Abstract
Objective:
Data regarding the cardiac abnormalities associated with Stanford type B aortic dissection (TBAD) and whether these abnormalities are related to outcomes are limited. We describe the prevalence of cardiac abnormalities in TBAD patients as detected by echocardiography.
Methods:
This is a retrospective review of patients with TBAD presenting between 1990 and 2016. Echocardiograms performed within 6 weeks of acute TBAD were reviewed. Cardiac function, valve abnormalities, and stigmata of hypertensive heart disease including left ventricular hypertrophy (LVH) were ascertained. Characteristics of patients who did and did not receive echocardiograms were compared. Outcomes of patients with and without evidence of LVH on echocardiography were also compared.
Results:
Of 239 patients with TBAD, 90 had echocardiograms performed within 6 weeks of acute TBAD (74% male, mean age 57.8 ± 13.2 years). Echocardiograms were obtained at a median of 2 (range 0–41) days from acute TBAD. Patients who had echocardiograms were more likely to present with malperfusion (28% vs. 14%, P<.01) and had a trend towards increased operative repair during the subacute phase (17.4% vs 9.5%, P=.07) compared to patients who did not receive an echocardiogram. A majority of patients (57%) had at least mild LVH, including 39% of patients without a prior diagnosis of hypertension. Fibrocalcific changes associated with hypertension including aortic sclerosis and mitral annular calcification were noted in 40% and 11% of the patients respectively. Among patients with LVH, there was a trend towards higher all-cause mortality (35% vs. 23%, P=.21) and a younger age at death (58 ± 14 vs. 66 ± 13 years, P=.19) despite a similar age at TBAD onset. In a multivariable analysis controlling for age, sex and admission eGFR, LVH independently predicted all-cause mortality (hazard ratio, 2.38 [95% CI 1.02 – 5.56], P=.04).
Conclusions:
LVH and other findings of hypertensive heart disease are common in patients with TBAD. LVH predicted all-cause mortality after TBAD in this small group of patients. Further exploration of the relationship between the chronic effects of hypertension and using LVH as an objective biomarker to risk stratify TBAD patients and long-term outcomes after TBAD is warranted.
Keywords: Type B aortic dissection, Echocardiography, Hypertensive heart disease, Left ventricular hypertrophy
Introduction
Stanford type B aortic dissection (TBAD) carries a 5-year mortality of 30–40%.1 Medical management is the mainstay of therapy for patients with uncomplicated TBAD. Previous studies investigating survival after medical management of acute TBAD have focused on predictors intrinsic to the aorta, such as aortic diameter and dissection-related aneurysmal degeneration.2–4 Data regarding the cardiac abnormalities associated with TBAD and whether these abnormalities are related to outcomes after TBAD are limited. Echocardiography has broad utility in the diagnosis and management of cardiac disease, allowing for real-time, precise anatomical definition, and physiological interrogation of cardiac structures with minimal patient risk and discomfort. Common clinical applications for echocardiography include assessment of myocardial and valvular function and identification of structural abnormalities. Practices of obtaining transthoracic echocardiography (TTE) during the routine management of TBAD vary by institution and are not routine in all practices. We seek to describe the prevalence of cardiac abnormalities in TBAD patients as detected by echocardiography, and to assess the utility of TTE in the workup of TBAD.
Methods
The Institutional Review Board at the University of Washington approved this study of patients with TBAD presenting to UW Medicine hospitals (University of Washington Medical Center and Harborview Medical Center) between 1990 and 2016 (IRB #49069). Patients were identified using the discharge diagnoses International Classification of Diseases (ICD)-9 of 441.00 and ICD-10 of I71.00. Records were reviewed to confirm the TBAD diagnosis. Patients were excluded if the aortic dissection etiology was related to trauma, or if upon review of imaging the TBAD was due to isolated abdominal aortic dissection. Participants underwent informed consent or were enrolled retrospectively with a waiver of consent for medical record review if they were had died by the time of the study or stopped receiving care at UW Medicine since 2012. Demographic data, comorbid conditions, TBAD characteristics, findings on TTEs, and long-term outcomes were reviewed.
