Abstract
Purpose:
Hyposecretion of aqueous humor has been postulated to adversely affect trabecular meshwork health and outflow resistance. However, the effect of medications that reduce aqueous humor production on outflow facility in living human eyes is unclear. In this study we evaluated the effect of timolol, an aqueous humor flow suppressant, on outflow facility in healthy eyes.
Design:
Prospective, before-and-after study.
Methods:
In a multicenter study, 113 healthy participants over age 40 years were included. Intraocular pressure (IOP) was measured in the sitting position by using a pneumatonometer and outflow facility was measured in the supine position by 2-minute pneumatonography. After one week of self-administering timolol 0.05% twice daily in each eye, both measurements were repeated.
Results:
Mean IOP decreased from 15.1 ± 3.0 mmHg at baseline to 12.4 ± 2.4 mmHg (P < 0.001) after one week of timolol use. Mean outflow facility decreased from 0.23 ± 0.08 μL/min/mmHg at baseline to 0.18 ± 0.08 μL/min/mmHg (P < 0.001) after timolol. The change in outflow facility was negatively correlated with baseline outflow facility (r = −0.51, P < 0.001).
Conclusion:
Timolol reduces outflow facility in healthy human eyes, and this effect is greater in eyes with higher baseline outflow facility. This phenomenon may be related to reduced aqueous humor flow, but the precise mechanism remains to be determined.
Introduction
Glaucoma is a progressive optic neuropathy and is the second most common cause of blindness in the world, with over 2.5 million people affected in the United States alone.1 Elevated intraocular pressure (IOP) is the primary risk factor in glaucoma, and lowering IOP is currently the only effective treatment. IOP can be lowered in several ways including reduction of aqueous humor production, or improvement of aqueous humor outflow facility.
Timolol is a nonselective beta-adrenergic antagonist2 that has been widely used for glaucoma treatment since the late 1970s. It lowers IOP in healthy volunteers3–5 and in patients with open-angle glaucoma6–15 or ocular hypertension12, 14, 15 by suppressing aqueous humor production 33%−50% as measured by fluorophotometry.4, 5, 16–21
The effect of aqueous humor flow suppression on other parameters of aqueous humor dynamics, including aqueous humor outflow facility is not well understood. Previous investigators have suggested that suppression of aqueous production could cause underperfusion of the trabecular meshwork, leading to a reduction in outflow facility.22, 23 However, several studies in patients with ocular hypertension or glaucoma have reported that timolol had no significant effect on outflow facility measured by tonography11–13, 19, 24–30 or fluorophotometry,30 while some have even reported an increase in outflow facility20, 31, 32. In these previous studies, all outflow facilities were measured in patients with dysfunctional trabecular meshwork, as indicated by low outflow facility at baseline. The effect of aqueous humor suppression on outflow facility in healthy human eyes (with healthy trabecular meshwork) has not been reported. In this study, we evaluated the effect of timolol on outflow facility in healthy human subjects.
Methods
This prospective before-and-after study was approved by the Institutional Review Boards at Mayo Clinic, University of Michigan, and University of Nebraska Medical Center. The study followed the tenets of the Declaration of Helsinki and was in accordance with HIPAA regulations. All subjects provided written informed consent to participate after discussion of the nature and possible risks of the study.
Study Subjects
One hundred-thirteen healthy participants, ages 40 years and above were enrolled from local area residents, employees, and patients of the ophthalmology departments at Mayo Clinic Rochester, University of Michigan, and University of Nebraska as part of a multi-center study (NCT01677507). Each participant underwent a general health interview and comprehensive ophthalmologic examination including visual acuity, IOP by pneumatonometry, gonioscopy, slit-lamp biomicroscopy, A-scan biometry, ultrasound pachymetry and fundoscopy. Subjects were excluded if they had a history or evidence of intraocular surgery, laser treatment, ocular pathology (including narrow angles or any form of glaucoma), ocular trauma within the past 6 months, current use of any IOP-lowering medication, serious hypersensitivity to any components of the study medications, recent changes to systemic medications that may affect IOP, use of any glucocorticoid by any route, women who were known to be pregnant, and any contraindication for treatment with the study glaucoma medications including severe reactive airways disease and bradycardia.
Measurements
Intraocular Pressure (IOP)
Baseline IOP was measured in both eyes of each subject in the sitting position by using a pneumatonometer (Model 30 Classic, Reichert Inc., Buffalo, NY). The subject was then placed in a supine position and IOP was re-measured after 5 minutes. All IOP measurements were between 9 and 11 AM. Calibration of the tonometer was verified according to the manufacturer’s instruction and the tip was cleaned before each set of measurements. Topical proparacaine 0.5% was instilled before each IOP measurement and tonography. The right eye was always measured first.
Outflow Facility by Pneumatonography
Outflow facility was measured with the subject in the supine position by using a pneumatonometer with a 2-minute tonography option. Outflow facility was calculated from the pressure decay curves digitized from paper tracing and using Langham’s pressure volume relationship tables and a polynomial fitted to the decay curve, as described previously.33, 34 The right eye was always measured before the left eye.
