Abstract
Objective.
Previous studies have shown hydrogen sulfide (H2S) exerts potent proangiogenic properties under in vitro conditions and in rodent models. We sought to determine whether a novel H2S prodrug promotes peripheral revascularization in a swine model of acute limb ischemia (ALI).
Methods and Results.
ALI was induced in 17 female miniswine via intravascular occlusion of the external iliac. At day 7 post- ALI induction, miniswine (n = 17) were randomized to received placebo or the H2S pro-drug, SG-1002 (800 mg PO BID), for 35 days. At day 35 SG-1002 increased circulating levels of H2S (5.0 ± 1.2 μmol/L vs. 1.8 ± 0.50 μmol/L; p < 0.05), sulfane sulfur (10.6 ± 2.3 μmol/L vs. 2.6 ± 0.8 μmol/L; p < 0.05), and nitrite (0.5 ± 0.05 μmol/L vs. 0.3 ± 0.03 μmol/L; p < 0.005) compared to placebo. SG-1002 therapy increased angiographic scoring in ischemic limb vessel number (27.6 ± 1.6 vs. 22.2 ± 1.8; p < 0.05) compared to placebo. Treatment with SG-1002 preserved existing capillaries in ischemic limbs (128.3 ± 18.7 vs. 79.0 ± 9.8 capillaries/mm2; p < 0.05) compared to placebo. Interestingly, treatment with SG-1002 also improved coronary vasorelaxation responses to bradykinin and substance P in miniswine with ALI.
Conclusions.
Our results suggest that daily administration of the H2S prodrug, SG-1002, leads to an elevation in circulating H2S and NO signaling and preserves vessel number and density in ischemic limbs. Furthermore, SG-1002 therapy improved endothelial-dependent coronary artery vasorelaxation in the setting of ALI. Our data demonstrate that SG-1002 preserves the vascular architecture in ischemic limbs and exerts vascular protective effects in the coronary vasculature in a model of peripheral vascular disease.
Keywords: acute limb ischemia, peripheral artery disease, hydrogen sulfide, nitric oxide, swine
Here is the edited TOC summary: In this experimental study using a pig model of acute limb ischemia treatment with SG-1002 restored blood flow, enhanced circulating hydrogen sulfide and nitriate, and reduced oxidative stress and improved ex vivo coronary vascular function via endothelium-dependent vasodilation mechanism.
Introduction
Peripheral arterial disease (PAD) is a progressive, age-related disease affecting 6.5 million Americans over the age of 40 that significantly increases cardiovascular mortality and morbidity (1). PAD is caused by atherosclerosis resulting from obstructions in arteries limiting blood supply to organs other than the heart (2). In more severe manifestations, PAD can progress to critical limb ischemia (CLI); in which there is inadequate perfusion to meet resting tissue demands. CLI is a devastating disease affecting an estimated 500,000 to 1 million patients annually, and up to 10% of PAD patients older than 50 years will develop CLI within 5 years of diagnosis (2). Surgical and catheter-based revascularization are the preferred approaches for CLI although many patients with CLI are poor candidates for revascularization. Additionally, even after successful revascularization, stenosis or failure of the graft often reoccur resulting in a need for the development of new treatments for patients with CLI (3).
Furthermore, patients with large-vessel PAD are at higher risk of mortality from cardiovascular disease, specifically coronary heart disease (4-6). Patients with greater lower limb occlusion and lower ankle-brachial index are at an exceptionally high risk of death from cardiovascular etiology (7). Risk factors for PAD include smoking, obesity, hyperlipidemia, diabetes, and hypertension. In recent years, PAD has been categorized as a coronary heart disease equivalent meaning there is a >20% chance of a cardiovascular event in 10 years (8,9). Due to the severity of PAD, the high risk of coronary artery disease, and risk of amputation, there is a dire need for pharmacological therapies aimed at the restoration of peripheral blood flow and the reduction of related cardiovascular disease events.
Long thought to be a toxic gas, hydrogen sulfide (H2S) has recently become appreciated as a powerful, endogenous regulator of vascular homeostasis and cardiovascular health (10). H2S, a gaseous signaling molecule that is produced endogenously by 3 enzymes that include: cystathionine gamma lyase, cystathionine beta synthase, and 3-mercaptopyruvate sulfur transferase. The enzymes are constitutively active in a normal physiological state and produce low levels of H2S throughout the circulation and the body. H2S exerts vasodilatory and proangiogenic effects, has recently been shown to regulate angiogenesis and cytoprotective signaling via upregulation of endothelial nitric oxide synthase (10-12). SG-1002 is an α-sulfur H2S prodrug previously examined in animal models of heart failure (13) and in normal and heart failure patients (14, 15). These studies reveal cardioprotective actions of SG-1002 including reductions in myocardial fibrosis, increased myocardial vascularity, and increased nitric oxide (NO) bioavailability.
PAD patients experience profound vascular pathology that ultimately impacts vascular integrity and endothelial function from reduced NO bioavailability and deficient arteriolization with abnormal microvessel structure within the ischemic limb (16-18). Our laboratory has published data demonstrating significant reductions in circulating NO bioavailability in swine with CLI (19). Vessel reactivity is highly regulated by endothelial nitric oxide synthase (eNOS) derived NO and indirectly via H2S and that NO and H2S play a synergist role in maintenance of vascular tone (20-23). In the setting of CLI, endothelial dysfunction leads to eNOS uncoupling and decreased NO production. In order to reduce the cardiovascular risk associated with CLI, vascular integrity and function must be maintained and/or restored.
