Abstract
Advanced and metastatic squamous cell carcinomas (SCCs) are common and difficult-to-treat malignancies. We assessed 75 immunotherapy-treated SCC patients from a clinically annotated database of 2,651 patients, as well as 9,407 patients from a de-identified database for molecular features that might influence checkpoint blockade response. SCCs had higher tumor mutational burdens (TMB) than non-SCCs (P <0.0001). Cutaneous SCCs had the highest TMB (P <0.0001), with 41.3% demonstrating a very high TMB (≥50 mutations/mb). In immunotherapy-treated SCC patients, higher TMB (≥12 mutations/mb) correlated with a trend to higher clinical benefit rate (stable disease ≥6 months or partial/complete remission; 60% versus 29%; (high versus low TMB) p=0.06) and significantly longer median time-to-treatment failure (TTF) (9.9 versus 4.4 months, p=0.0058). Cutaneous SCCs had the highest clinical benefit [11/15 patients (73%) versus 20/60 (33%) non-cutaneous (p=0.008)], TTF (p=0.0015), and overall survival (OS; p=0.06) with immunotherapy treatment. In conclusion, amongst a diverse set of SCCs, higher TMB and cutaneous disease associated with better immunotherapy outcome.
Keywords: squamous cell carcinoma, immunotherapy, tumor mutational burden
INTRODUCTION
Squamous cell carcinomas (SCCs) occur in tissues that are lined with squamous epithelium. Common sites for SCC include the lung, head and neck, esophagus, and skin (1). Many types of SCCs are lethal, and several often present with locally advanced or metastatic disease. In contrast, the majority of cutaneous SCCs are cleared with local therapies (e.g., excision). However, cutaneous SCC can progress over time, leading to tissue destruction and morbidity. Rarely, cutaneous SCCs can metastasize to regional lymph nodes and distant sites (2,3), and treatment options for locally advanced or metastatic cutaneous SCCs are suboptimal and consist of radiation therapy and chemotherapy, though various other treatments have been tried (4).
Studies of the genomic landscape of SCCs (or “squamousness”) arising in diverse sites have suggested the targeting the PI3K-AKT-mTOR and/or cyclin pathway components (5–8). Studies have suggested that SCCs from different organs can share patterns of molecular alterations (6,7). Human papilloma virus (HPV) is a major cause of several SCCs including oropharyngeal, cervical, and cutaneous tumors (9). For the most part, HPV-negative SCCs harbor TP53 and cyclin mutations whereas HPV-positive patients harbor more PI3K pathway alterations (10,11). Distinct mutation profiles in HPV-positive and HPV-negative SCCs of the head and neck identify subgroups with poor outcomes after adjuvant chemoradiation. Mutations in TP53, NOTCH1, KDR, and the PI3K pathway have been recognized as possible targets for subgroup-specific treatment regimens (12).
High tumor mutational burden (TMB) has been acknowledged as a response biomarker for PD-1/PD-L1 blockade in multiple tumor types (13). Higher TMB correlates with better treatment outcomes, including higher response rates, longer progression-free survival (PFS), and longer overall survival (OS), in diverse cancers treated with immunotherapies compared to tumors with a low TMB (13). Patients with cancers harboring mismatch repair gene alterations, which are almost always associated with high TMB, also benefit from checkpoint inhibitors (14). Cutaneous SCCs have many molecular features that predict response to immunotherapy, including a high TMB, possibly due to ultraviolet (UV) light driven mutations and an increased disease risk among patients with immunosuppression (15,16). PD-1/PD-L1 blocking antibodies have been shown to be efficacious in the treatment of advanced SCCs. Nivolumab and pembrolizumab have both been approved for the treatment of advanced head and neck and lung SCC (17–19), and cemiplimab has been approved for the treatment of advanced cutaneous SCC (20). In this study, we explored the response to immunotherapy and the genomic features, including TMB, of a variety of SCCs. We observed high response rates in those tumors with a high TMB and in advanced cutaneous SCCs.
MATERIALS AND METHODS
Patient selection
A total of 2,651 patients were reviewed from a clinically annotated University of California San Diego (UCSD) database. Data for those with SCC and treated with immunotherapy were extracted for analysis. All patients had undergone hybrid capture-based next generation sequencing (NGS) (FoundationOne) and were treated at UCSD Moores Cancer Center. Immunotherapy agents included anti–PD-1 and anti-programmed death ligand 1 (PD-L1) and various combination regimens (Table 1). The TMB of 9,407 patients with SCC were reviewed from a large database (Foundation Medicine (FM)). This study was performed in accordance with UCSD Institutional Review Board guidelines for data analysis (NCT02478931) and for any investigational treatments, for which patients provided written consent. The Foundation Medicine data was approved by the Western Institutional Review Board (Protocol No. 20152817). This study was conducted in accordance with the Declaration of Helsinki.