Axial Length and Central Corneal Thickness
Axial length (AL) was measured by A-scan biometry and central corneal thickness (CCT) was measured by ultrasound pachymetry.
Study Medications
Intraocular pressure and outflow facility were measured at baseline prior to any medication use. Subjects were then instructed to instill timolol 0.5% solution, one drop every 12 hours in both eyes. The drug was self-administered for one week. After one week of treatment, IOP and outflow facility were remeasured by using the same techniques as used for the baseline measurements.
Statistical analysis
Significance of changes in IOP and outflow facility from baseline in response to timolol were determined by using generalized estimating equation models to account for possible correlation between fellow eyes of the same subject (R Core Team 2018, RStudio version 1.1.456, GEE version 4.13–19, Vienna, Austria). Correlation between baseline outflow facility and changes in outflow facility after timolol treatment was determined by linear regression analysis. Differences were considered significant if P was less than 0.05.
Results
One hundred-thirteen healthy participants (27 males and 86 females; 98 Caucasians, 11 African Americans, and 4 other races), ages 40 to 81 years (55.3 ± 8.9 years, mean ± SD) were included in the study (Table 1). Among these subjects, 200 eyes from 104 subjects had satisfactory 2-minute IOP recordings that were suitable for calculation of tonographic outflow facility. The other 26 eyes did not have outflow facility tracings of adequate quality for outflow facility calculation.
Table 1.
Subject Characteristics
| Characteristic | |
|---|---|
| Men | 27 |
| Women | 86 |
| Age (yrs) | |
| Mean ± SD | 55.3 ± 8.9 |
| Range | 40 – 81 |
| Race | |
| White | 98 |
| Black | 11 |
| Other | 4 |
| CCT (μm) | 551 ± 39 |
| Axial Length (mm) | 23.93 ± 1.23 |
SD = Standard deviation
CCT = Central corneal thickness
The mean IOP at baseline was 15.1 ± 3.0 mmHg (± SD, n = 200 eyes) and decreased to 12.4 ± 2.4 mmHg (P < 0.001) after 1 week of timolol therapy (Table 2, Figure 1). The mean outflow facility at baseline was 0.23 ± 0.08 μL/min/mmHg, and decreased after one week of timolol treatment to 0.18 ± 0.08 μL/min/mmHg (P < 0.001) (Figure 2), a mean change of 24.5%. In a subgroup analysis, the mean outflow facility decreased after one week of timolol treatment compared to baseline at each study site: from 0.22 ± 0.08 to 0.16 ± 0.07 μL/min/mmHg at the University of Michigan (P < 0.001); from 0.23 ± 0.07 to 0.20 ± 0.07 μL/min/mmHg at the Mayo Clinic (P = 0.01); and from 0.24 ± 0.08 to 0.17 ± 0.09 μL/min/mmHg at the University of Nebraska (P < 0.001).
Table 2.
Effect of Timolol on Intraocular Pressure and Outflow Facility
| Baseline | Timolol | P | |
|---|---|---|---|
| mean ± SD | mean ± SD | ||
| IOP (mmHg) | 15.1 ± 3.0 | 12.4 ± 2.4 | < 0.001 |
| Outflow Facility (μL/min/mmHg) | 0.23 ± 0.08 | 0.18 ± 0.08 | <0.001 |
Both IOP and outflow facility decreased significantly after 1 week of treatment with timolol in healthy human eyes.
IOP = Intraocular pressure
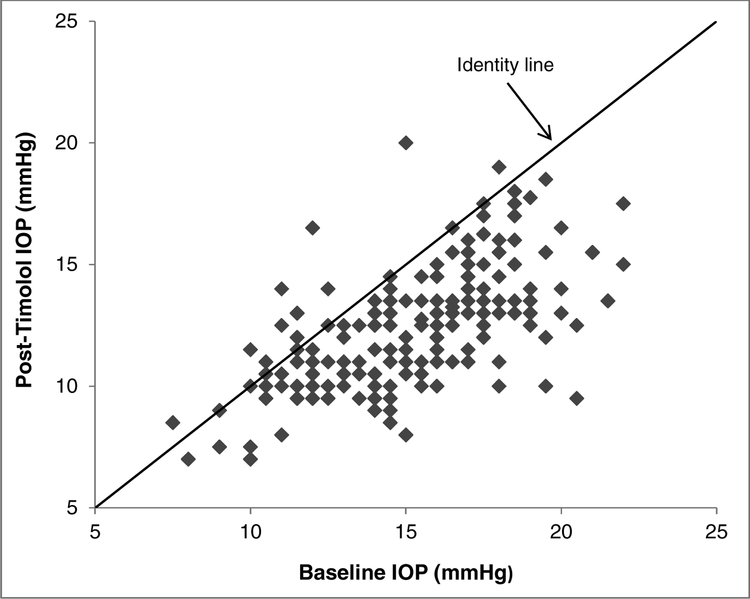
Figure 1.