Our lab has previously shown decreased H2S bioavailability in the setting of CLI (24). Mouse and pig studies have shown pharmacological levels of H2S attenuate inflammation, apoptosis, and oxidative stress as well as decrease myocardial injury (13, 25, 26). Previous studies have demonstrated clear pro-angiogenic actions of H2S and H2S donors under in vitro conditions and rodent models of hindlimb ischemia (27-32).
Although NO functions through a separate pathway, NO shares many of the same cardioprotective roles as H2S. Our lab has previously reported on the cardioprotective actions of NO and crosstalk between NO and H2S in the cardiovascular system (33, 34). King et al demonstrated physiological concentrations of H2S elicit powerful cytoprotective actions mediated in part by NO signaling (12). Recently our lab has shown that sustained release NO therapy results in upregulation of vascular angiogenic factors within ischemic skeletal muscle and myocardium in a swine model of metabolic syndrome with CLI (19, 35). SG-1002 has been investigated in the setting of pressure-overload heart failure showing H2S therapy to be a promising treatment of cardiovascular complications secondary to diabetes (36). One of the most promising findings showed SG-1002 prevented the transition from compensated to decompensated heart failure via increase NO bioavailability (15).
We developed a severe model of limb ischemia via intravascular placement of an occluder via carotid artery access into external iliac arteries of swine (37). Using this method, external iliac occlusion is maintained leaving no possibility of collateralization and significant increases in blood flow in ischemic hindlimbs, as seen in surgical femoral artery ligation models (37). Based on the potent vasodilatory and proangiogenic effects of H2S, we sought to determine whether a novel H2S prodrug, SG-1002, protects limb vascular from ischemic injury and improves coronary vascular function in a swine model of limb ischemia.
Materials and Methods
Animals.
Female Yucatan miniswine (n = 17), 10-12 months of age, were obtained from S&S Farms (Ramona, CA). Females were used in an effort to limit variables (male vs. female) between pigs. Pigs were maintained on standard commercial diet (Teklad Miniswine Diet 8753, Harlan Laboratories) with free access to water. Animals were handled in compliance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and approval from the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee.
Hydrogen sulfide prodrug, SG-1002 treatment.
Seventeen miniswine were enrolled with eight pigs assigned to the Placebo group and nine pigs assigned to the SG-1002 group in a blinded and randomized fashion. Starting 7 days after ALI induction and continuing for 35 days, animals received Placebo or SG-1002 tablets (800 mg/tablet) orally twice a day (Figure 1A). SG-1002 (sodium polysulthionate; Sulfagenix, Cleveland, OH) is an α-sulfur orally active H2S prodrug that generates therapeutic levels of free H2S (14).
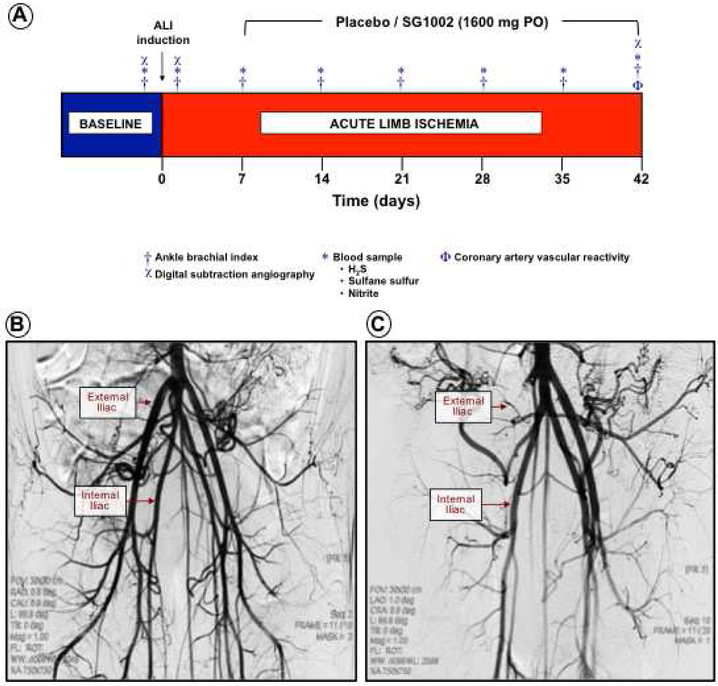
Figure 1. Large animal model of acute limb ischemia.
Pigs underwent intravascular induction of acute limb ischemia (ALI) and placebo or SG-1002 (1600 mg PO) administration starting 7 days post-ALI and continued for 35 days. Weekly ABI measurements were performed and blood samples obtained. At day 42 follow-up arteriograms were acquired, animals euthanized and nonischemic and ischemic limb tissues collected (Figure 1A). Under fluoroscopic guidance, contrast arteriography was performed to evaluate baseline vessel architecture (Figure 1B). ALI was generated by deployment of an AMPLATZER™ Vascular Plug II inside a GORE® VIABAHN® Endoprosthesis into the external iliac artery followed by an arteriogram to verify occlusion (Figure 1C).
Acute limb ischemia model.