Table 1:
Patient demographics by squamous cell carcinoma type (N=75 treated with immunotherapy; UCSD cohort)
| Variable | All patients (N = 75) | Cutaneous SCC (n = 15) | Other SCC (n = 60) | P-value1 |
|---|---|---|---|---|
| Median age (range) in years2 | 67 (33–90) | 67 (45–79) | 69 (33–90) | 0.4663 |
| Sex | ||||
| Male | 54 (72%) | 14 (93%) | 40 (67%) | 0.0534 |
| Female | 21 (28%) | 1 (7%) | 20 (33%) | |
| Ethnicity | ||||
| White | 61 (81%) | 14 (93%) | 47 (78%) | 0.2763 |
| Hispanic | 7 (9%) | 1 (4%) | 6 (10%) | 1.0000 |
| Black | 3 (4%) | 0 (0%) | 3 (5%) | 1.0000 |
| Asian | 3 (4%) | 0 (0%) | 3 (5%) | 1.0000 |
| Other | 1 (2%) | 0 (0%) | 1 (2%) | 1.0000 |
| Disease status2 | ||||
| Locally advanced | 23 (31%) | 5 (33%) | 18 (30%) | 0.7654 |
| Metastatic | 52 (69%) | 10 (67%) | 42 (70%) | |
| Genomics | ||||
| Median time (range) in months from biopsy used for genomic analysis to treatment with checkpoint blockade | 7.9 (−16.1–43.2) | 8.5 (−3.3–31.9) | 6.5 (−16.1–43.2) | 0.3535 |
| Median number (range) of genomic alterations3 | 7 | 10 (5–22) | 5 (0–12) | <0.0001 |
| Median TMB (range) mutations/mb3 | 10 (1–347) | 63 (12–347) | 6 (1–25) | <0.0001 |
| TMB low | 11 (27%) | 0 (0%) | 11 (38%) | 0.0175 |
| TMB intermediate | 18 (44%) | 2 (17%) | 16 (55%) | 0.0378 |
| TMB high | 12 (29%) | 10 (83%) | 2 (7%) | 0.0001 |
| MSI status4 | ||||
| MS-stable | 33 (97%) | 7 (88%) | 26 (100%) | 0.2353 |
| MS-high | 1 (3%) | 1 (12%) | 0 (%) | |
| Treatment | ||||
| Median number (range) of prior systemic therapies | 1 (0–5) | 1 (0–3) | 1 (0–5) | 0.1993 |
| PD-1/PD-L1 blockade monotherapy | 68 (91%) | 15 (100%) | 53 (88%) | 0.3327 |
| PD-1/PD-L1 blockade + chemotherapy | 1 (2%) | 0 (0%) | 1 (2%) | 1.0000 |
| PD-1/PD-L1 blockade + targeted therapy | 1 (2%) | 0 (0%) | 1 (2%) | 1.0000 |
| PD-1/PD-L1 blockade + investigational agent | 5 (6%) | 0 (0%) | 5 (8%) | 1.0000 |
| Median TTF (range) in months1,2 | 4.8 (0–32.1+) | Not reached (0–27.3+) (median follow up of 10.1 mos) | 4.2 (0.1–32.1+) |
0.0015 HR = 0.3 [95% CI, 0.2–0.5] |
| Median OS in months1,2 | 17.4 (0.1–32.1+) | Not reached (0.3–27.3+) (median follow up of 11.2 mos) | 12.5 (0.1–32.1+) | 0.0593 HR = 0.5 [95% CI, 0.2–1.0] |
| Overall benefit rate (SD for ≥ 6 months plus PR/CR) | 31/75 (41%) | 11/15 (73%) | 20/60 (33%) |
0.008 OR = 5.5 [95% CI 1.6–16.9] |
Calculated using Student’s T-test, Fisher’s exact test, and log-rank (Mantel-Cox) where applicable
At the time of initiation of checkpoint blockade
Not available (N = 3 patient with cutaneous SCC and N = 31 for other SCC)
Not available (N = 7 patients with cutaneous SCC and N = 34 for other SCC)
Abbreviations: HR = hazard ratio; mb = megabase; MSI = microsatellite instability; OR = odds ratio; OS = overall survival; SCC = squamous cell cancer; TMB = tumor mutational burden; Time to treatment failure
Next-generation sequencing (NGS) and assessment of tumor mutational burden (TMB)
The FoundationOne assay was used (hybrid-capture-based NGS; 236 (if sequenced prior to August 2014) or15 genes depending on the time period; http://www.foundationone.com/). Formalin-fixed paraffin-embedded tumor samples were submitted for NGS to FM by referring physicians as per their need to have NGS results on their patients. The methods and associated software information have been previously described (21). Average sequencing depth of coverage was greater than 250×, with >100× at >99% of exons.