Comparison of Post-timolol Intraocular Pressure (IOP) with Baseline IOP. IOP decreased from 15.1 ± 3.0 mmHg at baseline to 12.4 ± 2.4 mmHg after 1 week of timolol treatment (P < 0.001). Points below the identity line had a reduction of IOP after timolol treatment.
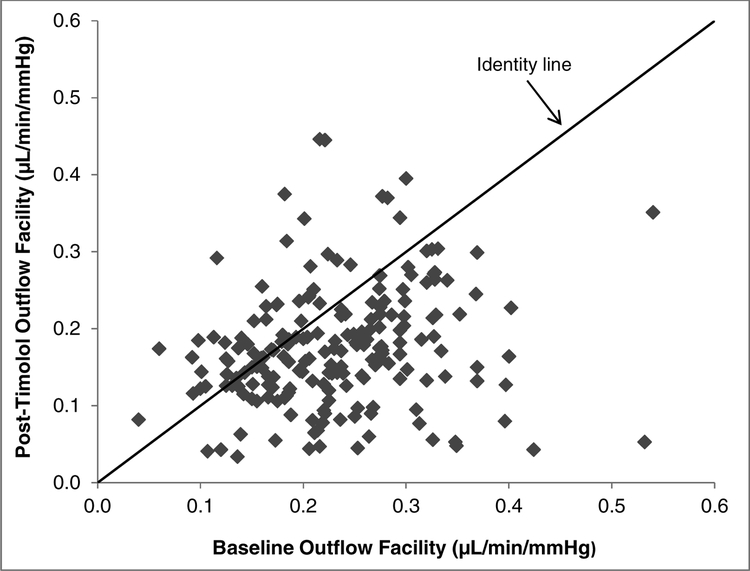
Figure 2.
Comparison of Post-timolol Outflow Facility with Baseline Outflow Facility. Outflow facility decreased from 0.23 ± 0.08 μL/min/mmHg at baseline to 0.18 ± 0.08 μL/min/mmHg after 1 week of timolol treatment (P < 0.001). Points below the identity line had a reduction of outflow facility after timolol treatment.
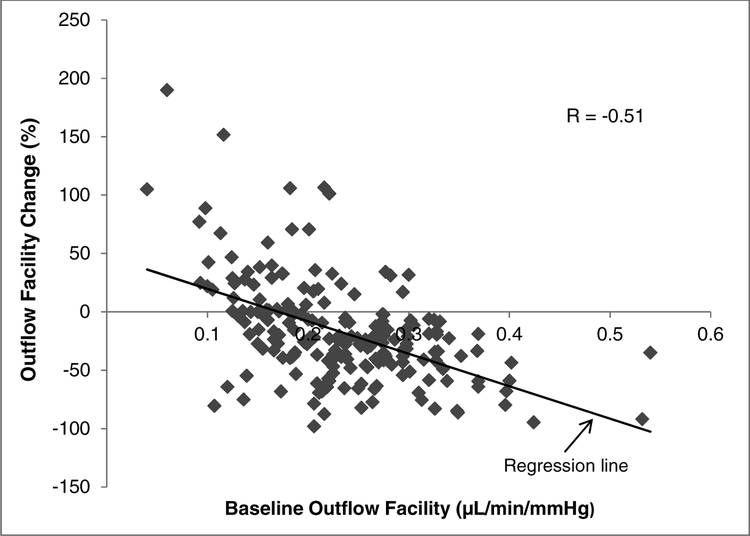
There was a moderate strength negative correlation between baseline outflow facility and the change in outflow facility after timolol treatment (r = −0.51, P < 0.001), with higher baseline outflow facility being associated with a greater decrease in outflow facility (Figure 3). Outflow facility decreased in 145 eyes (73%), from a mean baseline outflow facility of 0.26 ± 0.07 μL/min/mmHg to 0.16 ± 0.07 μL/min/mmHg after timolol treatment. In 53 eyes (26%), outflow facility increased from a mean at baseline of 0.17 ± 0.06 μL/min/mmHg to 0.22 ± 0.09 μL/min/mmHg after timolol treatment. Two eyes (1%) did not show any change in outflow facility.
Figure 3.
Correlation of Outflow Facility Change with Baseline Outflow Facility. Higher baseline outflow facility was associated with greater decrease in outflow facility after timolol treatment (P < 0.001).