Study indications and echocardiography measurements were abstracted from the TTE reports. Report interpretations by board certified cardiologists were reviewed. Left ventricular ejection fraction (EF) was used as a marker of systolic function, with EF ≥ 60% considered normal. Wall motion abnormalities were categorized as present or absent. LV diastolic function was categorized as normal, indeterminate or abnormal based on the interpreting cardiologist’s global assessment of diastolic function. A board certified cardiologist (RF) adjudicated cases with equivocal diastolic function based on the original TTE interpretation. LVH presence was determined from two-dimensional measurements of septal and posterior wall thickness and by the interpreting cardiologist’s global assessment of LV wall thickness (greater than 1.1 cm) at end diastole. Valve pathology was scored as trace, mild, moderate or severe. Aortic sclerosis and mitral annular calcification were categorized as present or absent. Pulmonary hypertension was defined as a pulmonary arterial systolic pressure (PASP) estimate greater than 30 mmHg, derived from the peak tricuspid regurgitant jet velocity and central venous pressure estimate.
Data were analyzed using Microsoft Excel 2013 (Microsoft, Redmond, WA), SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL) and Stata 14IC (StataCorp, College Station, TX). Continuous data are presented as means and standard deviation from the mean or median and interquartile range (IQR) or ranges where appropriate. Means of continuous data were compared using the Student’s t-test or Wilcoxon rank-sum test, while categorical data were compared using the Fisher exact test or Pearson Chi-square test where appropriate. A Kaplan-Meier analysis was performed to estimate survival in patients with and without LVH determined by echocardiography, and survival curves were compared using the log-rank test. The adjusted effects of risk factors for all-cause mortality were assessed using Cox proportional-hazards regression analysis. Covariates were selected a priori based on a previously published analysis investigating risk factors for all-cause mortality in TBAD patients receiving medical management 5 Estimated GFR (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) study equation, and expressed as a continuous variable in the regression analysis.6 Differences were considered statistically significant at a P-value <.05.
Results
Of 239 patients with TBAD, 90 (38.4%) had an echocardiogram (73.3% male, mean TBAD age 57.6 ± 13.2 years) performed within 6 weeks of the acute dissection as echocardiograms are not routinely performed in our practice for TBAD. The median interval to echo was 2 days (range 0–41 days). In this cohort, 77 (85.6%) were managed in the acute phase of TBAD at UW Medicine, and the remaining 13 patients were referred in the chronic phase of TBAD. Demographics, comorbid conditions and dissection characteristics of the patients with and without echocardiograms are summarized in Table I. There were no differences in age of acute TBAD, sex, or race between the two groups. Marfan syndrome was present in 6% of patients overall, with comparable prevalence between the groups. The patients who received a TTE had a higher percentage of uncontrolled hypertension and a higher percentage of methamphetamine or cocaine use (Table 1). A higher percentage of patients with echocardiograms were transferred from an outside hospital during the acute phase of TBAD (77.2% vs. 48.3%, P<.001) and had a higher percentage of malperfusion (24.6% vs. 11.3%, P=.012). There was a trend towards more operative repairs in this group (17.4% vs. 9.5%, P=.07). Additionally, patients who had echocardiograms had a longer median hospital length of stay (9.5 days (IQR 6, 20) vs. 7 days (IQR 4, 12), P=.001), and died at a younger age compared to those who did not have a TTE (60.9 ± 13.5 years vs. 71.5 ± 13.1 years, P<.001).
Table I.
Comparison of patients with type B aortic dissection who did and did not have an echocardiogram within 6 weeks after presentation for TBAD management.
| N (%)/Mean ± SD | TTE obtained (n=90) | No TTE obtained (n=149) | P |
|---|---|---|---|
| Age at dissection | 57.6 ± 13.2 | 59.7 ± 13 | .24 |
| Male | 66 (73.3) | 102 (68.5) | .42 |
| Race | .12 | ||
| American Indian or Alaska Native | 2 (2.2) | 3 (2.0) | |
| Asian | 7 (7.8) | 5 (3.4) | |
| Black or African American | 13 (14.4) | 11 (7.4) | |
| Caucasian | 56 (62.2) | 114 (76.5) | |
| Multiracial | 10 (11.1) | 12 (8.1) | |
| Native Hawaiian or Pacific Islander | 2 (2.2) | 1 (.7) | |
| Unknown | 0 | 3 (2.0) | |
| Comorbid conditions | |||
| Hypertension | 72 (80) | 115 (77.2) | .61 |
| Uncontrolled hypertensiona | 32 (35.6) | 27 (18.1) | .002 |
| On 3 or more antihypertensives | 9 (10) | 18 (12.1) | .62 |