Miniswine were subjected to acute limb ischemia (ALI) performed as previously described (19, 35, 37). Pigs were sedated with ketamine (15 mg/Kg) in combination with xylazine (1 mg/Kg) endotracheally intubated, mechanically ventilated, and anesthetized with isoflurane (1-3%) in oxygen. Preoperative analgesic buprenorphine (0.15 mg) and perioperative antibiotic cefazolin (1,000 mg) were administered. ECG, heart rate, respiration, oxygen saturation, blood pressure, and body temperature were monitored.
Under sterile conditions and fluoroscopic guidance, an 8-French introducer (Maximum Hemostasis Introducer 23-cm ACT Sheath, St. Jude Medical) was inserted into the right external carotid artery, advanced into the abdominal aorta, and placed just above the aortoiliac bifurcation. Heparin (300 U/Kg) was administered, and activated clotting time was monitored to ensure clotting times of > 250 sec. Contrast arteriography was performed to evaluate baseline vessel architecture (Figure 1B). A percutaneous guide wire (0.035 × 230 cm; Rosen Starter, Boston Scientific) was positioned in the right external iliac artery. A 7-French delivery guide catheter (Mach 1 MP1, Boston Scientific) containing a self-expanding endoluminal endoprosthesis consisting of an expanded polytetrafluoroethylene (ePTFE) lining with an external nitinol stent (6 mm × 15 cm; VIABAHN, W. L. Gore and Associates) advanced, positioned within the right external iliac artery, and deployed. The ePTFE-lined endoprosthesis served to inhibit artery recanalization by collateral vessels, typically observed in animal models of hindlimb ischemia. Following ePTFE-lined endoprosthesis placement, a self-expanding nitinol mesh occlusion device (8 × 7 mm; AMPLATZER Vascular Plug II, St. Jude Medical) was advanced, positioned, and deployed within the proximal portion of the ePTFE-lined stent. An arteriogram was performed to verify occlusion (Figure 1C). The guide wire, guide catheter, and introducer sheath were removed, and the left external carotid artery ligated. For blood sampling, an indwelling catheter was placed in the right jugular vein (7-10F Hickman, CR Bard). The incision was closed and Buprenex-SR (0.15 mg/Kg) was administered for analgesia.
Angiography.
Hindlimb vasculature was visualized using undiluted contrast (Oxilan 300, Guerbet). Arteriographs of the uninstrumented left and occluded right iliac arteries were obtained at baseline and day 42.
Ankle-brachial index.
Automated cuff blood pressure was used to measure limb blood pressure before ALI, immediately post- ALI, and day 7, 14, 21, 28, 35, and 42. Animals were sedated using isoflurane in oxygen by mask, pressure cuff secured to each limb, rapidly inflated to > 30 mmHg above the anticipated systolic pressure then deflated slowly for determination of systolic blood pressure in each of the forelimbs and hindlimbs. Ankle-brachial index (ABI) was calculated for each side of the animal by dividing systolic blood pressure measured at the hindlimb (ankle) by that measured at the contralateral hindlimb.
Euthanasia and tissue collection.
Animals were heparinized (300 U/Kg) then euthanized while under deep inhalant anesthesia. Gastrocnemius skeletal muscle tissue was collected from ischemic hindlimbs distal to the occlusion site and a corresponding region in the nonischemic limb. Hearts were excised and left anterior descending (LAD) and left circumflex (LCX) coronary arteries collected.
Quantitative angiographic score.
Collateral vessel growth in ischemic and nonischemic limbs was assessed using grid overlays of 2-mm squares as previously described (35). Quantitative vessel analysis was performed on angiographic images of ischemic and nonischemic limbs distal from the ePTFE-lined endoprosthesis at day 42. Angiogenic scores were calculated as percentage of the number of contrast-opacified vessels crossing the squares divided by total number of vessels.
Capillary density.
Skeletal muscle tissue samples from ischemic and nonischemic limbs were fixed in neutral buffered formalin (10%), paraffin-embedded, 4 μm sections cut, and immunostained with anti-CD31 antibody (Abcam, Cambridge MA) to identify blood vessels. For each tissue section, twenty 40× magnification images were captured and tissue area measured and number of CD31 positive blood vessels was counted (Image-Pro Premier). Total number of blood vessels per total area was calculated for each sample.
Measurement of nitrite.
Nitrite concentrations were quantified in plasma obtained at baseline and day 7, 14, 21, 28, and 35by ion chromatography (ENO20 Analyzer, Eicom) as previously described (19).
Measurement of H2S and sulfane sulfur levels.
H2S and sulfane sulfur levels were determined in plasma obtained at baseline and day 7, 14, 21, 28, and 35 using gas chromatography (Agilent 7890 Series GC, G660XA Series Chemiluminescence Detector; Agilent) according to previously described methods (12).
Coronary artery vascular reactivity.
Left anterior descending (LAD) and left circumflex (LCX) coronary arteries were cut into 3-mm rings, mounted in organ baths with warm Krebs buffer and oxygenated for isometric tension experiments. Coronary arteries were placed under 0.5 g of tension to obtain a stable and optimal baseline passive tension. Coronary artery viability and contractile responsiveness were assessed by potassium chloride (KCl, 100 mM). Rings were washed and precontracted with prostaglandin (PGF2a, 30 mM) and relaxation responses to endothelial-dependent bradykinin (10−11 to 10−6 M) and substance P (10−11 to 10−8 M) were measured. Endothelial-independent relaxation was determined by sodium nitroprusside (SNP, 10−9 to 10−5 M). Half maximal effective concentrations (EC50) calculated.