TMB was measured in mutations per megabase (Mb). To assess TMB, somatic mutations detected by NGS (interrogating 1.2 Mb of the genome) were calculated, and the values were extrapolated to the whole exome utilizing a validated algorithm (13,15). Bona fide oncogenic driver alterations and germline polymorphisms were excluded. TMB levels were divided into three groups (15): low (1–5 mutations/Mb), intermediate (6–19 mutations/Mb), and high (≥20 mutations/Mb), which divided approximately 50% of patients to low TMB, 40% intermediate TMB, and 10% high TMB. The number of patients with very high TMB (≥50 mutations/Mb) was also assessed. One hundred non-synonymous mutations per exome were used previously as a threshold (15). The threshold of 20 coding mutations per megabase was roughly equivalent to 400 non-synonymous mutations per exome (20 coding mutations/Mb * 30 Mb/exome * 2/3 non-synonymous/coding).
The microsatellite instability (MSI) status was calculated using 114 loci determined to be useful in detecting evidence of polymerase slippage and, therefore, MSI (22). The information from these loci were then used in principal component analysis to produce an MSI score.
Statistical analysis and outcome evaluation
Student’s T-test, Fisher’s exact test, and log-rank (Mantel-Cox) were used to assess categorical variables. P values ≤0.05 were considered significant. Stable disease (SD), partial and complete remission (CR and PR), and progressive disease (PD) were assessed based on physician notation. Physicians generally used RECIST imaging criteria (23). Time-to-treatment failure (TTF) and overall survival (OS) were calculated Kaplan-Meier survival analysis. Patients who died early were considered evaluable (as progressive disease). For patients who received multiple immunotherapy regimens, the treatment with first immunotherapeutic was used in this analysis. TTF was defined as a composite endpoint measuring the time from immunotherapy origination to treatment discontinuation for any reason, including disease progression, treatment toxicity, or death. OS was defined as the time from initiation of the immunotherapy until patient death. Patients were censored at date of last follow up for TTF and OS, if they had not progressed or died, respectively. Multivariate analyses were used to calculate independent variables associated with outcome. TMB was available on only 41 patients, and these were used in the calculation. Statistical analyses were carried out by SK using GraphPad Prism version 7.0 and IBM SPSS Statistics version 24.
RESULTS
Patient characteristics
Of the 2,651 patients with cancer of any histology and who had available data reviewed from a clinically annotated UCSD database, a total of 75 patients treated with immunotherapy for SCC were identified (Supplementary Fig. S1). Twenty-three patients had locally advanced disease, whereas 52 patients had metastatic SCC. Patients were treated with various immunotherapies, with the majority receiving anti–PD-1/PD-L1 monotherapy (N=68) (Table 1). Median age was 67 years (range, 33–90 years). Of the 75 patients, 15 had cutaneous SCC, and 60 had other types of SCC: head and neck cancer (N=35), non-small–cell lung cancer (NSCLC) (N=7), esophageal (N=3), cervical (N=2), anal (N=1), rectal (N=1), and urethral cancers (N=1).
TMB and other molecular alterations
The median TMB for SCC versus non-SCCs of the entire UCSD cohort (N = 2,651) was 6 vs. 2, respectively (P<0.0001) (Table 2, Supplementary Fig. S5). Overall, 33.9% of patients with SCC, compared to 9.8% of non-SCCs, had TMB ≥12 mutations/megabase (Mb)(P<0.0001), and 21.7% of patients with SCC, compared to 5.7% of patient with non-SCCs, had a TMB ≥20 mutations/Mb (P<0.0001). 10% of patients with SCC had a TMB ≥50 mutations/Mb compared to 2.5% of patients with non-SCC (P<0.0001).