| Diabetes mellitus | 12 (13.3) | 11 (7.4) | .13 |
| Coronary artery disease | 15 (16.7) | 41 (27.5) | .06 |
| Prior myocardial infarction | 2 (2.2) | 15 (10.1) | .02 |
| Congestive heart failure | 4 (4.4) | 10 (6.7) | .47 |
| Atrial fibrillation | 6 (6.7) | 9 (6) | .85 |
| Prior stroke | 6 (6.7) | 12 (8.1) | .69 |
| Chronic obstructive pulmonary disease | 4 (4.4) | 13 (8.7) | .21 |
| Obstructive sleep apnea | 5 (5.6) | 8 (5.4) | .95 |
| Chronic kidney disease | 9 (10) | 7 (4.7) | .11 |
| End stage renal disease | 2 (2.2) | 1 (.7) | .30 |
| Marfan syndrome | 5 (5.6) | 9 (6) | .88 |
| Family historyb | 15 (16.7) | 16 (10.9) | .22 |
| Ever smoker | 59 (65.6) | 117 (78.5) | .03 |
| Methamphetamine or cocaine use | 12 (13.3) | 8 (5.4) | .03 |
| Blood pressure | |||
| Peak systolic blood pressure on presentation | 180 (150, 196) | 184 (162, 204) | .32 |
| Peak diastolic blood pressure on presentation | 94 (78, 109) | 99 (87, 113) | .04 |
| Follow-up and mortality | |||
| Follow-up duration post TBAD diagnosis (y) | 3.4 ± 3.6 | 5.0 ± 5.4 | .01 |
| All-cause mortality | 27 (30) | 74 (49.7) | .003 |
| Age at death (y) | 60.9 ± 13.5 | 71.5 ± 13.1 | <.001 |
Hypertension described as untreated, difficult to control, poorly controlled, or noncompliant
Refers to family history of aortic or arterial aneurysm/dissection or sudden death. IQR = interquartile range, SD = standard deviation.
The predominant echocardiography study performed was a TTE (n=88, 97.8%). Two patients (2.2%) had transesophageal echocardiograms (TEE) performed. The most common indication for echocardiography was evaluation of cardiac function (62%), followed by evaluation of aortic dissection (18%), and assessment for ascending aortic dissection (11%). Only one TTE was ordered primarily for preoperative evaluation for TBAD repair.
Structural Abnormalities Detected by Echocardiography
Significant valve abnormalities were uncommon. Valve dysfunction of moderate or greater severity was identified for the following lesions: aortic stenosis (2%), aortic regurgitation (1%), and tricuspid regurgitation (2%). None of the patients had a bicuspid aortic valve and only two patients had mitral valve prolapse. None of the patients had mitral stenosis or mitral regurgitation of moderate or greater severity. Four patients had mechanical aortic valves with normal hemodynamic function. Aortic sclerosis was present in 40% of patients, and mitral annular calcification was present in 11% of patients.
Aortic measurements were not routinely documented. The ascending anterior-posterior aortic diameter was most commonly reported (n=50, mean 3.8 ± 0.5 cm, range 2.5 – 5 cm).
Functional and Hemodynamic Abnormalities Detected by Echocardiography
The left ventricular EF was normal in 82% of patients with echocardiograms, with a mean value of 61.4 ± 9.4%. Diastolic dysfunction was common in patients with and without a history of hypertension (35% vs. 35%, P=.96). Right ventricular systolic dysfunction and wall motion abnormalities were uncommon occurring in 5.5% and 5.5% of the patients respectively.
PASP was recorded in 46 patients, of whom 67% had pulmonary hypertension with a mean PASP of 35.7 ± 11.1 mmHg. The average right atrial pressure was slightly elevated at 7.4 ± 4.1 mmHg. Mean left ventricular EF and the prevalence of diastolic dysfunction did not differ significantly between patients with mean PASP ≥ 30 mmHg and those with PASP < 30 mmHg (60.8 ± 10.0% vs. 64.3 ± 5.2%, P=.19 and 32.4% vs. 46.7 %, P=.29, respectively), indicating that the elevated pulmonary arterial pressures were not explained by left heart dysfunction.
The overall prevalence of LVH was 57% (n=51). Interestingly, LVH was present in 39% of patients without a previous diagnosis of hypertension, compared to 61% of patients with a previous diagnosis of hypertension (P=.09).
Comparison of Patients With and Without LVH
While there were no differences in sex or age at acute TBAD among patients with and without LVH, TBAD patients with LVH were less likely to be Caucasian and presented with significantly higher blood pressure (Table II). Although most patients had an established diagnosis of hypertension at the time of presentation for acute TBAD management, patients with LVH were more likely to have hypertension that was described as refractory, poorly controlled, untreated, or non-adherent to antihypertensive treatment (Table II). Diastolic dysfunction was more common among those with LVH compared to those without LVH (45.1% vs. 21.1%, P=.02). The prevalence of pulmonary hypertension did not differ significantly between patients with and without LVH (62.5% vs. 59.1% respectively, P=.48). The mean descending thoracic aortic diameter at acute TBAD as assessed by computed tomography was equal between the two groups (3.7 ± 0.8 cm).