ELISA.
Plasma 8-Isoprostane was measured at baseline and day 7, 14, 21, 28, and 35 according to the manufacturer’s recommendations (Abcam, Cambridge, MA).
Statistical analysis.
Values are means ± SE. Differences between two groups were compared (Prism 6, GraphPad Software) using Student unpaired, 2-tailed t-test. Two-way ANOVA was used to test Placebo and SG-1002 effects over time and coronary relaxation responses. One-way ANOVA was used to test treatment and vasoactive agent doses on coronary artery EC50. If significant differences were found with ANOVA, Tukey multiple-comparison post-hoc tests were performed. P values < 0.05 were considered statistically significant.
Results
Ankle-brachial index unchanged in ischemic limbs.
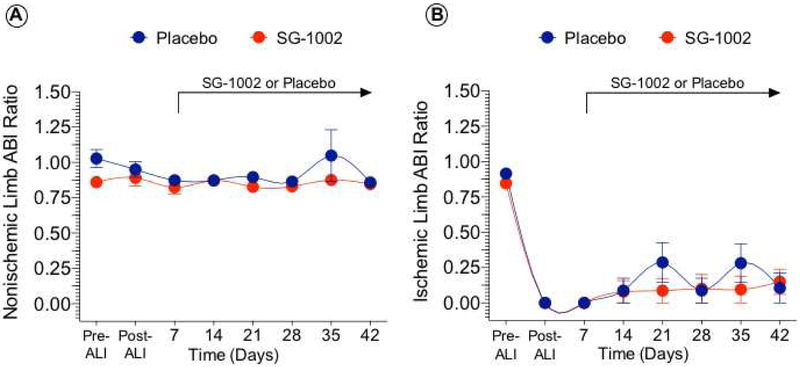
Measurement of blood pressure remained unchanged in nonischemic limbs (Figure 2). There was an expected immediate reduction in ischemic limb ABI following ALI. Ischemic limb ABI and continued to demonstrate a persistent ischemic state with no significant pressure differences in ischemic limbs between SG-1002 to Placebo.
Figure 2. Ankle-brachial index unchanged in ischemic limbs.
Ankle-brachial index (ABI) for nonischemic (Figure 2A) and ischemic hindlimbs (Figure 2B) was calculated from systolic pressures measured weekly in each of the forelimbs and hindlimbs. ABI improved but continued to demonstrate a persistent ischemic state with values below 0.35 at day 42 in both groups. No difference in ABI between the placebo and SG-1002 groups was observed at any time point. Values are means ± SEM.
Pharmacokinetics of SG-1002.
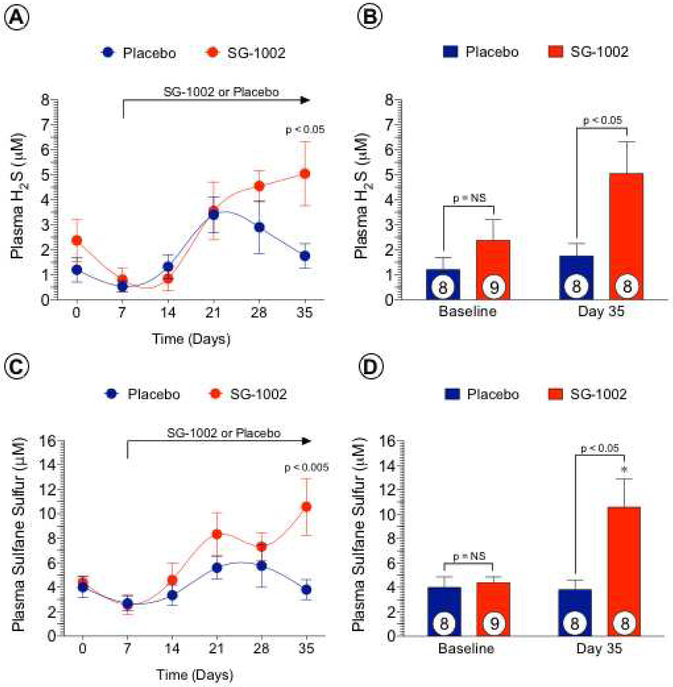
Beginning post- ALI day 7, SG-1002 or Placebo was administered twice daily for 35 days. There were no observable significant changes in plasma free H2S (Figures 3A, 3B) or SS (Figures 3C, 3D) with Placebo. In contrast, we observed a significant increase (p < 0.05) in free H2S at day 35 with SG-1002 compared to Placebo. We failed to observe increases in circulating free H2S at days 14, 21 and 28 in the SG-1002 treated group compared to Placebo, possibly due to reaction of H2S with reactive oxygen species upon release in vivo due to severe ischemia in the setting of ALI. We also observed a significant increase (p < 0.05) in the H2S storage form, SS at day 35 with SG-1002 compared to Placebo. In addition, circulating SS levels were significantly increased (p < 0.05) in the SG-1002 treated group at day 35 compared to baseline (p < 0.05) and day 7 (p < 0.01) levels. As with free H2S, we failed to observe statistically significant increases in plasma SS at days 14, 21 and 28 with SG-1002 treatment compared to Placebo.