Table 2:
Tumor mutational burden in 9,407 patients with squamous cell carcinoma
| Median TMB (IQR) | P value | Low TMB (<6 mutations/mb) | Intermediate TMB (6–19 mutations/mb) | High TMB (≥20 <50) mutations/mb) | Very High TMB (≥50 mutations/mb) | TMB <12 mutations/mb | TMB ≥12 mutations/mb | P value | Odds Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tumors UCSD cohort (N = 2651) | Percent of patients | Percent of patients | ||||||||
| Squamous tumors (N=180)** | 6 (13) | <0.0001 | 37.2 | 41.1 | 21.7 | 10.0 | 66.1 | 33.9 | <0.0001 | 4.7 (3.3–6.7) |
| Non-squamous tumors (N=2471) | 2 (5) | 74.8 | 19.5 | 5.7 | 2.5 | 90.2 | 9.8 | <0.0001 | 0.2 (0.1–0.3) | |
| Squamous tumors (FM cohort) (N = 9407)** | ||||||||||
| Cutaneous (N=426) | 40 (68) | <0.0001 | 22.3 | 16.0 | 61.7 | 41.3 | 33.1 | 66.9 | <0.0001 | 5.8 (4.7–7.2) |
| Non-cutaneous (N=8981) | 6 (9) | 42.2 | 47.6 | 10.2 | 3.0 | 74.2 | 25.8 | <0.0001 | 0.2 (0.1–0.2) | |
| Squamous tumor subsets | ||||||||||
| Cutaneous (N=426) | 40 (68) | <0.0001 | 22.3 | 16.0 | 61.7 | 41.3 | 33.1 | 66.9 | <0.0001 | 5.8 (4.7–7.3) |
| Lung (N=4096) | 8 (8) | 28.1 | 61.9 | 10.0 | 1.6 | 66.3 | 33.7 | <0.0001 | 1.8 (1.7–2.0) | |
| Head and neck (N=1938) | 4 (6) | 57.8 | 31.3 | 10.9 | 5.4 | 82.0 | 18.0 | <0.0001 | 0.5 (0.4–0.6) | |
| Esophageal (N=423) | 5 (4) | 57.2 | 40.7 | 2.1 | 0.5 | 89.8 | 10.2 | <0.0001 | 0.3 (0.2–0.4) | |
| Anal (N=390) | 5 (5) | 58.2 | 38.0 | 3.8 | 0.8 | 89.0 | 11.0 | <0.0001 | 0.3 (0.2–0.4) | |
| Cervical (N=541) | 5 (5) | 54.9 | 38.6 | 6.5 | 0.4 | 83.9 | 16.1 | <0.0001 | 0.5 (0.4–0.6) | |
| Urothelial (N=74) | 6 (6) | 43.2 | 44.6 | 12.2 | 0.0 | 81.1 | 18.9 | 0.09 | 0.6 (0.3–1.1) | |
P values of median TMB were determined using Mann-Whitney U test (for non-normally distributed data) for 2 group comparisons and Kruskal-Wallis for multiple group comparisons. P value, OR (≥12 to <12), and 95% CI for TMB >= 12 comparisons were determined using Fisher’s Exact.
The 180 UCSD patients with SCC and curated clinical data are a subset of the 9407 patients with SCC from the FM de-identified database.
Abbreviations: FM = Foundation Medicine: TMB = tumor mutational burden; UCSD = University of California San Diego
A total 9,407 patients with SCC had TMB testing performed (FM cohort) (Table 2). Malignancies in this de-identified dataset (which included the 180 patients with SCC in the UCSD clinically annotated dataset, of which 75 received immunotherapy) included cutaneous (N=426), lung (N=4,096), head and neck (N=1,938), esophageal (N=434), anal (N=390), cervical (N=541), and urothelial (N=74). The median TMB of cutaneous SCCs was 40 mutations/mb compared to 8 (lung), 4 (head and neck), 5 (urothelial), and 5 mutations/mb (esophageal, anal, and cervical) (P <0.0001). Overall, 66.9% of cutaneous SCCs had a TMB ≥12 compared to 33.7% of lung cancers (P <0.0001), and 61.7% of cutaneous SCCs had a high TMB compared to 10% of lung cancers. 41.3% of cutaneous SCCs had a very high TMB compared to 1.6% of lung cancers. Less than 1% of esophageal, anal, cervical, and urothelial tumors had a very high TMB.
Of the 41 patients who were treated with immunotherapy and whose tumors were analyzed for TMB (UCSD cohort), 11 (27%) were TMB low, 18 (44%) TMB intermediate, and 12 (29%) TMB high. Of those with high TMB, 4 (10%) had very high TMB (all cutaneous). For the 41 patients with TMB data available, dichotomizing TMB at <12 versus ≥12, yielded 21 patients in the lower group and 20 patients in the higher group. Of the 34 patients tested, only one patient had microsatellite instability high (MSI-H).
Supplementary Fig. S2 compares the molecular alterations in cutaneous versus non-cutaneous SCC in the 41 immunotherapy-treated patients with available data in the UCSD cohort (with all alterations identified listed in Supplementary Tables S1-S2). The most common alterations in cutaneous SCC involved the TP53, NOTCH1, CDKN2A, LRP1B, and FAT1 genes, whereas the most common alterations in non-cutaneous SCC were in the TP53, CDKN2A/B, FAT1, TERT, and PIK3CA genes (Supplementary Fig. S2).