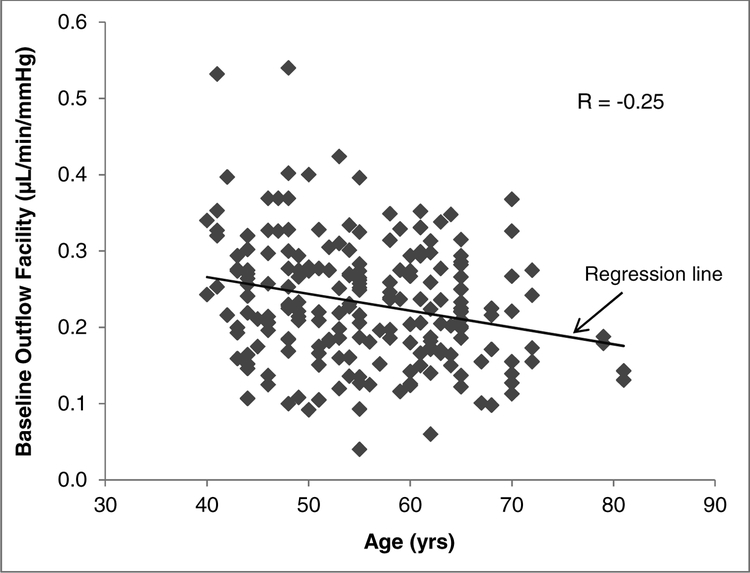
Baseline outflow facility was weakly correlated with age in our study population (r = −0.25, P < 0.001). Older subjects were associated with lower outflow facilities (Figure 4). Baseline outflow facility was not correlated with either central corneal thickness (r = 0.02, P = 0.78) or axial length (r = 0.07, P = 0.28). Also, there were no significant differences in baseline IOP and outflow facility between men and women (P = 0.29 for IOP and P = 0.07 for outflow facility).
Figure 4.
Correlation of Baseline Outflow Facility with Age. Older subjects were associated with lower outflow facilities (P < 0.001).
Discussion
Our study found a reduction in outflow facility after 1 week of treatment with timolol eye drops in healthy subjects. We are unaware of previous reports of this finding and could find no reference to it in a search in PubMed. Suppression of aqueous humor production is the main mechanism of action for IOP reduction by beta-adrenergic blockers, such as timolol, but a decrease in outflow facility potentially limits this effect. However, the effect of timolol on aqueous humor outflow facility reported in the literature is variable. While one animal study in cynomolgus monkeys suggested that timolol may lead to a reduction in outflow facility,22 studies in living humans have reported no change or even an increase in outflow facility in patients with ocular hypertension or open angle glaucoma.11–13, 19, 20, 24–32 An important distinction, though, is that none of these studies were performed in healthy human eyes.
One possible mechanism for alteration of outflow facility by timolol in our study population may be the blockade of beta-receptors in the trabecular outflow pathway. Thomas and Epstein31 reported a significant increase in outflow facility after two weeks of treatment with epinephrine, a beta-adrenergic agonist, in 16 glaucoma patients. However, after two weeks of combined therapy with timolol and epinephrine, a significant decrease in outflow facility was observed. They postulated that beta-adrenergic receptors mediating outflow may be located in the trabecular meshwork and stimulating them produces an increase in outflow facility, and this effect is blocked by timolol. However, they also observed that blocking the beta-receptors with timolol alone did not decrease outflow facility in glaucoma patients. Similarly, Robinson and Kaufman35 reported that pretreatment with timolol prevented the facility-increasing effect of topical epinephrine and norepinephrine in normotensive cynomolgus monkey eyes. They concluded that the facility-increasing effect of both epinephrine and norepinephrine is mediated by beta-2-adrenergic receptors in the trabecular meshwork endothelium. However, a single treatment with timolol alone had no effect on outflow facility in cynomolgus monkeys. This suggests that the decrease in facility after 1 week of treatment in our study population may not be from an immediate effect of timolol on beta-adrenergic receptors, but a slower onset mechanism of action related to aqueous humor flow suppression.
Hyposecretion of aqueous humor has been suggested as potentially damaging to the trabecular meshwork, leading to a reduction of outflow facility.23 Becker, in an unpublished observation of the effect of oral acetazolamide, found that healthy human subjects had an initial rapid decrease in IOP, but demonstrated a return toward baseline IOP after several weeks of treatment, and this was associated with a reduction in tonographic outflow facility.23 Kiland et al.22 in a study of healthy cynomolgus monkeys reported a trend towards a decrease in outflow facility after four weeks of treatment with timolol and dorzolamide, but this did not reach statistical significance. However, after four weeks of treatment with timolol + dorzolamide + prostaglandin, outflow facility was significantly reduced compared to baseline. They suggested that long-term use of medications that suppress aqueous humor formation, such as timolol may cause underperfusion of the trabecular meshwork, reducing the concentration of oxygen and nutrients reaching the trabecular meshwork, possibly leading to changes that decrease outflow facility, and this effect may be exacerbated by medications that redirect aqueous humor outflow away from the trabecular meshwork, such as prostaglandin analogues. Supporting these findings, Lutjen-Drecoll and Kaufman36 demonstrated by electron microscopy marked histological changes in the trabecular meshwork of healthy cynomolgus monkeys after 3–7 months of topical timolol given twice daily. Further, using a human anterior segment organ culture system, Johnson37 demonstrated that perfusion rates of less than 1 μL/min over 7–21 days resulted in marked trabecular cell loss. This finding suggests that pathological changes in the trabecular meshwork as a result of severe underperfusion may result in reduction of outflow facility. However, timolol eye drops alone may not reduce aqueous humor flow rate enough to induce pathologic changes. Of note, the 1 μL/min flow rate threshold identified by Johnson is lower than the nocturnal flow rate in humans, when aqueous humor production decreases by approximately 50%38 and lower than the flow rate typically achieved with timolol eye drops.4, 5, 16–21
Another possible explanation for the reduction in outflow facility by timolol is that changes in outflow facility in response to decreased aqueous humor flow are not pathologic, but are instead pressure regulatory mechanisms. Acott et al.39 hypothesized and reviewed the evidence that resistance within the conventional outflow pathway is continually adjusted in response to cell stretch from sustained IOP changes. The proposed mechanism involves extracellular matrix turnover, requiring hours to days, which would be consistent with the findings in our study. This mechanism also may explain why a reduction in outflow facility was detected in our study of healthy subjects, but not previous studies of glaucoma patients with presumably dysfunctional trabecular meshwork that may not be able to respond to pressure changes. Further potential evidence for a compensatory change in outflow facility in response to aqueous humor flow reduction comes from studies of circadian aqueous humor dynamics.40–42 In healthy subjects, the reduction of aqueous humor production at night is partially compensated by a reduction in outflow facility, resulting in a relatively stable circadian IOP when measured in a constant body position. However, it is not known if the change in outflow facility is a response to the reduction in flow rate, or a concurrent change that occurs due to another mechanism.
