Abstract
Introduction:
The dismally slow improvement in patient survival over the years for pancreatic cancer patients is mainly due to two factors: the late diagnosis, at which point the disease is spread to distant organs; and the fact that tumor cells are surrounded by a dense, highly immunosuppressive microenvironment. The tumor microenvironment not only shields pancreatic cancer cells from chemotherapy but also leaves it unsusceptible to various immunotherapeutic strategies that have been proven successful in other types of cancer.
Areas covered:
This review highlights the main components of the pancreatic tumor microenvironment, how they cross-talk with each other to generate stroma and promote tumor growth. Additionally, we discuss the most promising treatment targets in the microenvironment whose modulation can be robustly tested in combination with standard of care chemotherapy. Currently active clinical trials for pancreatic cancer involving components of the microenvironment are also listed.
Expert opinion:
Although immunotherapeutic approaches involving checkpoint inhibition are being pursued enthusiastically, there is still more work to be done with several other emerging immune targets that could provide therapeutic benefit.
Keywords: Pancreatic cancer, tumor microenvironment, stellate cells, fibroblasts, tumor-associated macrophages, myeloid derived suppressor cells
1. Introduction
Unlike other cancer types where significant advances have been made, therapies for the treatment of pancreatic cancer have largely failed to move through the clinical trials. After the approval of Gemcitabine by the Food and Drug Agency (FDA) in 1997, the most significant clinical benefit has been shown by FOLFIRINOX, which is a combination of oxaliplatin, irinotecan, leucovorin and 5-fluorouracil, showing a median survival of 11.1 months as compared to 6.8 month for gemcitabine [1]. A combination of Gemcitabine and nanoparticle albumin-bound Paclitaxel (Nab-Paclitaxel) is also used as an alternative frontline therapy [2]. However, when compared to FOLFIRINOX in a metastatic pancreatic cancer study, Gemcitabine plus Nab-Paclitaxel showed only a marginal advantage [3]. The study revealed that Gemcitabine plus Nab-Paclitaxel was better suited for older patients because of lower toxicity and ease of administration. Addition of immunotherapy to this regimen is the next hope towards increasing patient survival. The microenvironment in Pancreatic Ductal Adenocarcinoma (PDAC) constitutes up to 80% of the total tumor mass and as a major component, plays a vital role in creating an immunosuppressive environment, promoting disease progression and metastasis [4]. This stromal microenvironment, composed of cellular and acellular components, forms a physical barrier around the tumor cells, shielding them from immune surveillance. Chemoresistance of PDAC is also thought to be largely due to the robust stroma. Water retention capability of some extracellular matrix proteins discussed later, causes interstitial fluid pressure that makes it difficult to deliver anti-tumor drugs to the area.
Over the past years, there have been many efforts exploring different ways in which to manipulate the stromal microenvironment to achieve a more immunogenic environment, better drug delivery and ultimately improve patient survival in the clinical studies. In this review, we will highlight the major components of the pancreatic TME, their interaction with tumor cells and strategies to target TME that have clinical potential.
2. Major players in pancreatic tumor microenvironment
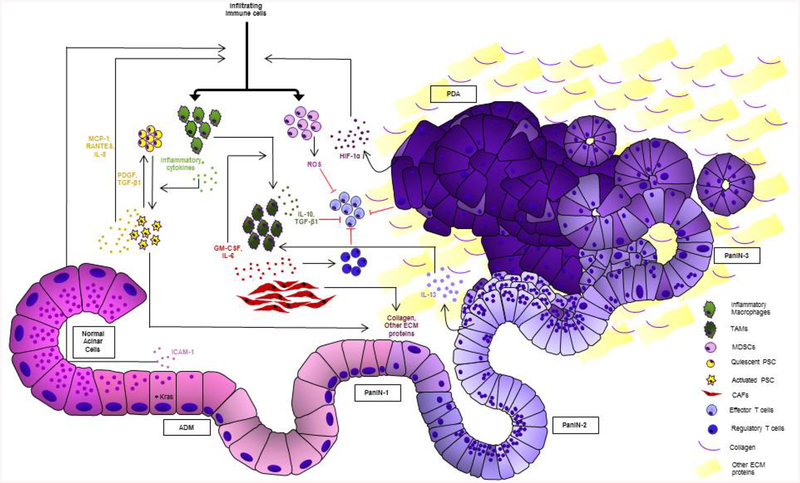
Over the past decade, studies have brought into light the profound importance of the pancreatic tumor microenvironment (TME) as a driving force in tumor development and progression. Not only does the desmoplastic stroma create a protective shield from therapeutics [5], it also aids in the process of epithelial to mesenchymal transition (EMT) and causes tumor cell dissemination into surrounding tissue. This process involves constant remodelling of the stroma, and activation of different signalling pathways in the TME [6,7]. The following sections enlist and discuss some of the major components of the TME and how they interact amongst themselves and the tumor cells to promote tumorigenesis. Figure 1 depicts the pancreatic tumor microenvironment and summarizes the cross-talk amongst different components.
Figure 1: Pancreatic tumor microenvironment and cross-talk within its components.
Schematic depicting pancreatic acinar cells progressing through precancerous lesion types to PDAC and their interaction with different stromal cell types. Activated stellate cells and transformed acinar cells undergoing acinar to ductal metaplasia (ADM), release cytokines that recruit M1 macrophages, generating an inflammatory environment. Fibroblasts and PanIN cells release factors that promote polarization of M1 macrophages to pro-tumor M2 macrophages. Activated stellate cells and fibroblasts also release proteins that make up a dense and fibrous extracellular matrix, a hallmark of pancreatic cancer. Tumor cells release HIF-1α that can attract MDSCs. MDSCs along with regulatory T cells, immunosuppressive factors released by TAMs/M2 macrophages and suppressive signals from tumor cells cause the suppression of effector T cells.