Figure 3. SG-1002 increases circulating levels of H2S and sulfane sulfur.
Baseline plasma was obtained before ALI induction, day 7 (prior to SG-1002 administration) and post-ALI days 14, 21, 28 and 35. Weekly circulating levels of H2S (Figure 3A) and sulfane sulfur (Figure 3C) were similar between the placebo and SG-1002 groups on days 0, 7, 14, 21, and 28. At day 35, levels of H2S (Figure 3B) and sulfane sulfur (Figure 3D) were higher in the SG-1002 group compared to placebo. Values are means ± SEM with number of animals per group shown. NS, not significant. *P < 0.05 vs. baseline.
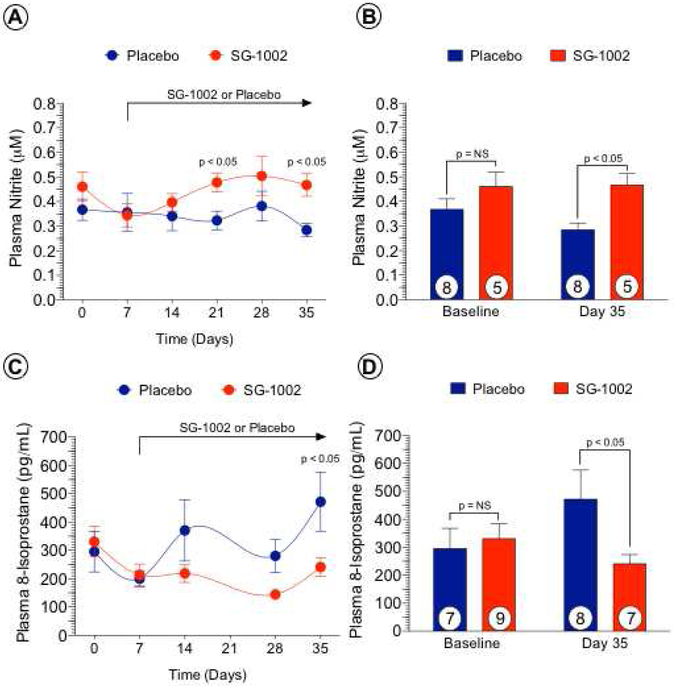
SG-1002 augments nitrite and attenuates oxidative stress following ALI.
We previously reported that H2S augments NO bioavailability via increased activation of eNOS and reduces oxidative stress (12). Therefore, we measured circulating levels of the NO-storage form, nitrite (Figures 4A, 4B). SG-1002 administration significantly increased plasma nitrite levels at days 21 (p < 0.05) and 35 (p < 0.05) compared to Placebo-treatment. One pathological consequence of chronic tissue ischemia is increased oxidative stress and circulating levels of the oxidative stress marker, 8-isoprostane. Plasma 8-isoprostane levels (Figures 4C, 4D) were significantly increased in Placebo-treated animals at day 35 compared to baseline (p < 0.05) and day 7 (p < 0.05). SG-1002 therapy significantly attenuated (p < 0.05) plasma 8-isoprostane at day 35 compared to Placebo.
Figure 4. SG-1002 increases circulating levels of nitrite and reduces oxidative stress.
Baseline plasma was obtained before ALI induction, day 7 (prior to SG-1002 administration) and post-CLI days 14, 21, 28 and 35. Weekly circulating levels of nitrite were increased in the SG-1002 group at days 21 and 35 compared to placebo (Figure 4A). Circulating nitrite levels were similar between groups on days 0, 7, 14, and 28. At day 35, levels of nitrite were higher in the SG-1002 group compared to placebo (Figure 4B). Plasma levels of the oxidative stress marker, 8-isoprostane, were similar between the placebo and SG-1002 groups on days 0, 7, 14, 21, and 28 (Figure 4C). At day 35, levels of 8-isoprostane were lower in the SG-1002 group compared to placebo (Figure 4D). Values are means ± SEM with number of animals per group shown. NS, not significant.
SG-1002 therapy improves extremity blood flow in ischemic limbs.
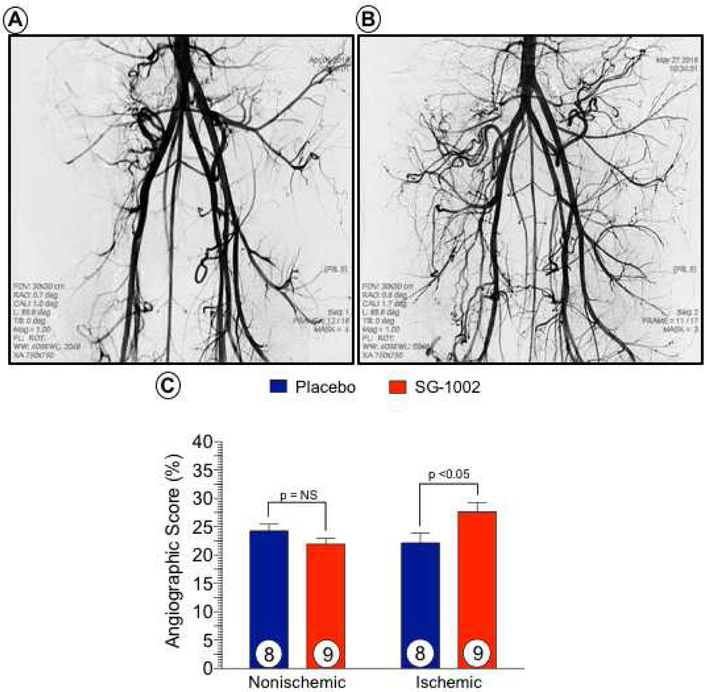
Representative, magnified-view contrast angiographic images of the peripheral circulation in Placebo and SG-1002 treated at day 42 are shown (Figure 5). Quantitative angiographic analysis revealed a significant increase (p < 0.05) in ALI limb perfusion with SG-1002 treatment compared to Placebo at day 42. No significant change (p = NS) was observed between Placebo ischemic and nonischemic limbs.