Outcomes by TMB and histology
No difference in clinical benefit (SD ≥6 months or PR/CR), TTF, and OS between SCC and non-SCC patients was seen. Less than half (41%, 31/75) of patients with SCC had clinical benefit. The median TTF for all patients was 4.8 months, and median OS was 17.4 months from time of first immunotherapy (Table 1). In comparison, the percent of immunotherapy-treated patients with non-SCC/non-melanoma (N=133) who attained clinical benefit was 36% (48/133) (p=0.4613), and the median TTF and OS for this group were 3.7 months (p=0.2068) and 12.2 months (p=0.4927), respectively. All comparis ons were to SCC patients treated with immunotherapy.
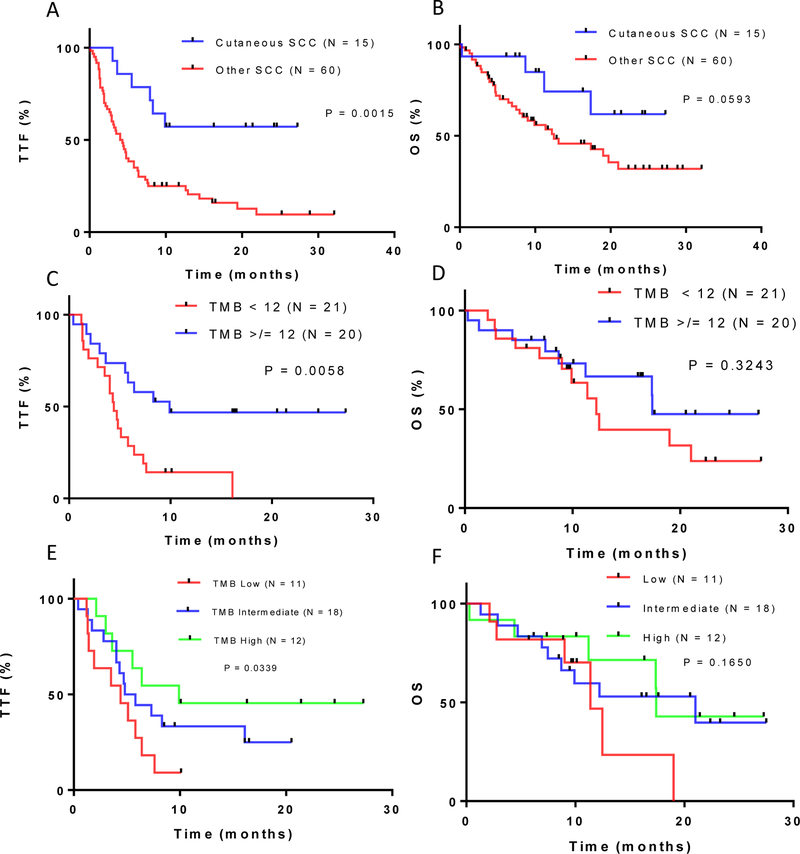
In univariate analysis of patients with SCC treated with immunotherapy, TMB [dichotomized at ≥12 mutations/Mb (N=20 patients) versus <12 (N=21 patients)] correlated with numerically higher rates of clinical benefit, although not statistically significant (SD ≥6 months or PR/CR; 60% versus 29%; ≥12 versus <12 p=0.06) and OS (17.4 versus 12.2 months; p=0.3). Patients with a TMB ≥12 mutations/Mb did have a significantly longer median TTF (9.9 versus 4.4 months)(p=0.0058)(Table 3, Fig. 1, Supplementary Fig. S3).
Table 3:
Univariate and multivariate analysis of factors affecting outcome for patients with SCCs treated with PD-1/PD-L1 blockade [TMB <12 and ≥ 12]. (See Supplementary Table 3 for analysis with TMB stratified by high, intermediate and low) (N=75 patients).