2.1. Pancreatic stellate cells (PSCs)
The lineage mapping of PSCs remains to be a topic of ongoing investigation, however some results from hepatic stellate cell studies support a mesodermal origin based on functional similarities [7,8]. Other studies suggest that they can originate from more than one source. In the context of pancreatic cancer and chronic pancreatitis, a fraction of stellate cells is recruited from the bone-marrow [9,10]. Infiltrating monocytes can also differentiate to PSCs under the influence of MCP-1 [11]. Under physiological conditions, stellate cells are known to play a role in the maintenance of the basement membrane [12]. They also synthesize matrix proteins, matrix metalloproteinases (MMP-2, MMP-9, MMP-13) and MMP inhibitors that regulate extracellular matrix (ECM) turnover [13,14]. On the other hand, under pathological conditions, PSCs can proliferate, get activated and exhibit increased production of ECM proteins (α-SMA, collagen) and MMPs [15]. Factors that can activate quiescent stellate cells range from inflammatory cytokines to platelet-derived growth factor (PDGF) and transforming growth factors TGF-α, TGF-β [16,17] [18,19]. Sources of these factors can be pancreatic acinar cells and other cell types found in the inflammatory microenvironment, as discussed later [20]. Importantly, tumor cells are also shown to induce stellate cell proliferation and matrix production [21]. Apart from receiving activating signals from other sources in a paracrine manner, PSCs themselves express factors like TGF-β1 and PDGF to perpetuate their own proliferation and activation [22]. Once activated, PSCs can secrete chemoattractant molecules such as IL-8, macrophage chemoattractant protein-1 (MCP-1), and RANTES to contribute to the inflammatory nature of the microenvironment [23].
2.2. Cancer associated fibroblasts (CAFs)
Primary function of fibroblasts is to facilitate tissue regeneration in the face of injury. Under physiological conditions, they are present in organ tissues in a quiescent state and become activated only upon receiving signals from surrounding damaged tissue. Once activated, they proliferate, generate growth factors and produce extracellular matrix (ECM) proteins to aid the healing process [24]. Activated fibroblasts that arise in the TME are called CAFs and are one of the most predominant cell types found in the stroma with several functional subtypes [25]. This heterogeneity in the CAF population is dependent on their interaction with cancer cells and on the local signals they receive from their immediate surroundings [26]. Activated CAF populations in turn have been shown to facilitate the growth and proliferation of tumor cells [27], and a recently published study also reported that Laminin-332 and α3β1 Integrin expression by CAFs can facilitate cell invasion [28]. CAFs can also carry out their effector functions via secreted cytokines. Amongst those, the granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-6 (IL-6) have recently emerged to be important in enhancing monocyte differentiation to pro-tumor macrophages [29] that further contribute to tumor progression.
2.3. Myeloid derived suppressor cells (MDSCs)
MDSCs are a group of bone-marrow derived cells, immature in nature, whose primary function is immuno-regulation and tissue repair. Owing to their ability to curtail immune responses, in the context of TME, MDSCs create an immunosuppressive environment. Pancreatic cancer cells can induce mobilization of MDSCs out of the bone marrow and into systemic circulation before they get recruited into the TME [30]. In pancreatic cancer patients, the number of circulating MDSCs has been shown to correlate with the stage of the cancer and metastasis [31]. As the primary tumor grows and a hypoxic environment is created, hypoxia inducible factors (HIF) such as HIF1α are upregulated, and serve as key mediators of MDSC recruitment to the pancreatic TME [32]. As mentioned above, the main role MDSCs play in the TME is that of immunosuppression. They can release reactive oxygen species (ROS) that can induce stress in other immune cells present in the TME [33]. Oxidative stress in T cells suppresses the expression of CD3 ζ chain, which hampers their proliferation [34]. Another important mechanism by which MDSCs induce immunosuppression in the TME in response to IL-10 secreted by T cells is by the upregulation of the programmed death-ligand 1 (PD-L1) [35]. Once the programmed death protein 1 (PD-1) present on activated T cells is engaged by the upregulated PD-L1 on MDSCs, T cells go into a cell cycle arrest, rendering the lymphocytes inactive.
Since MDSCs are a mixed population of immature immune cells that are not fully differentiated, they exhibit a certain amount of plasticity. There is evidence that MDSCs in a hypoxic environment can differentiate into macrophages through an upregulation of CD45 phosphatase and downregulation of STAT3 [36]. Tumor-associated macrophages are another major component of the TME, as discussed ahead.
2.4. Tumor-associated macrophages (TAMs)
TAMs found in the microenvironment arise from three main sources. 1) They originate from the tissue-resident population that arises from the embryonic yolk-sac [37], 2) they are attracted into the pancreas by various chemoattractants present in the tumor stroma such as IL-4, IL-13, intercellular adhesion molecule-1 (ICAM-1) and colony stimulating factor-1 (CSF-1) [38,39] and 3) they are generated by a polarization switch from inflammatory M1 macrophages to a tumor promoting M2-like phenotype [40,41]. While M1 macrophages are more predominant in the early stages of pancreatic lesion formation creating a fibrotic and inflammatory environment, M2-like macrophages are found in later stages of the progression and are referred to as TAMs. In humans, abundance of M2 macrophages in tumors is correlated to early metastasis, tumor recurrence and ultimately reduced overall survival [42,43]. These cells are known to secrete factors like IL-10 and TGF-β and promote tumor progression by creating a fibrotic and immunosuppressive environment [44,45]. TGF-β is known to have a paradoxical effect in pancreatic TME, where it inhibits cell growth in early stages but is known to have an immunosuppressive role as the disease progresses [41,46]. In turn, pancreatic cancer cells can also modulate polarization of macrophages to a tumor promoting M2 phenotype [41,47]. Tumor cells have been shown to produce IL-13, a polarization factor for M2 macrophages [41], and to enhance the differentiation of macrophages to an M2-like phenotype under hyperglycaemic conditions [48]. Various studies have shown that TAMs can propagate the disease by playing a significant role in tumor cell invasion and metastasis [49–52].