A reduction in outflow facility with timolol treatment also may help explain the frequently observed phenomenon of tachyphylaxis. Previous studies have reported that some patients using timolol exhibited a reduction in efficacy over a few days of therapy. This clinically observed phenomenon has been termed “short-term escape”.6, 7, 43, 44 One possible explanation for this phenomenon is the finding in a study in albino New Zealand rabbits that the number of beta-adrenergic receptors in ocular tissues increases within days of beginning timolol administration.45 Neufeld demonstrated that the number of beta receptors in ocular tissues increases with continued timolol therapy. The time course of this alteration of receptor numbers is a matter of days and would seem to correlate with the short-term escape.45 As well, Johnson et al.30 found that the degree of aqueous humor suppression decreased after six weeks of timolol treatment compared to one week of treatment. However, increase in the number of beta receptors does not explain the decrease in outflow facility with timolol. Instead, these may be concurrent effects that both reduce efficacy in some patients.
Our study found that eyes with higher baseline outflow facility had a larger decrease in outflow facility after timolol treatment. This is consistent with the notion that healthy trabecular meshwork cells are required to produce a decreased outflow facility as a compensatory response to decreased aqueous flow rate or intraocular pressure, and this phenomenon is not a pathologic effect of hypoperfusion. Although none of our subjects had glaucoma, it is certainly possible that the spectrum of baseline outflow facilities represents a spectrum of trabecular meshwork health. Interestingly, 26% of eyes demonstrated an increase in outflow facility after treatment. These were generally eyes with lower baseline outflow facility, potentially indicating subclinical trabecular meshwork dysfunction. This is consistent with studies that demonstrated an increase in outflow facility in patients with glaucoma and ocular hypertension treated with timolol.20, 31, 32 However, it is unknown what changes at the cellular level or extracellular matrix may occur that decrease flow resistance in eyes with low baseline outflow facility. It is also possible that this relationship is the result of regression to the mean, in which a group of eyes may have had outflow facility measurements lower than their normal, and the increase in outflow facility after treatment in this group was a return to baseline. Further research is required to clarify the association between baseline outflow facility and outflow facility change, and the inclusion of a placebo control arm would be helpful.
Our study also found that increasing age was associated with lower baseline outflow facility in our subjects. This is consistent with the findings of most previous studies that have reported age-related decreases in tonographic outflow facility in human eyes.46–50 However, this has not been a universal finding, as several studies reporting tonographic outflow facility in humans did not find a significant relationship between age and outflow facility.51–53 Two other studies reported trends suggesting lower outflow facility in older subjects, but did not reach statistical significance.54, 55 These apparently contradictory results may be a reflection of the inherent variability in tonography. In contrast, total outflow facility measured by 2-level constant pressure perfusion of the anterior chamber in rhesus monkeys consistently demonstrates a decrease in outflow facility with age.56–60 It is worth noting that, while our study found a statistically significant relationship between age and baseline outflow facility, the strength of the relationship was weak in our subjects.
Compared with previous studies investigating the effect of timolol on outflow facility, our study has several important advantages. Our study was a multi-center design with a standardized protocol. This design provided a much larger sample size than all previous studies, and thus was appropriately powered to detect this effect. One potential limitation of the study was that the measurements were performed by different people in different centers and this may have resulted in inter-observer variabilities. However, a subgroup analysis indicated that timolol treatment reduced outflow facility in the study populations at each site, suggesting that the measurements were consistent at each site and the results are generalizable to different healthy populations.
In summary, our study found that timolol reduced aqueous humor outflow facility in eyes with healthy trabecular meshwork (as indicated by normal baseline outflow facility) after one week of treatment. Eyes with higher baseline outflow facility showed a greater decrease in outflow facility. Compensatory physiologic changes in the trabecular meshwork in response to reduced aqueous flow rate and intraocular pressure may provide an explanation for the decreased outflow facility in healthy eyes. However, the precise mechanism remains to be determined. The reduction in outflow facility may partially negate the overall IOP lowering effect of timolol.