Figure 5. SG-1002 therapy increases collateral vessel number in ischemic hindlimbs.
Contrast angiographic images were acquired at day 42 post-ALI in placebo (Figure 5A) and SG-1002 (Figure 5B) treated animals. Quantitative angiographic scoring of contrast-opacified vessels was higher in ischemic limbs of SG-1002 treated pigs compared to placebo ischemic limbs (Figure 5C). There was no difference in collateral vessel number in the nonischemic limbs between the placebo and SG-1002 groups. Values are means ± SEM with number of animals per group shown. NS, not significant.
SG-1002 protects vasculature in ischemic limbs.
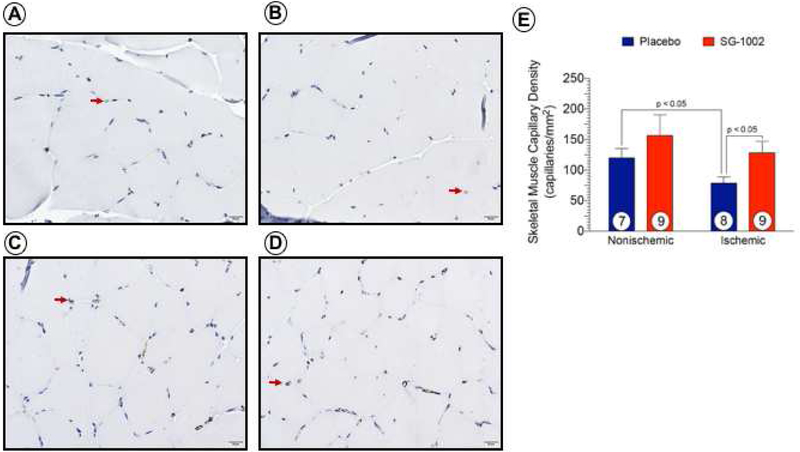
Representative micrographs of immunohistochemical stained vascular endothelium in ischemic and nonischemic hindlimb tissue at day 42 are shown (Figure 6). ALI resulted in a significant reduction (p < 0.05) in capillary number in Placebo-treated ischemic limbs compared to the nonischemic limb (Figure 6E). There was no difference in capillary number in SG-1002-treated limbs compared to nonischemic limbs. Animals receiving SG-1002 had significantly more (p < 0.05) capillaries in ischemic limbs compared to Placebo.
Figure 6. SG-1002 therapy preserves capillary density in ischemic hindlimbs.
Representative micrographs shown of CD31 immunostained muscle tissue obtained from placebo nonischemic (Figure 6A), ischemic (Figure 6B) and SG-1002 nonischemic (Figure 6C), ischemic (Figure 6D) hindlimbs at day 42 post-ALI. Arrows denote CD31 -positive staining of capillaries and capillary density quantified (Figure 6E). Values are means ± SEM with number of animals per group shown. NS, not significant.
Improved coronary vascular reactivity following SG-1002 therapy.