3. Targeting the tumor microenvironment
The dense desmoplasia surrounding the tumor cells is a huge obstacle for drug delivery, and studies that combined stromal targeting with standard of care chemotherapy showed an increase in efficacy [2]. However, more recent studies suggest that completely depleting tumor stroma may in fact, have a more detrimental prognosis indicating that the tumor stroma, in addition to having largely tumor promoting characteristics, stifles tumor growth to some extent. For example, Ozdemir et al showed that complete depletion of tumor stroma by targeting CAFs accelerated the progression of PDAC with reduced overall survival [53]. Such studies underscore the highly complex nature of tumor stroma and that targeting the pancreatic tumor stroma doesn’t simply require complete ablation but in fact needs to be careful modulated. The key components of the stroma, as discussed above, are stellate cells, CAFs, MDSCs and the TAMs, that cross-talk with each other and the tumor cells and create an immunosuppressive environment, refractive to therapy. But the acellular component of the TME, comprised of ECM proteins such as collagen I, III, IV, hyaluronic acid, fibronectin, laminin etc. are equally important. These ECM proteins can provide a scaffold for cytokines and growth factors, interact with tumor cells directly to enhance growth, and create a physical barrier for chemotherapeutics and immune cells. Therefore, it is imperative for any therapeutic strategy against pancreatic cancer, that a combination of drugs simultaneously targeting the stromal components and the tumor cells be used. Table 1 summarizes some of the currently active clinical trials for pancreatic cancer that are using agents targeting the pancreatic TME alone or in combination with chemotherapeutics after surgical removal of the tumors. Some of the most promising TMA targeting strategies are discussed in detail as follows. Figure 2 broadly summarizes the main targeting strategies in pancreatic TME, some of which are discussed in detail as follows.
Table 1.
Currently active and/or recruiting clinical trials for pancreatic cancer, testing drugs that target different components of the tumor microenvironment, alone or in combination with standard of care chemotherapy or other therapies. Clinical trials currently investigating TME targeting strategies
| Clinical Trial Identifier | Study drugs/ Biologicals/ Other interventions | Phase |
|---|---|---|
| Checkpoint inhibition | ||
| NCT03331562 | Pembrolizumab, Paricalcitol | Phase II |
| NCT03727880 | Pembrolizumab, Defactinib | Phase II |
| NCT02646748 | Pembrolizumab, Itacitinib, INCB050465 | Phase I |
| NCT03681951 | GSK3145095, Pembrolizumab | Phase I, Phase II |
| NCT03184870 | BMS-813160, Nivolumab, Nab-paclitaxel, Gemcitabine, 5-fluorouracil, Leucovorin, Irinotecan | Phase I, Phase II |
| NCT02734160 | Galunisertib, Durvalumab | Phase I |
| NCT03481920 | PEGPH20, Avelumab | Phase I |
| NCT02311361 | Durvalumab, Tremelimumab | Phase I, Phase II |
| NCT02777710 | Pexidartinib, Durvalumab | Phase I |
| NCT02826486 | BL-8040, Pembrolizumab | Phase II |
| NCT02648282 | Cyclophosphamide, GVAX, Pembrolizumab | Phase II |
| NCT02930902 | Pembrolizumab, Paricalcitol, Gemcitabine, Nab-paclitaxel, surgical resection | Phase I |
| NCT03153410 | Cyclophosphamide, GVAX, Pembrolizumab, IMC-CS4 | Phase I |
| NCT02713529 | AMG820, Pembrolizumab | Phase I, Phase II |
| NCT02451982 | Cyclophosphamide, GVAX, Nivolumab, Urelumab | Phase I, Phase II |
| NCT03190265 | Cyclophosphamide, Nivolumab, Ipilimumab, CRS-207, GVAX | Phase II |
| NCT03161379 | Cyclophosphamide, Nivolumab, GVAX | Phase II |
| NCT02243371 | CRS-207, GVAX, Nivolumab, | Phase II |
| NCT03519308 | Nivolumab, Paricalcito, Gemcitabine, Nab-paclitaxel | Phase I |
| NCT02868632 | MEDI4736, Tremelimumab | Phase I |
| NCT01473940 | Ipilimumab, Gemcitabine | Phase I |
| NCT02588443 | RO70097890, Gemcitabine, Nab-paclitaxel | Phase I |
| NCT02807844 | MCS110, PDR001 | Phase I, Phase II |
| Targeting CAF mediated immunosuppression | ||
| NCT03277209 | Plerixafor | Phase I |
| NCT02826486 | BL-8040, Pembrolizumab | Phase II |
| Targeting MDSC recruitment | ||
| NCT02345408 | CCX872-B, FOLFIRINOX | Phase I |
| NCT03681951 | GSK3145095, Pembrolizumab | Phase I, Phase II |
| Targeting stromal depletion | ||
| NCT02715804 | PEGPH20, Gemcitabine, Nab-paclitaxel | Phase III |
| NCT02910882 | PEGPH20, Gemcitabine, Radiation | Phase II |
| NCT03481920 | PEGPH20, Avelumab | Phase I |
| GVAX | ||
| NCT02451982 | Cyclophosphamide, GVAX, Nivolumab, Urelumab | Phase I, Phase II |
| NCT02648282 | Cyclophosphamide, GVAX, Pembrolizumab | Phase II |
| NCT02243371 | CRS-207, GVAX, Nivolumab | Phase II |
| NCT03153410 | Cyclophosphamide, GVAX, Pembrolizumab, IMC-CS4 | Phase I |
| NCT03161379 | Cyclophosphamide, Nivolumab, GVAX | Phase II |
| NCT03190265 | Cyclophosphamide, Nivolumab, Ipilimumab, CRS-207, GVAX | Phase II |
| Others | ||
| NCT02030860 | Paricalcitol, Gemcitabine, Abraxane | N/A |
| NCT02929797 | CD8+NKG2D+ AKT Cell | Phase I |
| NCT02562898 | Ibrutinib, Paclitaxel, Gemcitabine | Phase I, Phase II |
| NCT02923921 | AM0010, FOLFOX | Phase III |
| NCT02550327 | Anakinra, Gemcitabine, Nab-paclitaxel, Cisplatin | Phase I |
| NCT02559674 | ALT-803, Gemcitabine, Nab-paclitaxel | Phase I |
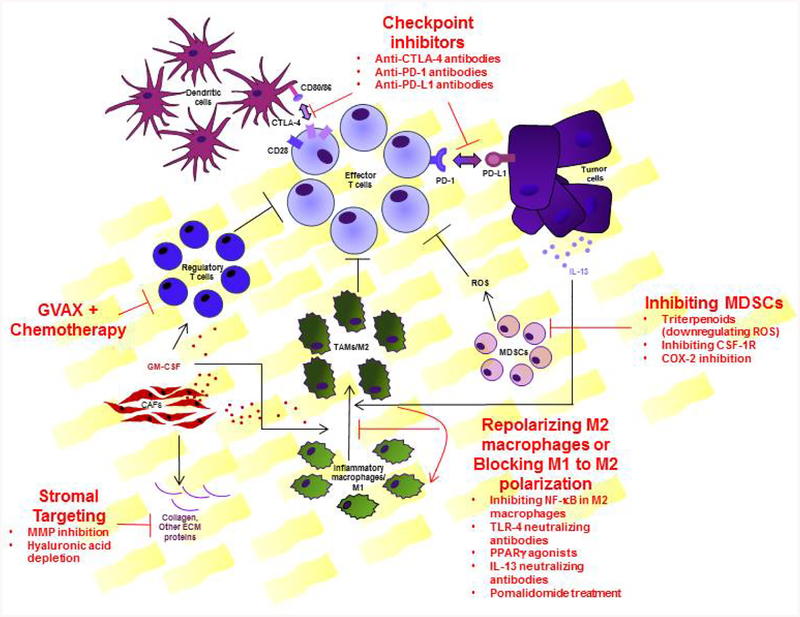
Figure 2: Therapeutic targets in the tumor microenvironment.