Supplementary Material
Timolol decreased outflow facility in healthy human eyes.
This effect was greater in eyes with higher baseline outflow facility.
Older age was associated with lower outflow facility.
Acknowledgements/Disclosure
Funding/Support: This study was supported by National Institutes of Health (Grant # EY022124) (SEM), National Institutes of Health (Grant # EY007003, University of Michigan Core Grant), and unrestricted departmental grants from Research to Prevent Blindness, New York, New York (Mayo Clinic and University of Michigan) (AJS, SEM). Dr. Gulati has received funding from National Eye Institute (Grant # K23EY023266). The funding organizations had no role in study design; collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The authors alone are responsible for the content and writing of the paper.
Financial Disclosures: Arthur J. Sit: Aerie Pharmaceuticals, Inc. (Consultant, research support), Allergan, Inc. (Consultant), Injectsense, Inc. (Consultant), PolyActiva, Pty (Consultant). Carol B. Toris: Nicox (Research funding), Ivantis (Research funding). Sayoko E. Moroi: Aerie Pharmaceuticals, Inc. (Grant support), Allergan (Grant support), Bausch & Lomb (Consultant), Icare USA (Grant support), Wolters Kluwer Health (Royalty), National Science Foundation (Grant # AWD010114). The following authors have no financial disclosures: Arash Kazemi, Jay W. McLaren, Matthew G. Trese, Vikas Gulati, Shan Fan, David M. Reed, Tyler Kristoff, and Jesse Gilbert. All authors attest that they meet the current ICMJE criteria for authorship.
Other acknowledgements: Diana K. Burnett (University of Michigan) contributed to the data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci 1997;38(1):83–91. [PubMed] [Google Scholar]
- 2.Scriabine A, Torchiana ML, Stavorski JM, Ludden CT, Minsker DH, Stone CA. Some cardiovascular effects of timolol a new beta-adrenergic blocking agent. Arch Int Pharmacodyn Ther 1973;205(1):76–93. [PubMed] [Google Scholar]
- 3.Katz IM, Hubbard WA, Getson AJ, Gould AL. Intraocular pressure decrease in normal volunteers following timolol ophthalmic solution. Invest Ophthalmol 1976;15(6):489–92. [PubMed] [Google Scholar]
- 4.Dailey RA, Brubaker RF, Bourne WM. The effects of timolol maleate and acetazolamide on the rate of aqueous formation in normal human subjects. Am J Ophthalmol 1982;93(2):232–7. [DOI] [PubMed] [Google Scholar]
- 5.Wayman L, Larsson LI, Maus T, Alm A, Brubaker R. Comparison of dorzolamide and timolol as suppressors of aqueous humor flow in humans. Arch Ophthalmol 1997;115(11):1368–71. [DOI] [PubMed] [Google Scholar]
- 6.Boger WP 3rd Puliafito CA, Steinert RF, Langston DP. Long-term experience with timolol ophthalmic solution in patients with open-angle glaucoma. Ophthalmology 1978;85(3):259–67. [DOI] [PubMed] [Google Scholar]
- 7.Boger WP 3rd Steinert RF, Puliafito CA, Pavan-Langston D. Clinical trial comparing timolol ophthalmic solution to pilocarpine in open-angle glaucoma. Am J Ophthalmol 1978;86(1):8–18. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman TJ. Timolol maleate--a new glaucoma medication? Invest Ophthalmol Vis Sci 1977;16(8):687–8. [PubMed] [Google Scholar]
- 9.Zimmerman TJ, Kaufman HE. Timolol, dose response and duration of action. Arch Ophthalmol 1977;95(4):605–7. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman TJ, Kaufman HE. Timolol. A beta-adrenergic blocking agent for the treatment of glaucoma. Arch Ophthalmol 1977;95(4):601–4. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman TJ, Harbin R, Pett M, Kaufman HE. Timolol and facility of outflow. Invest Ophthalmol Vis Sci 1977;16(7):623–4. [PubMed] [Google Scholar]
- 12.Zimmerman TJ, Kass MA, Yablonski ME, Becker B. Timolol maleate: efficacy and safety. Arch Ophthalmol 1979;97(4):656–8. [DOI] [PubMed] [Google Scholar]
- 13.Cyrlin MN, Thomas JV, Epstein DL. Additive effect of epinephrine to timolol therapy in primary open angle glaucoma. Arch Ophthalmol 1982;100(3):414–8. [DOI] [PubMed] [Google Scholar]
- 14.Camras CB. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology 1996;103(1):138–47. [DOI] [PubMed] [Google Scholar]
- 15.Watson P, Stjernschantz J. A six-month, randomized, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. The Latanoprost Study Group. Ophthalmology 1996;103(1):126–37. [DOI] [PubMed] [Google Scholar]