| Variable | Group (N) | SD ≥ 6 months plus PR/CR1 N = 31 (%) | OR2 (95% CI) | P univariate | P multivariate* | Median TTF (mos) | HR2 (95% CI) | P univariate | P multivariate* | Median OS | HR3 (95% CI) | P univariate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | <60 (N = 18) | 6 (33%) | 0.6 [0.2–1.8] | 0.5844 | 3.9 | 1.8 [0.9–3.4] | 0.0848 | 11.4 | 1.6 [0.8–3.4] | 0.1724 | ||
| ≥60 (N = 57) | 25 (44%) | 1.6 [0.5–5.0] | 5.0 | 0.6 [0.3–1.2] | 19.7 | 0.6 [0.3–1.3] | ||||||
| Sex | Male (N = 54) | 21 (39%) | 0.7 [0.3–1.8] | 0.6032 | 4.8 | 1.0 [0.5–1.7] | 0.9261 | 17.4 | 1.0 [0.5–2.0] | 0.9157 | ||
| Female (N = 21) | 10 (48%) | 1.4 [0.5–3.7] | 4.8 | 1.0 [0.6–1.8] | 17.4 | 1.0 [0.5–2.2] | ||||||
| Ethnicity | White (N = 61) | 25 (41%) | 0.9 [0.3–3.2] | >0.9999 | 5.0 | 0.8 [0.4–1.5] | 0.4328 | 17.4 | 1.0 [0.4–2.5] | 0.9361 | ||
| Other (N = 14) | 6 (43%) | 1.1 [0.3–3.2] | 2.9 | 1.3 [0.6–2.6] | 11.4 | 1.0 [0.4–2.3] | ||||||
| Disease status | Locally advanced (N = 23) | 10 (43%) | 1.1 [0.4–3.2] | 0.8052 | 5.5 | [0.9 [0.5–1.6] | 0.8419 | 17.4 | 0.7 [0.3–1.4] | 0.3422 | ||
| Metastatic (N = 52) |
21 (40%) | 0.9 [0.3–2.2] | 4.7 | 1.1 [0.6–1.8] | 13.1 | 1.4 [0.7–2.9] | ||||||
| TMB | TMB < 12 mut/mb (N = 21)* | 6 (29%) | 0.3 [0.1–0.9] | 0.0616 | 0.285 | 4.4 | 2.7 [1.3–5.7] | 0.0058 | 0.209 | 12.2 | 1.6 [0.6–3.7] | 0.3243 |
| TMB ≥ 12 mut/mb (N = 20)* | 12 (60%) | 3.8 [1.1–12.3] | 9.9 | 0.3 [0.2–0.7] | 17.4 | 0.6 [0.2–1.5] | ||||||
| Treatment | ≤1 prior therapy (N = 46) |
20 (44%) | 1.3 [0.5–3.4] | 0.8101 | 4.8 | 0.9 [0.5–1.6] | 0.7385 | 19.7 | 0.8 [0.4–1.5] | 0.4657 | ||
| 2 or more prior therapies (N = 29) | 11 (38%) | 0.8 [0.3–2.0] | 5.2 | 1.1 [0.6–1.9] | 12.2 | 1.2 [0.7–2.5] | ||||||
| PD-1/PD-L1 blockade monotherapy (N = 68) |
29 (43%) | 1.9 [0.3–9.8] | 0.6926 | 5.1 | 1.1 [0.4–2.6] | 0.8822 | 17.4 | 0.7 [0.2–2.3] | 0.5067 | |||
| PD-1/PD-L1 blockade + other (N = 7) | 2 (29%) | 0.5 [0.1–2.9] | 4.3 | 0.9 [0.4–2.3] | 8.9 | 1.4 [0.4–4.7 | ||||||
| Histology | Cutaneous SCC (N = 15) |
11 (73%) | 5.5 [1.5–16.9] | 0.008 | 0.459 | Not reached | 0.3 [0.2–0.5] | 0.0015 | 0.397 | Not reached | 0.5 [0.2–1.0] | 0.0593 |
| Non-cutaneous (N = 60) |
20 (33%) | 0.2 [0.1–0.6] | 4.2 | 3.5 [2.0–6.2] | 12.5 | 2.1 [1.0–4.5] |
41 patients were included in the multivariate analysis. P values ≤ 0.1 in univariate were included in the multivariate analysis. Outcome numbers may be different than in Table 1 because only 41 patients with available TMB were included in the multivariate analysis in this table, while in Table 1, all 75 patients were analyzed.
Response included patients with stable disease ≥ 6 months, partial responders, and complete responders
Calculated using Fischer’s exact test
Calculated using long-rank (Mantel-Cox)
Other therapy: chemotherapy (N = 1), targeted therapy (N = 1), and investigational agent (N = 5)
Abbreviations: HR = hazard ratio; mb = megabase; MSI = microsatellite instability; OR = odds ration; OS = overall survival; SCC = squamous cell carcinoma; TMB = tumor mutational burden; TTF = time to treatment failure
Figure 1: TTF and OS for patients with advanced SCC treated with PD-1/PD-L1 blockade.
(A) Kaplan Meier analysis of time-to-treatment failure (TTF) for cutaneous squamous cell carcinoma (SCC) vs. other SCCs. (B) Kaplan Meier analysis for overall survival (OS) for cutaneous SCC vs. other SCCs. (C) Kaplan Meier analysis for TTF for tumor mutational burden (TMB) <12 vs ≥12 mutations/Mb. (D) Kaplan Meier analysis for OS for TMB <12 vs. ≥12. (E) Kaplan Meier analysis for TTF for all SCCs categorized by TMB low vs. intermediate vs. high. (F) Kaplan Meier analysis for OS for all SCCs categorized by TMB low vs. intermediate vs. high. Number of patients/group indicated.