Several components outside and within the microenvironment contribute to effector T cell suppression. Dendritic cells in the regional lymph nodes expressing CD80/86 on the surface, bind to CTLA-4 present on the T cells blocking appropriate priming for T cells. This can be targeted by anti-CTLA-4 antibodies. Tumor cells express the ligand PD-L1, which can also render the effector T cells inhibited and unable to perform effector functions once bound to the PD1 receptor on T cells. This can be targeted by anti-PD1 and anti-PD-L1 antibodies. Regulatory T cells (Tregs), whose primary function is to curtail effector T cell function, are heavily recruited in the microenvironment by GM-CSF. GVAX in combination with chemotherapeutics leads to reduced Treg recruitment and enrichment of effector T cells. T cell suppression can also be reduced by targeting MDSCs via COX-2 inhibition, targeting the CSF-1 receptor or by using triterpenoids that are shown to reduce MDSCs in the tumors by downregulating ROS production. Alternatively activated or M2 macrophages are one of the major orchestrators of the immunosuppressive environment, making them an important target. M2 polarization can be caused by IL-13, making IL-13 neutralizing antibodies a potential therapeutic. Pomalidomide treatment can also reduce M1 to M2 polarization. Lastly, the dense stroma in the tumor poses an enormous challenge in PDAC therapy and its depletion is essential. Targeting the stromal proteins by MMP inhibition and hyaluronic acid depletion are promising strategies for better delivery of any therapy including chemotherapeutic drugs.
3.1. Immunotherapy, targeting the cellular component of the TME
3.1.1. Enhancing T cell-mediated immunity
Unlike other solid tumors, pancreatic cancer is unique in having a strongly immunosuppressive microenvironment, adding to the complexity of immunotherapeutic targeting. T cells are the main effectors that can potentially target tumor cells through cytotoxic activity. But their cytotoxic function is preceded by a complex process involving priming, antigen presentation by dendritic cells and tumor infiltration. To evade T cell cytotoxicity, tumors develop mechanisms that inhibit various steps in this process. Increased recruitment of regulatory T cells, expression of PD-L1 (ligand for PD1 expressed on T cells) being some of them. Therefore, employing strategies to overcome these inhibitory mechanisms is essential for successful immunotherapy in PDAC. Enhancing T cell-mediated immunity, especially using the chimeric antigen receptor (CAR) T cell therapy has been successful in the treatment of patients with lymphoma and acute myeloid leukemia [54], but is still under investigation as a treatment for pancreatic cancer [55]. Mesothelin is an attractive target for CAR-T cell therapy since it is expressed in 80% of pancreatic cancers and is correlated with an unfavourable patient outcome [56]. Beatty et al showed that adoptive transfer of mesothelin specific mRNA CAR-T (CARTmeso) cells was safe in patients with minimal off-target effects and infiltrated primary and metastatic sites [57,58]. Apart from mesothelin, some of the other antigens used as a target for CAR-T cell therapy include prostrate stem cell antigen (PSCA), Muc-1, carcinoembryonic antigen (CEA), or fibroblast activation protein (FAP) [59].
Checkpoint inhibition in pancreatic cancer, even in combination with chemotherapy has not shown any significant improvement in therapy [60,61]. However, alteration of the TME prior to checkpoint inhibition such that it becomes more immunogenic may result in better outcomes. Treatment with the GM-CSF vaccine (GVAX) in combination with chemotherapy was shown to deplete regulatory T cells from pancreatic tumors and form lymphoid aggregates within the tumor, making the microenvironment less immunosuppressive [62]. When used in combination with Ipilimumab (checkpoint inhibitor-monoclonal antibody targeting CTLA-4), the overall survival was better than Ipilimumab alone (3.6 months for Ipilimumab; 5.7 months for combination treatment) [63]. Focal adhesion kinase (FAK) has recently been identified as being upregulated in PDAC and is correlated with poor CD8+ T cell infiltration. A study targeting FAK in neoplastic tumor cells showed that when used alone, FAK inhibitor showed limited tumor progression, lesser fibrosis and fewer immunosuppressive cells in KPC mouse tumors [64]. The study also showed that after FAK inhibitor treatment, KPC tumors which were not susceptible to checkpoint inhibition became more responsive to PD-1 antagonists. FAK inhibitors are being tested in the clinic for other solid tumor malignancies ( NCT00787033) and are an attractive drug candidate for being tested in pancreatic cancer patients.
Another promising strategy for enhancing T cell mediated immunity is by using CD40 agonist antibodies [65,66]. CD40 is a molecule expressed mainly on antigen presenting cells and some fibroblasts and endothelial cells. Engagement of the CD40 receptor with the CD40 ligand, which is expressed on T cells, macrophages and smooth muscle cells [67], is known to enhance the activation of antigen presenting cells, priming them for cytotoxic T cell responses [68]. In pancreatic cancer, CD40 stimulation has been evaluated in combination with Gemcitabine treatment with the understanding that the chemotherapeutic aids the release of tumor antigens, followed by antigen presentation by APCs that are then “licenced” with the help of a CD40 agonist. This leads to the stimulation of T cells that are cytotoxic against the cells expressing tumor antigens [69].