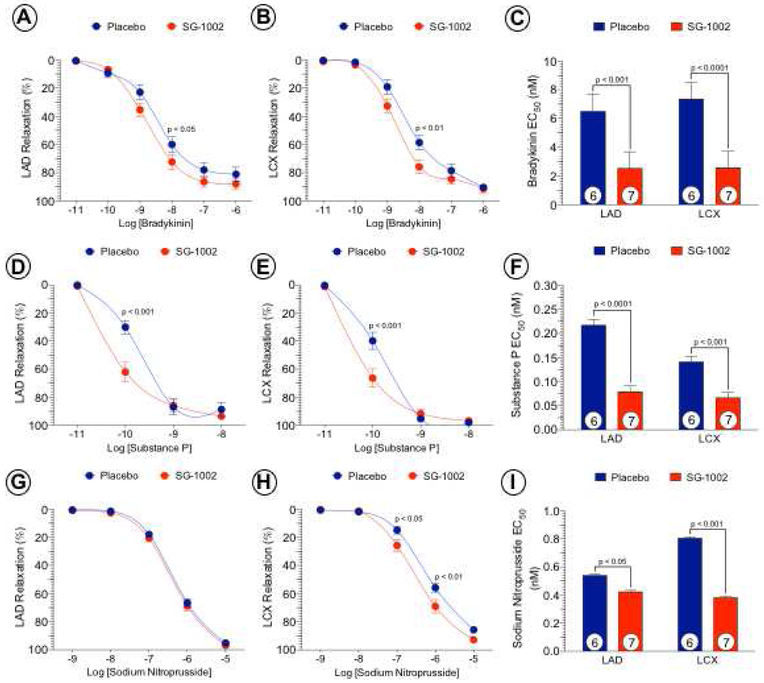
We investigated the effects of systemic administration of H2S therapy on coronary vascular reactivity in vitro (Figure 7). Vascular responses to endothelial-dependent vasodilator agents, bradykinin and substance P in coronary arterial rings isolated on day 42 from Placebo and SG-1002 treated groups are shown. Vascular relaxation of the LAD and LCX coronary arteries to bradykinin at 10−8 M was significantly (p < 0.05 and p < 0.01, respectively) improved in SG-1002 treated animals compared to Placebo. Furthermore, the EC50 to bradykinin was significantly lower in the LAD (p < 0.001) and LCX (p < 0.0001) with SG-1002 compared to Placebo. Relaxation to substance P at 10−10 M was significantly improved in LAD (p < 0.001) and LCX (p < 0.001) rings following SG-1002 treatment compared to Placebo. Substance P EC50 was significantly lower in LAD (p < 0.0001) and LCX (p < 0.0001) arteries following SG-1002 therapy compared to Placebo. While there were no differences observed in the LAD to the endothelial-independent vasodilator sodium nitroprusside, LCX vasorelaxation response was significantly enhanced at 10−7 M (p < 0.05) and 10−6 M (p < 0.01) following administration of SG-1002 along with the EC50 compared to Placebo.
Figure 7. SG-1002 improves coronary vascular reactivity by an NO-dependent mechanism.
Left anterior descending (LAD) and left circumflex (LCX) coronary arteries were isolated from placebo and SG-1002 treated groups for isometric tension experiments. Coronary arteries were precontracted with PGF2a and relaxation responses to endothelial-dependent bradykinin (Figure 7A and 7B) and substance P (Figure 7D and 7E). Endothelial-independent relaxation was determined by sodium nitroprusside (Figure 7G and 7H). Half maximal effective concentrations (EC50) were calculated (Figure 7C, 7F and 7I). Values are means ± SEM with number of animals per group shown.
Discussion
Peripheral arterial disease results from arterial obstruction, limiting blood supply to organs other than the heart. The lower extremities are most common site for PAD and with increasing ischemia, PAD patients have gait disturbance and tissue loss, resulting in increased risk for major amputation. Currently, there are no medical therapies effective in improving perfusion to the lower extremity in CLI patients (38-40). Surgical and catheter-based revascularization are the preferred approaches for CLI; however, many CLI patients are poor candidates for revascularization. Even after ‘successful’ revascularization, grafts often fail, or stenosis can reoccur after catheter-based treatment (3). There is a tremendous need to develop new treatments for patients with CLI who are not candidates for revascularization.
Patients with PAD have approximately a three-fold higher risk of cardiovascular and allcause mortality compared to those without PAD and exhibit impaired NO synthesis and endothelial dysfunction (41, 42). The vascular dysfunctions associated with PAD increase the risk of vascular complications in other areas, including the coronary circulation (43). One of the earliest manifestations of cardiovascular diseases, including PAD and CLI, is a loss of endothelial function characterized by dysfunction of eNOS and reduced NO bioavailability. Not only does NO promote distant organ protection (44), but with NO deficiency, the dysfunctional endothelium also becomes the source of mediators that are detrimental to the arterial wall, including endothelin-1, thromboxane A2, prostaglandin H2, and reactive oxygen species (45).
In this study, we used a porcine model of acute hindlimb ischemia that has sustained severe ischemia using endovascular occlusion of the external iliac artery and exclusion of its collateral vessels by means of a covered sent graft and vascular plug (37). This model is different than other published large animal models of PAD as they are limited by rapid collateralization not seen in PAD patients clinically and diminishes the time frame of ischemia to less relevant durations. While the existing small animal models of hindlimb ischemia have been effective for the study of collateral production, they do not produce the degree or duration of ischemia (mostly recovered within 2 weeks after ischemia) and they are associated with profound tissue damage from open arterial ligation (46). A limitation of our model is that the onset of ischemia is not the typical progressive atherosclerotic occlusion of PAD. The initial ischemic injury is acute with hallmarks of acute limb ischemia; however, after 1 week, these animals have recovered the ability to ambulate as seen clinically in PAD.
Previous studies have shown hydrogen sulfide exerts a number of cytoprotective and cardioprotective properties (47). Moreover, H2S plays an important role in modulating endothelial cell proliferation associated with ischemic vascular remodeling in response to experimental hindlimb ischemia (32). Studies have shown H2S and NO are reduced in PAD patients (24, 48). In our study, plasma H2S levels were unchanged from baseline during the first 2 weeks of ischemia in both Placebo and SG-1002 groups. Interestingly, there was a transient increase in plasma H2S levels observed in Placebo treated pigs at day 21 which returned to baseline levels by day 35. Kolluru et al also reported plasma H2S levels to be transiently elevated over the first 5 days and then returning to pre-ischemia levels following induction hindlimb ischemia in mice (49). Whether the increased circulating H2S levels are due to inducing limb ischemia acutely in healthy animals as opposed to the slow progressive tissue ischemia that occurs in patients suffering from atherosclerotic disease has yet to be investigated.