In patients with SCC, when TMB was examined with a three-way stratification, TMB low (<6 mutations/Mb), intermediate (6–19 mutations/Mb), and high (≥20 mutations/Mb), a similar pattern emerged. Immunotherapy-treated patients with high TMB tumors had a longer median TTF than those with intermediate or low TMB tumors (9.9, 5.3, and 4.4 months, respectively; p=0.0339). Other associations were not statistically significant (Fig. 1, Supplementary Fig. S3, Supplementary Table S3). Cutaneous SCCs had better outcomes after immunotherapy than non-cutaneous SCCs.
Comparing cutaneous to non-cutaneous SCCs treated with immunotherapy (Table 3), we observed higher rates of clinical benefit (SD ≥6 months or PR/CR) for cutaneous disease – 73% (11/15) versus 33% (20/60)(p = 0.008). The median TTF was longer (not reached versus 4.2 months (p=0.0015)) and a trend to longer median OS was observed (not reached versus 12.5 months (p=0.0593)(Table 2, Fig. 1) for cutaneous SCC patients. In univariate analysis of SCCs, both high TMB and cutaneous SCC correlated with better outcomes after immunotherapy. However, in multivariate analysis, none of the comparisons reached significance, perhaps because of the limited number of patients (N=41) with available TMB values (Table 3, Supplementary Table S2).
A case report of a patient with cutaneous SCC
A 64–year-old man developed progressive irritation in the socket of his right prosthetic eye. A CT scan demonstrated a hypervascular 3 cm right orbital mass that displaced the prosthesis 1.9 cm posteriorly. A biopsy of the mass was consistent with invasive cutaneous SCC. Further staging revealed disease involving his right parotid gland, and he underwent resection of the orbital tumor and a neck dissection. He was treated with adjuvant radiation therapy and cetuximab. However, he developed progressive disease involving his right hilum. He was started on treatment with pembrolizumab and achieved a complete response (Supplementary Fig. S4) seven months after starting therapy. Pembrolizumab was discontinued after 14 months, and he remains in an ongoing complete response. He experienced no treatment related toxicities.
DISCUSSION
This study evaluated the genomic landscape and mutational burden of diverse SCCs. Response to checkpoint blockade and correlation with histology and TMB were also assessed. Cutaneous SCC appeared to be sensitive to checkpoint blockade, indicated by frequent and durable responses. This finding is similar to the outcome reported in a phase 1 study of the PD-L1 inhibitor cemiplimab in advanced cutaneous SCC (20). Response rates of this magnitude to single-agent PD-1/PD-L1 inhibition have only otherwise been seen in classical Hodgkin lymphoma (24).
Response rates to checkpoint blockade in cutaneous SCC are likely driven by the high mutational burden of this disease. This study, as well as others, reported cutaneous SCC to have the highest TMB of all SCC malignancies (15). In our study, 41.3% of cutaneous SCCs had a very high TMB compared to 5.4% or less in other major subtypes of SCC. Both melanoma and cutaneous basal cell carcinoma have a high mutational burden and frequent responses to checkpoint blockade (25,26). Indeed, TMB has been shown to be predictive of response to immunotherapy across diverse cancers (13). However, even amongst a group of SCCs, which in our study had high rates of clinical benefit after immunotherapy (rate of SD≥6 months or PR/CR = 41%), higher TMB was shown to be associated with a longer TTF. Future prospective trials in patients with SCC may warrant stratification by TMB.
Our study had several limitations. First, though univariate analysis demonstrated that both higher TMB and cutaneous SCCs were associated with better outcomes after immunotherapy, multivariate analysis was not able to determine if either of these variables independently predicted outcome. This may have been due to the fact that the number of patients was relatively small and no patients with cutaneous SCC and a low TMB treated with checkpoint blockade were included. Therefore, it was not possible to determine if TMB could segregate responders from non-responders with cutaneous SCC. PD-L1 expression by immunohistochemistry and microsatellite instability are both predictors of response to immunotherapy (14,27). Unfortunately, we did not have this data available for the majority of our samples. Finally, patients in this study were assessed retrospectively and were treated with a variety of immunotherapeutics, although the majority (91%) received anti–PD-1/PD-L1 monotherapy.