- 16.Yablonski ME, Zimmerman TJ, Waltman SR, Becker B. A fluorophotometric study of the effect of topical timolol on aqueous humor dynamics. Exp Eye Res 1978;27(2):135–42. [DOI] [PubMed] [Google Scholar]
- 17.Brubaker RF, Nagataki S, Bourne WM. Effect of chronically administered timolol on aqueous humor flow in patients with glaucoma. Ophthalmology 1982;89(3):280–3. [DOI] [PubMed] [Google Scholar]
- 18.Coakes RL, Brubaker RF. The mechanism of timolol in lowering intraocular pressure. In the normal eye. Arch Ophthalmol 1978;96(11):2045–8. [DOI] [PubMed] [Google Scholar]
- 19.Schenker HI, Yablonski ME, Podos SM, Linder L. Fluorophotometric study of epinephrine and timolol in human subjects. Arch Ophthalmol 1981;99(7):1212–6. [DOI] [PubMed] [Google Scholar]
- 20.Lin LL, Galin MA, Obstbaum SA, Katz I. Longterm timolol therapy. Surv Ophthalmol 1979;23(6):377–80. [DOI] [PubMed] [Google Scholar]
- 21.Larsson LI. Aqueous humor flow in normal human eyes treated with brimonidine and timolol, alone and in combination. Arch Ophthalmol 2001;119(4):492–5. [DOI] [PubMed] [Google Scholar]
- 22.Kiland JA, Gabelt BT, Kaufman PL. Studies on the mechanism of action of timolol and on the effects of suppression and redirection of aqueous flow on outflow facility. Exp Eye Res 2004;78(3):639–51. [DOI] [PubMed] [Google Scholar]
- 23.Does hyposecretion of aqueous humor damage the trabecular meshwork? J Glaucoma 1995;4(5):303–5. [PubMed] [Google Scholar]
- 24.Kass MA, Korey M, Gordon M, Becker B. Timolol and acetazolamide. A study of concurrent administration. Arch Ophthalmol 1982;100(6):941–2. [DOI] [PubMed] [Google Scholar]
- 25.Moss AP, Ritch R, Hargett NA, Kohn AN, Smith H Jr. Podos SM. A comparison of the effects of timolol and epinephrine on intraocular pressure. Am J Ophthalmol 1978;86(4):489–95. [DOI] [PubMed] [Google Scholar]
- 26.Sonntag JR, Brindley GO, Shields MB. Effect of timolol therapy on outflow facility. Invest Ophthalmol Vis Sci 1978;17(3):293–6. [PubMed] [Google Scholar]
- 27.Berson FG, Epstein DL. Separate and combined effects of timolol maleate and acetazolamide in open-angle glaucoma. Am J Ophthalmol 1981;92(6):788–91. [DOI] [PubMed] [Google Scholar]
- 28.Sonntag JR, Brindley GO, Shields MB, Arafat NI, Phelps CD. Timolol and epinephrine. Comparisin of efficacy and side effects. Arch Ophthalmol 1979;97(2):273–7. [DOI] [PubMed] [Google Scholar]
- 29.Alexander DW, Berson FG, Epstein DL. A clinical trial of timolol and epinephrine in the treatment of primary open-angle glaucoma. Ophthalmology 1988;95(2):247–51. [DOI] [PubMed] [Google Scholar]
- 30.Johnson TV, Fan S, Zhan G, Camras CB, Toris CB. Efficacy and mechanisms of intraocular pressure reduction with latanoprost and timolol in participants with ocular hypertension: a comparison of 1 and 6 weeks of treatment. J Glaucoma 2010;19(6):356–64. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JV, Epstein DL. Timolol and epinephrine in primary open angle glaucoma. Transient additive effect. Arch Ophthalmol 1981;99(1):91–5. [DOI] [PubMed] [Google Scholar]
- 32.Airaksinen PJ. The long-term hypotensive effect of timolol maleate compared with the effect of pilocarpine in simple and capsular glaucoma. Acta Ophthalmol (Copenh) 1979;57(3):425–34. [DOI] [PubMed] [Google Scholar]
- 33.Langham ME, Leydhecker W, Krieglstein G, Waller W. Pneumatonographic studies on normal and glaucomatus eyes. Adv Ophthalmol 1976;32:108–33. [PubMed] [Google Scholar]
- 34.Kazemi A, McLaren JW, Lin SC, et al. Comparison of Aqueous Outflow Facility Measurement by Pneumatonography and Digital Schiotz Tonography. Invest Ophthalmol Vis Sci 2017;58(1):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson JC, Kaufman PL. Effects and interactions of epinephrine, norepinephrine, timolol, and betaxolol on outflow facility in the cynomolgus monkey. Am J Ophthalmol 1990;109(2):189–94. [DOI] [PubMed] [Google Scholar]
- 36.Lutjen-Drecoll E, Kaufman PL. Long-term timolol and epinephrine in monkeys. II. Morphological alterations in trabecular meshwork and ciliary muscle. Trans Ophthalmol Soc U K 1986;105 (Pt 2):196–207. [PubMed] [Google Scholar]