Other ways of enhancing T cell mediated immunotherapy include MAP kinase pathway inhibition. It is widely known that more than 90% of the pancreatic cancer patients harbour a KRAS mutation. Combination of the KRAS/MAP kinase pathway inhibition and PD-L1 checkpoint blockade showed a synergistic effect and tumor regression [70].
Due to the highly fibrotic nature of the pancreatic TME that poses a physical barrier for immune cell entry, design of any therapeutic regimen should incorporate a strategy to reprogram the immunosuppressive microenvironment.
3.1.2. Reprograming macrophage polarization
Macrophages are inarguably one of the key players in the entire tumor development process. Recruitment of inflammatory macrophages (M1 polarized) into the pancreas is mediated by several chemokines, cytokines and growth factors such as CCL2, CCL5, GM-CSF, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and ICAM-1 [39,71–74]. Other cytokines present in the inflamed pancreatic milieu cause polarization of the M1 macrophages to an alternative active form referred to as M2 macrophages. A recent study showed that the expression of IL-13 at the PanIN lesions correlated with the presence of M2 macrophages and that it can cause M1 to M2 polarization [41]. These M2 macrophages thrive in and foster an environment that is fibrotic and immunosuppressive in nature, perpetuates tumor growth, facilitates metastasis, and ultimately leads to full-blown carcinoma. Therefore, they are also referred to as tumor-associated macrophages (TAMs). Presence of these macrophages in pancreatic cancer patients correlates with poor prognosis [75]. Given the critical role played by these cells in tumor biology, it stands to reason that fine-tuning the polarization of these macrophages in combination with adjuvant interventions presents a potential therapeutic opportunity. A recently published study shows that treatment of pancreatic tumors with an agent known as Pomalidomide led to reduced M2 macrophages in the microenvironment through downregulation of interferon regulatory factor 4 (IRF4) [45]. Other studies have shown that repolarization of M2 macrophages to an M1-like phenotype can be achieved by targeting nuclear factor-κB (NF-κB) signalling and can also result in tumoricidal activity [76]. Interestingly, both studies also indicated that targeting TAMs, not only induces tumoricidal activity, but also enhances T cell mediated cytotoxic effects, making the TME more immunogenic in nature. Another study delineated the role of tumor necrosis factor (TNF) in modulating macrophage polarization [77]. The study showed that TNF can do so in two different ways. It can negatively regulate M2 macrophage associated gene expression and can downregulate IL-13 production from eosinophils in the environment, which as discussed earlier, is a key regulator of M1 to M2 polarization. Other factors that can potentially repolarize M2 macrophages to an M1 phenotype include TLR4 neutralizing antibodies, PPAR-gamma agonists or Trabectadin [78].
Studies highlighted above, underscore the enormous potential of macrophages in reshaping the pancreatic TME. R programing of TAMs to an M1 phenotype modulates the TME such that it also helps improve immunosurveillance from other immune cells as well as improve T cell mediated immunity [79].
3.1.3. Targeting MDSCs
Given their enormous role in causing an immunosuppressive TME, MDSCs are considered a potential target for therapy. Use of an agent called as Triterpenoid in pre-clinical models of lung and colon carcinoma showed reduced MDSC function through downregulation of reactive oxygen species (ROS) and inhibition of STAT3 [80]. The same study showed that Triterpenoid treatment was well tolerated in patients. However, when used in patients with unresectable tumors in combination with Gemcitabine, Triterpenoid had no significant effects on MDSCs in peripheral blood, but increase T cell response. MDSCs express CSF-1R on their surface, which is a receptor for the growth factor CSF-1. One study showed that blocking this interaction led to depletion of MDSCs in the tumors along with some macrophages. It also showed that when used in combination with checkpoint inhibition, there was significant pancreatic tumor regression [81]. Downstream of growth factor receptors, the JAK-STAT signalling pathway is known to be essential in MDSC function. However, limited data is available to evaluate the potential of STAT inhibition in reducing MDSC function in pancreatic cancer. Rosiglatizone, an FDA approved drug, which suppresses JAK-STAT signalling, in combination with Gemcitabine showed reduced MDSC accumulation in the tumor and extended overall survival in a pre-clinical pancreatic cancer model [82]. LTP-1, an anti-mitotic agent and STAT3 inhibitor was shown to inhibit pancreatic tumor cell growth in vitro and tumor formation in vivo, but its mechanism of action on MDSCs was not explored [83]. Another agent, Sildenafil (phosphodiesterase-5 inhibitor) is known to inhibit MDSC function by downregulating arginase-1, IL4Ra and ROS leading to NK cell mediated cytotoxicity in the tumor [84]. In addition to these agents, cyclooxygenase-2 (COX-2) inhibition was also shown to inhibit recruitment and immunosuppressive function of MDSCs by targeting arginase-1 levels in a model of lung carcinoma [85]. These studies highlight several avenues that could potentially prove beneficial in the treatment of pancreatic cancer as adjuvant therapies to standard of care chemotherapeutics. In fact, Gemcitabine was discovered to target the splenic MDSC population years after it was approved for use in pancreatic cancer patients [86]. Also, gemcitabine treatment after tumor resection was shown to enhance NK cell mediated cytotoxic effects [87]. Therefore, at least a part of the effects seen by gemcitabine could be through its role in depleting MDSCs.
3.2. Targeting the non-cellular components of the TME
The non-cellular components of the TME mainly comprise of ECM proteins and matrix metalloproteases, both of which can be good therapeutic targets. ECM proteins are secreted into the stroma mainly by the fibroblasts and PSCs in the TME. These proteins provide structural integrity and are directly involved in promoting tumor cell proliferation and migration [88]. They are generally found to be upregulated in pancreatic cancer. Fibronectin, which is secreted by fibroblasts, can bind integrin molecules on cell surfaces and regulate cell survival, adhesion and migration, but abrogation of this interaction has not produced good results in the clinic despite promising pre-clinical results [89]. Some of the other potential strategies for targeting the non-cellular components of the TME are discussed as follows.