In conclusion, SCCs appeared to have high clinical benefit rates after checkpoint blockade, which, in our series, were 73% for cutaneous SCC and 33% for non-cutaneous SCC. The high clinical benefit rates, especially in cutaneous SCCs, may be, at least in part, related to their relatively higher TMB than other SCCs, most likely due to the effects of UV light on cutaneous SCCs (28). As mentioned, TMB has been previously correlated with immunotherapy response (13). In our cohort of patients, 60% with SCCs having a TMB ≥12 mutations/Mb showed clinical benefit (versus 29% of patients with TMB <12 mutations/Mb).
Patients with high TMB and those with cutaneous SCC also showed significantly longer TTF, and cutaneous disease also was associated with a trend towards longer OS. Three patients with non-cutaneous SCCs and low TMB also responded, perhaps due to other factors such as CD274 (PD-L1) amplification, which has been reported to correlate with response to anti–PD1/PD-L1 immunotherapies (29,30). Taken together, our results demonstrated that SCC, especially those of cutaneous origin and those with higher mutational burdens, are susceptible to checkpoint blockade.
Supplementary Material
Acknowledgments
Funding: Funded in part by National Cancer Institute grant P30 CA023100 and the Joan and Irwin Jacobs Fund philanthropic fund.
Footnotes
Conflict of Interest: Dr. Goodman receives speaking fees from Seattle Genetics and consulting fees from Jazz Pharmaceuticals. Dr. Montesion, Dr. Frampton, and Dr. Miller are employees of Foundation Medicine. Dr. Frampton and Dr. Miller are equity holders of Foundation Medicine. Dr. Kurzrock receives research funding from Genentech, Merck, Serono, Pfizer, Sequenom, Foundation Medicine, Konica Minolta, Grifols, and Guardant, as well as consultant fees from X Biotech, Loxo, Neomed, and Actuate Therapeutics, speaker fees from Roche, and has an ownership interest in CureMatch Inc.
References
- 1.Yan W, Wistuba II, Emmert-Buck MR, Erickson HS. Squamous Cell Carcinoma - Similarities and Differences among Anatomical Sites. Am J Cancer Res. 2011;1:275–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9:713–20. [DOI] [PubMed] [Google Scholar]
- 3.Kato S, Kurasaki K, Ikeda S, Kurzrock R. Rare Tumor Clinic: The University of California San Diego Moores Cancer Center Experience with a Precision Therapy Approach. Oncologist. 2018;23:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–26. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwaederle M, Elkin SK, Tomson BN, Carter JL, Kurzrock R. Squamousness: Next-generation sequencing reveals shared molecular features across squamous tumor types. Cell Cycle. 2015;14:2355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holsinger FC, Piha-Paul SA, Janku F, Hong DS, Atkins JT, Tsimberidou AM, et al. Biomarker-directed therapy of squamous carcinomas of the head and neck: targeting PI3K/PTEN/mTOR pathway. J Clin Oncol. 2013;31:e137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nature Reviews Cancer. 2010;10:550–60. [DOI] [PubMed] [Google Scholar]
- 10.Gross AM, Orosco RK, Shen JP, Egloff AM, Carter H, Hofree M, et al. Multi-tiered genomic analysis of head and neck cancer ties TP53 mutation to 3p loss. Nat Genet. 2014;46:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV-tumors. Genome Med. 2013;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinhofer I, Stenzinger A, Eder T, Konschak R, Niehr F, Endris V, et al. Targeted next-generation sequencing identifies molecular subgroups in squamous cell carcinoma of the head and neck with distinct outcome after concurrent chemoradiation. Ann Oncol. 2016;27:2262–8. [DOI] [PubMed] [Google Scholar]
- 13.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16:2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Medicine. 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. [DOI] [PubMed] [Google Scholar]
- 17.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine. 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 20.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. New England Journal of Medicine. 2018;0:null. [DOI] [PubMed] [Google Scholar]
- 21.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall M, Gowen K, Sanford E. Evaluation of microsatellite instability (MSI) status in 11,573 diverse solid tumors using comprehensive genomic profiling (CGP). J Clin Oncol 2016;34(15):1523. [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 24.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–20. [DOI] [PubMed] [Google Scholar]
- 25.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. New England Journal of Medicine. 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 26.Goodman AM, Kato S, Cohen PR, Boichard A, Frampton G, Miller V, et al. Genomic landscape of advanced basal cell carcinoma: Implications for precision treatment with targeted and immune therapies. Oncoimmunology [Internet]. 2017. [cited 2018 Jun 18];7 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5790366/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–56. [DOI] [PubMed] [Google Scholar]
- 28.Martincorena I, Roshan A, Gerstung M, Ellis P, Loo PV, McLaren S, et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman AM, Piccioni D, Kato S, Boichard A, Wang H-Y, Frampton G, et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol [Internet]. 2018. [cited 2018 Jun 18]; Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2684636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roemer MGM, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016;34:2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.