- 37.Johnson DH. Human trabecular meshwork cell survival is dependent on perfusion rate. Invest Ophthalmol Vis Sci 1996;37(6):1204–8. [PubMed] [Google Scholar]
- 38.Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture]. Invest Ophthalmol Vis Sci 1991;32(13):3145–66. [PubMed] [Google Scholar]
- 39.Acott TS, Kelley MJ, Keller KE, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther 2014;30(2–3):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Fan S, Gulati V, et al. Aqueous humor dynamics during the day and night in healthy mature volunteers. Arch Ophthalmol 2011;129(3):269–75. [DOI] [PubMed] [Google Scholar]
- 41.Nau CB, Malihi M, McLaren JW, Hodge DO, Sit AJ. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci 2013;54(12):7623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sit AJ, Nau CB, McLaren JW, Johnson DH, Hodge D. Circadian variation of aqueous dynamics in young healthy adults. Invest Ophthalmol Vis Sci 2008;49(4):1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boger WP 3rd. Timolol: short term “escape” and long term “drift”. Ann Ophthalmol 1979;11(8):1239–42. [PubMed] [Google Scholar]
- 44.Boger WP 3rd Steinert RF, Thomas JV. Timolol in the therapy of “ocular hypertension”. Surv Ophthalmol 1980;25(3):195–202. [DOI] [PubMed] [Google Scholar]
- 45.Neufeld AH, Zawistowski KA, Page ED, Bromberg BB. Influences on the density of beta-adrenergic receptors in the cornea and iris--ciliary body of the rabbit. Invest Ophthalmol Vis Sci 1978;17(11):1069–75. [PubMed] [Google Scholar]
- 46.Becker B The decline in aqueous secretion and outflow facility with age. Am J Ophthalmol 1958;46(5 Part 1):731–6. [DOI] [PubMed] [Google Scholar]
- 47.Gaasterland D, Kupfer C, Ross K, Gabelnick HL. Studies of aqueous humor dynamics in man. 3. Measurements in young normal subjects using norepinephrine and isoproterenol. Invest Ophthalmol 1973;12(4):267–79. [PubMed] [Google Scholar]
- 48.Gaasterland D, Kupfer C, Milton R, Ross K, McCain L, MacLellan H. Studies of aqueous humour dynamics in man. VI. Effect of age upon parameters of intraocular pressure in normal human eyes. Exp Eye Res 1978;26(6):651–6. [DOI] [PubMed] [Google Scholar]
- 49.Croft MA, Oyen MJ, Gange SJ, Fisher MR, Kaufman PL. Aging effects on accommodation and outflow facility responses to pilocarpine in humans. Arch Ophthalmol 1996;114(5):586–92. [DOI] [PubMed] [Google Scholar]
- 50.Weekers R, Watillon M, De Rudder M. Experimental and clinical investigations into the resistance to outflow of aqueous humour in normal subjects. Br J Ophthalmol 1956;40(4):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant WM. Clinical measurements of aqueous outflow. AMA Arch Ophthalmol 1951;46(2):113–31. [DOI] [PubMed] [Google Scholar]
- 52.De Roetth A Jr. Knighton WS. Clinical evaluation of the aqueous-flow test; a preliminary report. AMA Arch Ophthalmol 1952;48(2):148–53. [DOI] [PubMed] [Google Scholar]
- 53.Boles-Carenini B, Cambiaggi A. Are aqueous humor dynamics influenced by aging. Am J Ophthalmol 1957;44(3):395–401. [DOI] [PubMed] [Google Scholar]
- 54.Spencer RW, Helmick ED, Scheie HG. Tonography; technical difficulties and control studies. AMA Arch Ophthalmol 1955;54(4):515–27. [PubMed] [Google Scholar]
- 55.Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol 1999;127(4):407–12. [DOI] [PubMed] [Google Scholar]
- 56.Gabelt BT, Crawford K, Kaufman PL. Outflow facility and its response to pilocarpine decline in aging rhesus monkeys. Arch Ophthalmol 1991;109(6):879–82. [DOI] [PubMed] [Google Scholar]
- 57.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res 2005;24(5):612–37. [DOI] [PubMed] [Google Scholar]
- 58.Kiland JA, Croft MA, Gabelt BT, Kaufman P. Atropine reduces but does not eliminate the age-related decline in perfusion outflow facility in monkeys. Exp Eye Res 1997;64(5):831–5. [DOI] [PubMed] [Google Scholar]
- 59.Kiland JA, Gabelt BT, Kaufman PL. Effect of age on outflow resistance washout during anterior chamber perfusion in rhesus and cynomolgus monkeys. Exp Eye Res 2005;81(6):724–30. [DOI] [PubMed] [Google Scholar]
- 60.Kiland JA, Gabelt BT, Kaufman PL. Relationship of aqueous outflow resistance to age and total volume perfused in rhesus and cynomolgus monkeys. Invest Ophthalmol Vis Sci 2011;52(9):6820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.