Table II.
Comparison of patients with type B aortic dissection with and without left ventricular hypertrophy as detected by on echocardiography completed within 6 weeks of the acute presentation
| N (%)/Mean ± SD | LVH (n=51) | No LVH (n=39) | P |
|---|---|---|---|
| Transferred from an outside hospital | 42 (82.4) | 27 (69.2) | .14 |
| Age at dissection diagnosis | 57.7 ± 13.5 | 57.3 ± 13.0 | .89 |
| Male | 36 (70.6) | 30 (76.9) | .50 |
| Race | .04 | ||
| American Indian or Alaska Native | 2 (3.9) | 0 | |
| Asian | 5 (9.8) | 2 (5.1) | |
| Black or African American | 11 (21.6) | 2 (5.1) | |
| Caucasian | 26 (50.9) | 30 (76.9) | |
| Multiracial | 7 (13.7) | 3 (7.7) | |
| Native Hawaiian or Pacific Islander | 0 | 2 (5.1) | |
| Comorbid conditions | |||
| Hypertension | 44 (86.3) | 28 (71.8) | .09 |
| Uncontrolled hypertensiona | 25 (49.0) | 7 (17.9) | <.01 |
| On 3 or more antihypertensives | 7 (13.7) | 2 (5.1) | .18 |
| Coronary artery disease | 7 (13.7) | 8 (20.5) | .39 |
| Chronic obstructive pulmonary disease | 1 (2.0) | 3 (7.7) | .31 |
| Admission eGFR | 58.9 ± 22.5 | 68.5 ± 28.1 | .08 |
| Marfan syndrome | 0 | 5 (12.8) | .01 |
| Family historyb | 7 (13.7) | 8 (20.5) | .39 |
| Ever smoker | 36 (70.6) | 23 (59.0) | .25 |
| Methamphetamine or cocaine use | 8 (15.7) | 4 (10.3) | .45 |
| Blood pressure | |||
| Peak systolic blood pressure at presentation | 190 (151, 211) | 160 (143, 185) | .01 |
| Peak diastolic blood pressure at presentation | 99 (86, 114) | 87 (72, 100) | .02 |
| Systolic blood pressure at time of echo | 122.9 ± 16.1 | 122.5 ± 20.9 | .94 |
| Diastolic blood pressure at time of echo | 67.3 ± 13.7 | 65.1 ± 16.6 | .52 |
| Outcomes | |||
| Operative management during initial hospitalization | 11 (21.6) | 5 (12.8) | .28 |
| Initial hospitalization length of stay (days) | 11 (7, 21) | 9 (6, 12) | .17 |
| Disposition after acute episode hospitalization | .69 | ||
| Discharged alive | 46 (90.2) | 37 (94.9) | |
| Transferred | 1 (2) | 1 (2.6) | |
| Died | 4 (7.8) | 1 (2.6) | |
| Follow-up duration (years) | 3.1 ± 3.4 | 5.0 ± 3.8 | .02 |
| Age at death | 58.2 ± 13.7 | 65.6 ± 12.9 | .19 |
| All-cause mortality | 18 (35.3) | 9 (23.1) | .21 |
| Cause of death | .24 | ||
| Aortic-related | 9 (50.0) | 4 (44.4) | |
| Cardiac-related | 2 (11.1) | 3 (33.3) | |
| Malignancy | 0 | 1 (11.1) | |
| Other | 4 (22.2) | 1 (11.1) | |
| Unknown | 3 (16.7) | 0 | |
Hypertension described as untreated, difficult to control, poorly controlled, or noncompliant
Refers to family history of aortic or arterial aneurysm/dissection or sudden death.
On univariate analysis, all-cause mortality was higher in TBAD patients with LVH compared to those without LVH despite a similar age at acute TBAD, though this was not statistically significant (P=.21). After adjustment for age, sex, and admission eGFR, LVH independently predicted all-cause mortality (hazard ratio (HR), 2.38 [95% CI 1.02 – 5.56], P=.04), as shown in Table III. A Kaplan-Meier survival analysis of patients with and without LVH is shown in Figure 1. When peak systolic blood pressure and subsequently diastolic blood pressure were substituted for LVH in the multivariable model, neither one independently predicted all-cause mortality (HR 1.01 [95% CI .99 – 1.02], P=.09 and HR 1.01 [95% CI .99 – 1.03], P=.19, respectively).