3.1.2. Matrix metalloproteinase (MMP) inhibition
MMPs are proteolytic enzymes and are known for their wide ranged role in cancer initiation, growth and metastasis [90]. Unfortunately, clinical studies on pancreatic cancer patients have only shown modest success involving MMP inhibitors. A phase-1 study with the drug Marmistat (multifamily MMP inhibitor) on pancreatic cancer patients with unresectable tumors showed an overall survival equivalent to that with gemcitabine but failed to show any synergy with gemcitabine when used in combination [91]. As we learn more about the variety of MMPs found in pancreatic stroma and the diverse roles they play, it is becoming clear that efforts should be more targeted towards specific MMPs. Studies have shown that MMPs such as MMP-9 are upregulated in pancreatic cancer and are essential in mediating cell invasion [92]. In a phase III clinical trial with advanced and metastatic PDAC patients, Tanomastat, which inhibits MMP-9 along with MMP-2, MMP-3 and MMP-13 had minimal success [93]. Lack of success with MMP inhibitors is partly due to structural similarities between different MMPs resulting in off-target effects. Over the past few years, numerous miRNAs have been identified as post-transcriptional MMP regulators and can target MMP molecules with relatively greater specificity [94]. Also, miRNAs can target more than one MMP and may be beneficial in targeting a network of pathways. MMPs can also be regulated differently by more than one miRNAs. For example, miR-143 was shown to decrease MMP-2 and MMP-9 expression in pancreatic cancer cells [95], whereas miR-21, miR-221 and miR-222 led to their upregulation [96,97]. From these studies it becomes clear that even though MMPs are attractive therapeutic targets, a closer examination of their varied roles in pancreatic TME and their regulation via miRNAs needs to be carried out before they can be further tested in clinical trials.
3.2.2. Hyaluronic acid depletion
Extracellular matrix (ECM) proteins found in the vicinity of tumor cells and the cellular component of the TME, are extremely important in conferring physical properties to the stroma. Apart from collagen, which is the most abundant ECM protein, hyaluronic acid (HA) can also provide support and structure to the stroma and more importantly, it is targetable by therapeutics. HA is a glycosaminoglycan secreted by tumor cells and forms a gel-like elastic matrix between collagen fibres that helps retain growth factors and cytokines in the stroma. It can also bind to surface receptors on tumor cells and promote proliferation, migration and invasion [98]. HA’s capacity to absorb and retain water causes an increase in the interstitial fluid pressure (IFP) that hinders drug delivery to the tumor cells [99,100]. Given the multifaceted role played by HA in pancreatic stroma, HA targeting has been of great interest and has shown to cause tumor depletion in several preclinical tumor models [99,101]. Targeting HA was shown to enhance drug delivery by relieving the IFP [102]. A recent study showed that pancreatic tumors treated with Halofuginone, an anti-fibrotic agent, targeted PSCs and led to reduced HA in the tumors and showed overall reduced ECM production [54]. PEGylated recombinant hyaluronidase (PEGPH20) in combination with gemcitabine showed an improvement in overall survival and is emerging as an attractive adjuvant therapy [103]. Currently, patients are being recruited for a phase I clinical trial where PEGPH20 will be given in combination with a checkpoint inhibitor Avelumab to assess overall response rate in patients with metastatic or locally advanced pancreatic cancer ( NCT03481920).
4. Conclusion
Numerous studies over the past decade have made clear that the tumor microenvironment has a major contribution in promoting the hallmarks of pancreatic cancer. Not only do stromal cells promote the proliferation of cancer cells, they also provide a protective shell to decrease efficacy of chemotherapeutic drugs. Therefore, a combination therapy approach targeting stroma and cancer cells could be most efficient. However, studies have suggested that complete abrogation of the stroma can have unwanted detrimental effects on tumor growth and metastasis. This is due to the complexity of the TME which consists of a plethora of target cells and molecules present in the milieu of tumor cells (Figure 1). Therefore, rather than total depletion of one or more components of the TME, therapies should be aimed at modulating the composition of the tumor stroma to achieve the correct alignment of different mechanisms in the microenvironment with an overall goal to promote efficient targeting of tumor cells.
5. Expert opinion
New advances in the field show that despite the low immunogenicity of the PDAC microenvironment, careful modulation can make pancreatic tumors amenable to immunotherapy [104]. To improve chemotherapeutic responses and patient survival, strategic multipronged targeting of interdependent mechanisms in the TME that orchestrate disease progression will be key for advancement of therapy. Currently, there is a huge emphasis on checkpoint inhibitors for PDAC therapy as indicated by the clinical trials listed in Table 1, but it is important to note that T cells may not be the ultimate targetable factor in the goal of developing anti-tumor immunity. There are several mechanisms in play that provide an opportunity for intervention, some of which have been discussed in this review. Repolarization of TAMs into inflammatory/M1 macrophages poses an attractive target, as not only do M1 macrophages possess phagocytic capability to destroy tumor cells [105], but can also release tumor antigens in doing so. Released tumor antigens can then boost antigen presentation and stimulate T cell responses. However, translating macrophage-oriented therapies into clinic requires further investigation into identifying the most effective therapeutic targets and identifying any compensatory mechanisms that the tumors might develop in response to depleting TAMs from the TME. Further research is also required to determine how the depletion of acellular TME components effects tumor growth, resistance to chemotherapy and metastasis. Disappointing clinical studies involving MMP inhibitors, discussed earlier, underscore the need for intricate evaluation of the cost and benefit of depleting tumor stroma. Although there is limited success with current clinical trials, they still help to gain insight into the previously obscure nature of various TME components and how they interact with each other. Based on the new knowledge in this ever-advancing field of study, we will be able to further characterize the known therapeutic targets and perhaps discover better ones in future work.
Highlights.
