infected cells.
Recent work by the Miao lab has shown that, in addition to exposing intracellular bacteria to extracellular antimicrobial molecules, the pyroptotic lytic cell death immobilized bacteria into intracellular traps(9). Our data point to an additional mechanism responsible for the microbicidal action of pyroptosis. We have shown here that recombinant GSDMD was able to kill B. thailandensis in vitro after processing into the N-terminal fragment by active caspase-1. Moreover, bacteria recovered from GSDMD-deficient macrophages were more resistant than those recovered from wild type cells to the toxic action of hydrogen peroxide or β-defensin-3 suggesting that GSDMD-dependent sublethal damaging of bacterial membrane synergizes with the action of antimicrobial agents. These findings are in agreement with previous works (7) (13) (14)and expand the repertoire of the antimicrobial mechanisms deployed by induction of pyroptosis.
It is interesting to note that our study refined our understanding of the dynamic of the gentamycin protection assay. The accepted interpretation of this assay is that the integrity of the cellular membrane excludes gentamicin and therefore allows measurement of replication of intracellular bacteria. Induction of pyroptosis, or other forms of lytic cell death, in wild type but not inflammasome or GSDMD-deficient cells, would allow penetration of antibiotic and killing of bacteria. Our results show that regardless of the presence of gentamicin/kanamycin, wild type cells restrict B. thailandensis intracellular replication much more efficiently than GSDMD-deficient cells. This indicates that, at least in the case of B. thailandensis, the restriction of intracellular bacteria replication by pyroptotic wild type cells observed in the gentamycin protection assay is due to the toxic effect of GSDMD rather than that of the antibiotic (to which B. thailandensis is highly resistant). The importance of this result is that it demonstrates that pyroptosis can be directly microbicidal, an activity previously unappreciated.
Supplementary Material
Key points.
GSDMD-deficient mice are more susceptible to melioidosis
In vivo production of IL-1β by neutrophils is independent of GSDMD
GSDMD and pyroptosis are directly microbicidal
ACKNOWLEGMENTS
We are grateful to Thirumala Kanneganti (StJude Children’s Research Hospital) for providing Gsdmd−/− mice, to Hao Wu (Harvard Medical School) for the pDB.His.MBP-GSDMD vector, and to Bob Dickinson for help with flow cytometry.
This work was supported by The National Institutes of Health (grants AI130763, AI110783) to F.R.
REFERENCES
- 1.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, and Aderem A. 2010. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen I, and Miao EA. 2015. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 265: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade WA, and Zamboni DS. 2018. Inflammasome-dependent Mechanisms Involved in Sensing and Restriction of Bacterial Replication. Curr Issues Mol Biol 25: 99–132. [DOI] [PubMed] [Google Scholar]
- 4.Jimenez Fernandez D, and Lamkanfi M. 2015. Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem 396: 193–203. [DOI] [PubMed] [Google Scholar]
- 5.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, and Dixit VM. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, and Lieberman J. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Gao W, and Shao F. 2017. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42: 245–254. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen I, Zhang Y, Krantz BA, and Miao EA. 2016. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med 213: 2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC. 2018. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48: 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semino C, Carta S, Gattorno M, Sitia R, and Rubartelli A. 2018. Progressive waves of IL-1beta release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways. Cell Death Dis 9: 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, Holley CL, Boucher D, Shakespear MR, Kapetanovic R, Rolfes V, Sweet MJ, Stow JL, and Schroder K. 2018. Interleukin-1beta Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep 24: 1425–1433. [DOI] [PubMed] [Google Scholar]
- 13.Qiu S, Liu J, and Xing F. 2017. ‘Hints’ in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell Death Differ 24: 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, and Shao F. 2016. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535: 111–116. [DOI] [PubMed] [Google Scholar]
- 15.Wiersinga WJ, van der Poll T, White NJ, Day NP, and Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4: 272–282. [DOI] [PubMed] [Google Scholar]
- 16.West TE, Frevert CW, Liggitt HD, and Skerrett SJ. 2008. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans R Soc Trop Med Hyg 102 Suppl 1: S119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, and Re F. 2011. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 7: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahoo M, Del Barrio L, Miller MA, and Re F. 2014. Neutrophil elastase causes tissue damage that decreases host tolerance to lung infection with burkholderia species. PLoS Pathog 10: e1004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Sahoo M, Lantier L, Warawa J, Cordero H, Deobald K, and Re F. 2018. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog 14: e1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, and Miao EA. 2013. Caspase-11 protects against bacteria that escape the vacuole. Science 339: 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahoo M, Lantier L, and Re F. 2016. Role of Canonical and Non-canonical Inflammasomes During Burkholderia Infection. Curr Top Microbiol Immunol 397: 199–214. [DOI] [PubMed] [Google Scholar]
- 22.Karki R, Lee E, Place D, Samir P, Mavuluri J, Sharma BR, Balakrishnan A, Malireddi RKS, Geiger R, Zhu Q, Neale G, and Kanneganti TD. 2018. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell 173: 920–933 e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider KS, Gross CJ, Dreier RF, Saller BS, Mishra R, Gorka O, Heilig R, Meunier E, Dick MS, Cikovic T, Sodenkamp J, Medard G, Naumann R, Ruland J, Kuster B, Broz P, and Gross O. 2017. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep 21: 3846–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.