Table III.
Adjusted risk factors for all-cause mortality by Cox proportional-hazards regression analysis in patients with type B aortic dissection who had echocardiograms within 6 weeks after presentation (n=90).
| Covariate | HR [95% CI] | P |
|---|---|---|
| Age at diagnosis | 1.02 [.99 – 1.06] | .16 |
| Male | .81 [.34 – 1.90] | .63 |
| Admission eGFR | 1.01 [.99 – 1.02] | .44 |
| Left ventricular hypertrophy on echocardiography | 2.38 [1.02 – 5.56] | .04 |
CI = confidence interval, eGFR = estimated glomerular filtration rate, HR = hazard ratio.
Figure 1.
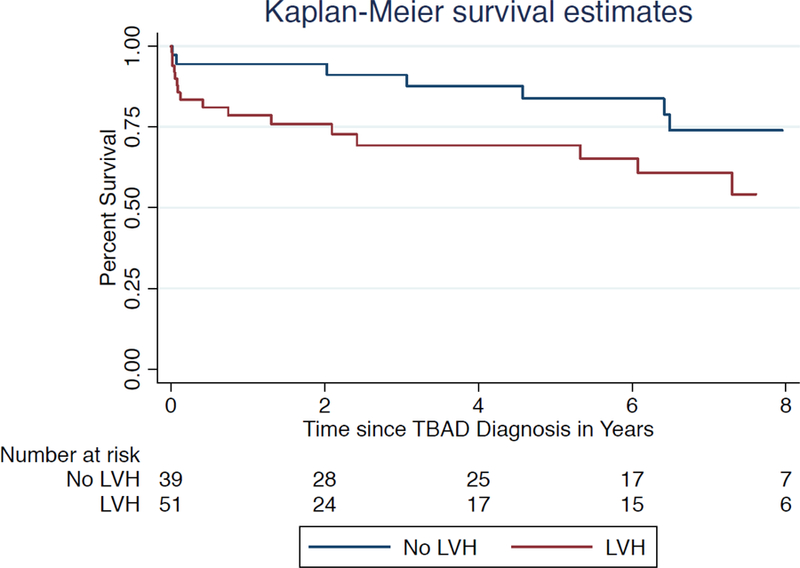
Kaplan-Meier survival analysis of 90 patients with echocardiograms showing survival among patients with and without a finding of left ventricular hypertrophy.
Discussion
In this retrospective study, we found an intriguing association between LVH and early mortality after TBAD. While LVH is well-recognized as a risk factor for adverse cardiovascular outcomes and mortality in general7–12, it has not been associated specifically with increased all-cause mortality after TBAD.
LVH from hypertensive heart disease is a reactive form of myocardial remodeling in response to the chronic increases in afterload against which the heart must contract and as such, is an early marker of end-organ damage from hypertension.13 Hypertension is a well-known risk factor for TBAD. It is worth noting that the TBAD patients with LVH presented with markedly elevated systolic and diastolic blood pressures compared to patients without LVH. Not surprisingly, LVH is a marker of resistant hypertension and may identify patients who will have difficult-to-control hypertension after hospital discharge.14 Previous studies have noted up to a 75% prevalence of LVH in TBAD patients,15,16 and an autopsy study demonstrated a high prevalence of marked ventricular hypertrophy in patients who died of thoracic aortic dissections.17
We demonstrated that LVH was common in patients presenting with TBAD regardless of a prior diagnosis of hypertension. This is not surprising considering that left ventricular mass may increase before hypertension is detected clinically13, thus LVH is likely an objective marker of uncontrolled hypertension compared to blood pressure measurements which could be influenced temporally by acute pain and anxiety. In our analysis, neither peak systolic nor diastolic blood pressure on presentation was independently associated with mortality while LVH was. Intriguingly, previous studies have not shown a significant difference in long-term outcomes between patients with controlled and uncontrolled hypertension post TBAD.18,19
As LVH progresses, systolic and diastolic dysfunction can occur. We observed a low prevalence of systolic dysfunction in our cohort, which may reflect the low rate (10%) of severe hypertrophy in this group. In contrast, 45% of patients with LVH had diastolic dysfunction, which is striking. For context, the prevalence of diastolic dysfunction on a population-based level is anticipated to be lower. A recent sample of patients with a mean age of 61.0 ± 9.5 years was approximately 23.8%.20 Diastolic dysfunction has also been associated with increased all-cause mortality in general.21
LVH is more common among ethnic minorities, and is particularly prevalent among African Americans. Hypertension is also more common in African Americans compared to Caucasians, although the mechanism for this difference has not been fully elucidated.22 A cardiac MRI study found that African Americans have a 2- to 3- fold higher prevalence of LVH compared to Caucasians, even after adjustment for multiple covariates, including body composition, systolic blood pressure, and socioeconomic status.23 Interestingly, in the same study, the association between black ethnicity and LVH was stronger in hypertensive patients compared to normotensive patients in multivariable analysis, and African Americans had more concentric hypertrophy (which develops in response to a chronic increase in afterload from hypertension) compared to Caucasians. The authors concluded that differences in blood pressure likely explain the ethnic differences in LVH prevalence. Ethnic disparities in blood pressure control do not appear to be secondary to differences in modifiable health behaviors alone – rather, it is possible that genetic, societal, and environmental factors are all contributory.24 We did not evaluate racial disparities in this study as this was not the original intent of the design, however, these findings are worth assessing in future studies of TBAD.