Desmoplasia in the stromal microenvironment makes up to ~80% of total tumor mass in PDAC, creating a physical barrier and conferring chemoresistance
Pancreatic TME is largely non-immunogenic and tumor promoting in nature
PSCs, CAFs, MDSCs and TAMs are major cellular components of the TME, that cross-talk amongst each other and with tumor cells to activate signaling pathways beneficial to tumor growth and metastasis
Remodeling of the stroma to enhance anti-tumor immunity requires simultaneous targeting of TME and tumor cells
Majority of ongoing clinical trials for PDAC are utilizing small molecule inhibitors and monoclonal antibodies targeting TME along with frontline chemotherapy
Funding
This work was supported by a grant from the National Institutes of Health (CA200572) to P Storz.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
6. References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med, 364(19), 1817–1825 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med, 369(18), 1691–1703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahara J, Shimizu K, Otsuka N, Akao J, Takayama Y, Tokushige K. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol, 82(2), 245–250 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Neesse A, Michl P, Frese KK et al. Stromal biology and therapy in pancreatic cancer. Gut, 60(6), 861–868 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Dauer P, Nomura A, Saluja A, Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology, 17(1), 7–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsey S, Langhans SA. Crosstalk of Oncogenic Signaling Pathways during Epithelial-Mesenchymal Transition. Front Oncol, 4, 358(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bynigeri RR, Jakkampudi A, Jangala R et al. Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J Gastroenterol, 23(3), 382–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahina K, Tsai SY, Li P et al. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology, 49(3), 998–1011 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrache F, Pendyala S, Bhagat G, Betz KS, Song Z, Wang TC. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut, 57(8), 1113–1120 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Scarlett CJ, Colvin EK, Pinese M et al. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS One, 6(10), e26088(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ino K, Masuya M, Tawara I et al. Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells. PLoS One, 9(1), e84889(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riopel MM, Li J, Liu S, Leask A, Wang R. beta1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab Invest, 93(1), 31–40 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Ferdek PE, Jakubowska MA. Biology of pancreatic stellate cells-more than just pancreatic cancer. Pflugers Arch, 469(9), 1039–1050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips PA, McCarroll JA, Park S et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut, 52(2), 275–282 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachem MG, Schneider E, Gross H et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology, 115(2), 421–432 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Mews P, Phillips P, Fahmy R et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut, 50(4), 535–541 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahara H, Sato K, Yamazaki Y et al. Transforming growth factor-alpha activates pancreatic stellate cells and may be involved in matrix metalloproteinase-1 upregulation. Lab Invest, 93(6), 720–732 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Apte MV, Haber PS, Darby SJ et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut, 44(4), 534–541 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelmann R, Ruf D, Wagner M, Adler G, Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol, 280(1), G164–172 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol, 44(4), 249–260 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Bachem MG, Schunemann M, Ramadani M et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology, 128(4), 907–921 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Schneider E, Schmid-Kotsas A, Zhao J et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol, 281(2), C532–543 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Andoh A, Takaya H, Saotome T et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology, 119(1), 211–219 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer, 16(9), 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Nielsen MFB, Mortensen MB, Detlefsen S. Typing of pancreatic cancer-associated fibroblasts identifies different subpopulations. World J Gastroenterol, 24(41), 4663–4678 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuzillet C, Tijeras-Raballand A, Ragulan C et al. Inter- and intra-tumoral heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang RF, Moore T, Arumugam T et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res, 68(3), 918–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaco ACM, Rezaei M, Caliandro MF et al. The Interaction between Laminin-332 and alpha3beta1 Integrin Determines Differentiation and Maintenance of CAFs, and Supports Invasion of Pancreatic Duct Adenocarcinoma Cells. Cancers (Basel), 11(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H, Seo Y, Loke KM et al. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clin Cancer Res, 24(21), 5407–5421 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Porembka MR, Mitchem JB, Belt BA et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother, 61(9), 1373–1385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother, 58(1), 49–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Bi Y, Shen B et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res, 74(3), 727–737 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Corzo CA, Cotter MJ, Cheng P et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol, 182(9), 5693–5701 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci U S A, 93(23), 13119–13124 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinton L, Solito S, Damuzzo V et al. Activated T cells sustain myeloid-derived suppressor cell-mediated immune suppression. Oncotarget, 7(2), 1168–1184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Cheng P, Condamine T et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity, 44(2), 303–315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Herndon JM, Sojka DK et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity, 47(2), 323–338 e326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habtezion A, Edderkaoui M, Pandol SJ. Macrophages and pancreatic ductal adenocarcinoma. Cancer Lett, 381(1), 211–216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Liou GY, Doppler H, Necela B et al. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov, 5(1), 52–63 (2015).Article shows the importance of inflammatory macrophages in generating an immunosuppressive microenvironment during early development of pancreatic lesions.
- 40.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol, 33(3), 119–126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Liou GY, Bastea L, Fleming A et al. The Presence of Interleukin-13 at Pancreatic ADM/PanIN Lesions Alters Macrophage Populations and Mediates Pancreatic Tumorigenesis. Cell Rep, 19(7), 1322–1333 (2017).Article highlights the cross-talk between tumor cells and macrophages via IL-13, that promotes polarization of macrophages to a tumor promoting M2 phenotype.
- 42.Kurahara H, Shinchi H, Mataki Y et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res, 167(2), e211–219 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Chen SJ, Zhang QB, Zeng LJ et al. Distribution and clinical significance of tumour-associated macrophages in pancreatic ductal adenocarcinoma: a retrospective analysis in China. Curr Oncol, 22(1), e11–19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Baruch A Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol, 16(1), 38–52 (2006). [DOI] [PubMed] [Google Scholar]
- 45**.Bastea LI, Liou GY, Pandey V et al. Pomalidomide alters pancreatic macrophage populations to generate an immune-responsive environment at precancerous and cancerous lesions. Cancer Res, (2019).Article exhibits the role of Pomalidomide as an agent modulating pancreatic tumor stroma to promote an anti-tumor microenvironment via TAMs.
- 46.Zhang H, Liu C, Kong Y, Huang H, Wang C, Zhang H. TGFbeta signaling in pancreatic ductal adenocarcinoma. Tumour Biol, 36(3), 1613–1618 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Meng F, Li C, Li W, Gao Z, Guo K, Song S. Interaction between pancreatic cancer cells and tumor-associated macrophages promotes the invasion of pancreatic cancer cells and the differentiation and migration of macrophages. IUBMB Life, 66(12), 835–846 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol, 92(6), 543–552 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell, 124(2), 263–266 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta, 1796(1), 11–18 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer, 9(4), 239–252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raul Casso GM. Role of tumor associated macrophages in regulating pancreatic cancer progression. World Journal of Immunology, (2016). [Google Scholar]
- 53**.Ozdemir BC, Pentcheva-Hoang T, Carstens JL et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell, 25(6), 719–734 (2014).Article underscores the intricate role of CAFs in the TME and that their complete depletion from the tumors results in unfavorable outcomes.