In addition to LVH, we observed a relatively high prevalence of aortic sclerosis (40%). The reported prevalence in similarly-aged patients in the general population is approximately 10% for those less than 60 years old, and does not reach 40% until the eighth or ninth decade in most studies. 25 In a TBAD specific study, the prevalence of aortic sclerosis was 25% in TBAD patients with an age distribution similar to our cohort.16 Additionally, mitral annular calcification was present in 11% of our cohort with a mean age of less than 60 years old, while a previous study investigating mitral annular calcification in a multi-ethnic cohort of patients in their early 70s without manifest cardiovascular disease reported a 9% prevalence of mitral annular calcification.26 These findings suggest that aortic sclerosis and mitral annular calcification may develop at an earlier age in patients with TBAD compared to the general population. While these lesions are rarely hemodynamically significant, they are markers for increased cardiovascular risk that signal the need to aggressively control hypertension and other atherosclerotic risk factors. Our finding of these lesions likely represent the high burden of hypertension in patients with TBAD.25–31
Obtaining a TTE in cases of TBAD is not routine in all practices and there are additional methods to detect LVH in addition to TTE including ECG and computed tomography (CT) scans (Figure 2). However, ECG criteria are relatively specific for LVH, but not sensitive for the diagnosis,32 and the finding of LVH on a CT scan is not routinely described on the imaging interpretation. The most recent American College of Cardiology Foundation/American Heart Association thoracic aortic disease guidelines state that the perioperative evaluation for patients with planned aortic repair may include echocardiography to help quantitate risk, but do not make specific recommendations for patients with TBAD.33 Based on our study findings, we recommend that providers consider obtaining a TTE to assess for LVH in patients with TBAD during the acute/subacute phase. Adding this to the workup of TBAD patient can potentially allow additional risk stratification by identifying TBAD patients with resistant hypertension and as such have a more malignant phenotype of TBAD. These patients may benefit from intensified outpatient follow-up and blood pressure checks. Additionally, identifying TBAD with LVH would also allow tailoring of the antihypertensive therapy regimen to include agents that induce LVH regression. A recent meta-analysis of antihypertensive therapy in patients with LVH demonstrated that a reduction of >10 mmHg of diastolic blood pressure allowed for significant decrease in LVH and that inhibition of the renin-angiotensin system was the most effective antihypertensive strategy for inducing LVH regression.34 Further exploration of the relationship between the chronic effects of hypertension and using LVH as an objective biomarker to risk stratify TBAD patients and long-term outcomes after TBAD is warranted.
Figure 2.
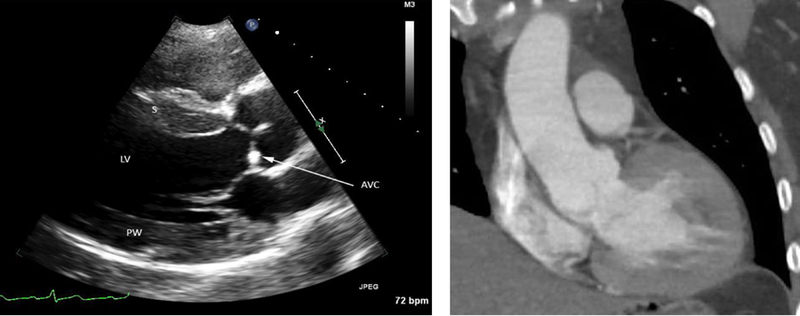
Imaging findings in a 43 year old man who presented with a systolic blood pressure of 290 mmHg and type B aortic dissection. Left panel: Parasternal long axis view on transthoracic echocardiogram of the left ventricle (LV) showing cardiac findings characteristic of chronic hypertension. The septal (S) and posterior wall (PW) thickness is greater than 1.5 cm (referenced to 1 cm-spaced tick marks along the right side of the image), consistent with moderate concentric hypertrophy. There is mild aortic valve calcification (AVC, arrow). Right panel: Coronal view of a chest computed tomography scan from the same patient demonstrating concentric hypertrophy of the left ventricle.
Another possible explanation to our findings is that hypertension alone may not be a singular explanation It is possible that the finding of LVH in the setting of is related to a shared genetic or biochemical pathway abnormality between the heart and aorta. For example, matrix metalloproteinase 9 expression is elevated in patients with LVH and thoracic aortic dissection, and thus could represent such a shared abnormality.35,36 Additional work to explore this observation is also warranted.
Mild pulmonary hypertension was also common in our cohort and not explained by left heart dysfunction. Many disease processes can contribute to pulmonary hypertension, including pulmonary arterial abnormalities, cardiac disease, COPD, obstructive sleep apnea, and chronic thromboembolic disease, among others. While only 4.3% of patients with TTEs had a past medical history of COPD, the actual prevalence of COPD in this cohort may be significantly higher given that high percentage of previous and current smokers, thus may partly explain the high rate of pulmonary hypertension. Further study is warranted to investigate the etiology and clinical significance of pulmonary hypertension in patients with TBAD.
The study has several limitations. This is exploratory work that is done via retrospective chart review design in a cohort derived from a large referral population over a relatively long time interval. The sample size is small thus likely underpowered for substantial risk stratification analysis. Moreover, echocardiography utilization and reporting practices were not standardized thus there is potential for bias. Additionally, patients who underwent TTE were more acutely ill, given their transfer to a tertiary care center and higher rates of malperfusion. Additional work delineating detailed causes specific mortality is also warranted.
In summary, our findings highlight the potential role of LVH as a biomarker for group of TBAD patients who are at risk for early mortality. This, however, is hypothesis-generating and will require prospective validation in larger cohorts.
Conclusion
LVH and other findings of hypertensive heart disease are common in patients with TBAD. LVH predicted all-cause mortality after TBAD in this small group of patients. Further exploration of the relationship between the chronic effects of hypertension and using LVH as an objective biomarker to risk stratify TBAD patients and long-term outcomes after TBAD is warranted.
Acknowledgements:
We thank Kevin C. Cain, PhD for his statistical consultation and Ellen Cho for her help with data abstraction.
Funding
This project was made possible in part by funding from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423 (SS), the Endovascular Training and Research Fund (SS) and the University of Washington House staff Association (AT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presentation Information
This study was presented at the Western Vascular Society 32 nd Annual Meeting on September 23, 2017 and the Pacific Northwest Vascular Society Annual Meeting on November 11, 2017.
Disclosures
None
References
- 1.Nauta FJ, Trimarchi S, Kamman AV, Moll FL, van Herwaarden JA, Patel HJ et al. Update in the management of type B aortic dissection. Vasc Med 2016;21(3):251–263. [DOI] [PubMed] [Google Scholar]
- 2.Grommes J, Greiner A, Bendermacher B, Erlmeier M. Frech A, Belau P, et al. Risk factors for mortality and failure of conservative treatment after aortic type B dissection. J Thorac Cardiovasc Surg 2014;148(5):2155–2160. [DOI] [PubMed] [Google Scholar]
- 3.Kudo T, Mikamo A, Kurazumi H, Suzuki R, Morikage N, Hamano K. Predictors of late aortic events after Stanford type B acute aortic dissection. J Thorac Cardiovasc Surg 2014;148(1):98–104. [DOI] [PubMed] [Google Scholar]
- 4.Ray HM, Durham CA, Ocazionez D, Charlton-Ouw KM, Estrera AL, Miller CC 3rd, et al. Predictors of intervention and mortality in patients with uncomplicated acute type B aortic dissection. J Vasc Surg 2016;64(6):1560–1568. [DOI] [PubMed] [Google Scholar]
- 5.Estrera AL, Miller CC 3rd, Safi HJ, Goodrick JS, Keyhani A, Porat EE, et al. Outcomes of medical management of acute type B aortic dissection. Circulation 2006;114(1 Suppl):I384–9. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol 2000;11:A0828. [Google Scholar]
- 7.Chatterjee S, Bavishi C, Sardar P, Agarwal V, Krishnamoorthy P, Grodzicki T, et al. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol 2014;114(7):1049–52. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Doyle JT, McNamara PM, Quickenton P, Gordon T. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation 1975;51(4):606–13. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322(22):1561–6. [DOI] [PubMed] [Google Scholar]