- 54.Elahi-Gedwillo KY, Carlson M, Zettervall J, Provenzano PP. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res, 79(2), 372–386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han S, Latchoumanin O, Wu G et al. Recent clinical trials utilizing chimeric antigen receptor T cells therapies against solid tumors. Cancer Lett, 390, 188–200 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Einama T, Kamachi H, Nishihara H et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas, 40(8), 1276–1282 (2011). [DOI] [PubMed] [Google Scholar]
- 57*.Beatty GL, Haas AR, Maus MV et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res, 2(2), 112–120 (2014).Article shows that mesothelin is an effective target for generting CAR-T cells.
- 58**.Beatty GL, O’Hara MH, Lacey SF et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology, 155(1), 29–32 (2018).Article highlights the ability of CAR-TMeso cells to penetrate into metastatic tumor sites of pancreatic cancer patients.
- 59.Akce M, Zaidi MY, Waller EK, El-Rayes BF, Lesinski GB. The Potential of CAR T Cell Therapy in Pancreatic Cancer. Front Immunol, 9, 2166(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aglietta M, Barone C, Sawyer MB et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol, 25(9), 1750–1755 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Lu C, Paschall AV, Shi H et al. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J Natl Cancer Inst, 109(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutz ER, Wu AA, Bigelow E et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res, 2(7), 616–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le DT, Lutz E, Uram JN et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother, 36(7), 382–389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H, Hegde S, Knolhoff BL et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med, 22(8), 851–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther, 17(2), 175–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother, 62(5), 949–954 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mach F, Schonbeck U, Sukhova GK et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A, 94(5), 1931–1936 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature, 393(6684), 480–483 (1998). [DOI] [PubMed] [Google Scholar]
- 69**.Byrne KT, Vonderheide RH. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep, 15(12), 2719–2732 (2016).Article shows that CD40 agonist treatment in pancreatic cancer leads to recruuitment of T cells into the tumors making them immunogenic.
- 70.Ebert PJR, Cheung J, Yang Y et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity, 44(3), 609–621 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Azenshtein E, Luboshits G, Shina S et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res, 62(4), 1093–1102 (2002). [PubMed] [Google Scholar]
- 72.Monti P, Leone BE, Marchesi F et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res, 63(21), 7451–7461 (2003). [PubMed] [Google Scholar]
- 73.Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol, 227(1), 17–28 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Uutela M, Wirzenius M, Paavonen K et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood, 104(10), 3198–3204 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol, 37(7), 8657–8664 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Hagemann T, Lawrence T, McNeish I et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med, 205(6), 1261–1268 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kratochvill F, Neale G, Haverkamp JM et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep, 12(11), 1902–1914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui R, Yue W, Lattime EC, Stein MN, Xu Q, Tan XL. Targeting tumor-associated macrophages to combat pancreatic cancer. Oncotarget, 7(31), 50735–50754 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol, 31(6), 212–219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagaraj S, Youn JI, Weber H et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res, 16(6), 1812–1823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu Y, Knolhoff BL, Meyer MA et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res, 74(18), 5057–5069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother, 62(2), 225–236 (2013).Article shows that JAK-STAT inhihibitor Rosiglitazone results in reduces accumulation of MDSCs in pancreatic tumors leading to extended overall survival.
- 83.Huang HL, Chao MW, Chen CC et al. LTP-1, a novel antimitotic agent and Stat3 inhibitor, inhibits human pancreatic carcinomas in vitro and in vivo. Sci Rep, 6, 27794(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tai LH, Alkayyal AA, Leslie AL et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology, 7(6), e1431082(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodriguez PC, Hernandez CP, Quiceno D et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med, 202(7), 931–939 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res, 11(18), 6713–6721 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Gurlevik E, Fleischmann-Mundt B, Brooks J et al. Administration of Gemcitabine After Pancreatic Tumor Resection in Mice Induces an Antitumor Immune Response Mediated by Natural Killer Cells. Gastroenterology, 151(2), 338–350 e337 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Weniger M, Honselmann KC, Liss AS. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers (Basel), 10(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Topalovski M, Brekken RA. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett, 381(1), 252–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knapinska AM, Estrada CA, Fields GB. The Roles of Matrix Metalloproteinases in Pancreatic Cancer. Prog Mol Biol Transl Sci, 148, 339–354 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer, 87(2), 161–167 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang K, Chen D, Jiao X et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest, 91(3), 426–438 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Moore MJ, Hamm J, Dancey J et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12–9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol, 21(17), 3296–3302 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Li L, Li H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol Ther, 14(9), 796–805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coons LB, L’Amoreaux WJ, Rosell-Davis R, Starr-Spires L. Fine structure of the fat body and nephrocytes in the life-stages of Dermacentor variabilis. Exp Appl Acarol, 8(1–2), 125–142 (1990). [DOI] [PubMed] [Google Scholar]
- 96.Giovannetti E, Funel N, Peters GJ et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res, 70(11), 4528–4538 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Xu Q, Li P, Chen X et al. miR-22½22 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget, 6(16), 14153–14164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol, 18(4), 244–250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21(3), 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacobetz MA, Chan DS, Neesse A et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut, 62(1), 112–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson CB, Shepard HM, O’Connor PM et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther, 9(11), 3052–3064 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Baumgartner G, Gomar-Hoss C, Sakr L, Ulsperger E, Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors--experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett, 131(1), 85–99 (1998). [PubMed] [Google Scholar]
- 103**.Hingorani SR, Harris WP, Beck JT et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res, 22(12), 2848–2854 (2016).Article shows that PEGPH20 treatment along with Gemcitabine provides therapeutic benefit to pancreatic cancer patients with high hyaluronic acid expression.
- 104.Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: Challenges and opportunities. Gastroenterology, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng M, Chen JY, Weissman-Tsukamoto R et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A, 112(7), 2145–2150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]