- 10.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52(25):2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beache GM, Herzka DA, Boxerman JL, Post WS, Gupta SN, Faranesh AZ, et al. Attenuated myocardial vasodilator response in patients with hypertensive hypertrophy revealed by oxygenation-dependent magnetic resonance imaging. Circulation 2001;104(11):1214. [DOI] [PubMed] [Google Scholar]
- 12.Burke AP, Farb A, Liang YH, Smialek J, Virmani R. Effect of hypertension and cardiac hypertrophy on coronary artery morphology in sudden cardiac death. Circulation 1996;94(12):3138. [DOI] [PubMed] [Google Scholar]
- 13.Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation 1994;90(1):179–185. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Nasothimiou EG, Chang CL, Sever PS, Dahlöf B, Poulter NR. Baseline predictors of resistant hypertension in the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT): a risk score to identify those at high-risk. J Hypertens 2011;29:2004–2013. [DOI] [PubMed] [Google Scholar]
- 15.Iarussi D, Caruso A, Galderisi M, Covnio FE, Dialetto G, Bossone E et al. Association of left ventricular hypertrophy and aortic dilation in patients with acute thoracic aortic dissection. Angiology 2001;52(7):447–55. [DOI] [PubMed] [Google Scholar]
- 16.Epperlein S, Mohr-Kahaly S, Erbel R, Kearney P, Meyer J. Aorta and aortic valve morphologies predisposing to aortic dissection. An in vivo assessment with transesophageal echocardiography. Eur Heart J 1994;15(11):1520–7. [DOI] [PubMed] [Google Scholar]
- 17.Prakash SK, Haden-Pinneri K, Milewicz DM. Susceptibility to acute thoracic aortic dissections in patients dying outside the hospital: an autopsy study. Am Heart J 2011;162(3):474–9. [DOI] [PubMed] [Google Scholar]
- 18.Durham CA, Aranson NJ, Ergul EA, Wang LJ, Patel VI, Cambria RP et al. Aneurysmal degeneration of the thoracoabdominal aorta after medical management of type B aortic dissections. J Vasc Surg 2015;62(4):900–6. [DOI] [PubMed] [Google Scholar]
- 19.Onitsuka S, Akashi H, Tayama K, Okazaki T, Ishihara K, Hiromatsu S, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg 2004;78(4):1268–73. [DOI] [PubMed] [Google Scholar]
- 20.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306(8):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 22.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension 2011;57(6):1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population. Hypertension 2005;46:124–129. [DOI] [PubMed] [Google Scholar]
- 24.Redmond N, Baer HJ, Hicks LS. Health behaviors and racial disparity in blood pressure control in the National Health and Nutrition Examination Survey. Hypertension 2011;57:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 2014;63(25 Pt A):2852–61. [DOI] [PubMed] [Google Scholar]
- 26.Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2010;213(2):558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS, et al. Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy). Am J Cardiol 2005;95(1):132–6. [DOI] [PubMed] [Google Scholar]
- 28.Fox CS, Guo CY, Larson MG, Vasan RS, Parise H, O’Donnell CJ, et al. Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol 2006;97(10):1502–5. [DOI] [PubMed] [Google Scholar]
- 29.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29(3):630–4. [DOI] [PubMed] [Google Scholar]
- 30.Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003;107(11):1492–6. [DOI] [PubMed] [Google Scholar]
- 31.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341(3):142–7. [DOI] [PubMed] [Google Scholar]
- 32.Woythaler JN, Singer SL, Kwan OL, Meltzer RS, Reubner B, Bommer W et al. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol 1983;2(2):305–11. [DOI] [PubMed] [Google Scholar]
- 33.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55(14):e27–e129. [DOI] [PubMed] [Google Scholar]
- 34.Zhang K, Chen J, Liu Y, Wang T, Wang L, Wang J, et al. Diastolic blood pressure reduction contributes more to the regression of left ventricular hypertrophy: a meta-analysis of randomized controlled trials. J Hum Hypertens 2013;27(11):698–706. [DOI] [PubMed] [Google Scholar]
- 35.Saglam M, Karakaya O, Esen AM, Barutcu I, Dogan S, Karavelioglu Y, et al. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med 2006;208(2):117–22. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Wu D, Choi JC, Minard CG, Hou X, Cselli JS, et al. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J Surg Res 2014;189(2):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]