H2S is known to have proangiogenic activity in vascular endothelial cells and to promote vessel growth via VEGF signaling in primary endothelial cells (50). Although H2S and NO signal via distinct molecular pathways, there is compelling evidence of crosstalk between these two physiological gases as the cardioprotective mechanism of H2S to be mediated in part via NO subsequent to eNOS activation (12, 15). Studies have shown that angiogenesis is promoted in animals receiving H2S donors in an NO-dependent manner via increased eNOS activity (27). Results of the current study demonstrate that increasing circulating H2S via SG-1002, maintains circulating NO and improves vascular function. Animals treated with SG-1002 maintained levels of circulating nitrite, increased contrast-angiographic index and preservation of capillary density in the ischemic limb compared to Placebo. Whether the increased vessel and capillary density in SG-1002 ischemic limbs are due to activation of the NO/eNOS pathway has yet to be determined.
Patients with PAD have an increased risk for cardiovascular ischemic events due to concomitant coronary artery disease (3). We have recently shown that administration of SG-1002 increases NO bioavailability in normal subjects and heart failure patients (14). Using isolated coronary arteries harvested from Placebo and SG-1002 treated animals, we evaluated vascular responses to a number of vasodilator substances. Coronary artery relaxation in response to endothelium-dependent vasodilators, bradykinin and substance P as well as the endothelium-independent vasodilator, sodium nitroprusside demonstrates increased vasorelaxation to bradykinin of left circumflex coronary arteries from SG-1002 treated animals and improved vasorelaxation responses to substance P in LCX and LAD arteries. We also observed enhanced vasomotor responses to sodium nitroprusside in LCX from SG-1002 treated animals compared to Placebo. One limitation of this study is that we did not investigate coronary vascular function from normal, non-ischemic pigs treated with Placebo or SG-1002; however, the purpose of this study was to determine the potential of SG-1002 to be used as a therapy for patients suffering from debilitating limb ischemia. These data suggest that administration of an H2S prodrug significantly improves coronary vascular reactivity in the setting of ALI and may further protect the coronary circulation against vascular dysfunction and coronary artery disease.
By utilizing an intravascular approach in a large animal, we produced a model of severe occlusion leading to ALI. In the present study, ABI measurements remained below 0.35 in SG-1002 and Placebo treated animals at all time points. This ALI model is extremely severe and likely mimics some ALI disease components that occur in patients. In this study, and in our previous studies utilizing this model, we failed to observe significant improvements in hindlimb blood pressure with either NO or H2S therapeutics. It is not clear if any agent can significantly improve blood flow in this very severe model. Future studies will investigate the effects of H2S therapy less severe models or slowly progressing models of ALI. The results of this study provide compelling evidence that H2S therapy may prove beneficial for the treatment of peripheral vascular disease, but it is important to consider other H2S releasing agents that are more potent in terms of overall H2S release or H2S donors with superior pharmacokinetics with sustained H2S release over a prolonged period of time. One major limitation with H2S therapy is the lack H2S releasing drugs that increase H2S bioavailability for hours instead of seconds or minutes. While the present study sheds novel insight into H2S therapy in the setting of ALI, more studies are warranted to investigate the mechanisms by which H2S promotes vasodilation, revascularization, and attenuates oxidative stress in the setting of chronic tissue ischemia.
Conclusions
Our results suggest that daily administration of the H2S prodrug, SG-1002, leads to elevations in circulating H2S and NO signaling along with increases in vessel number and density in ischemic limbs. Furthermore, SG-1002 therapy improved endothelial-dependent coronary artery vasorelaxation in the setting of ALI. Our data demonstrate that SG-1002 is proangiogenic in ischemic limbs and exerts vascular protective effects in the coronary vasculature in a model of peripheral vascular disease.
Key Findings:
The authors found that treatment with SG-1002 restored blood flow, enhanced circulating hydrogen sulfide and nitriate, and reduced oxidative stress.
Take Home Message:
Strategies to enhance hydrogen sulfide augment vasodilation to treat limb ischemia.
Clinical significance.
In a clinically relevant porcine model of peripheral vascular disease, treatment with a novel H2S prodrug restored ischemic limb blood flow, enhanced circulating hydrogen sulfide and nitrite levels, reduced oxidative stress, and improved ex vivo coronary vascular function via endothelium-dependent vasodilation mechanism.
Acknowledgements
We thank Amy Scarborough, Sarah Boisvert, Chelsea Organ and Rishi Trivedi for their invaluable assistance with technical aspects of the study. We thank Sulfagenix, Inc. (Cleveland, OH) for the generous supply of SG-1002 and W. L. Gore and Associates (Flagstaff, AZ) for the generous donation of the Viabahn® Endoprosthesis. Histological processing and histomorphometry was provided by Histo Scientific Research Laboratory, Jackson, VA.
Funding Sources
Supported in part by 1 U54 GM104940 entitled “Louisiana Clinical and Translational Science Center” from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. This work was also supported by grants from the National Heart, Lung, and Blood Institute (National Institutes of Health; 1R01 HL092141 (DJL), 1R01 HL093579 (DJL), 1U24 HL 094373 (DJL), 1P20 HL113452 (DJL).
Abbreviations
- ABI
ankle brachial index
- ALI
acute limb ischemia
- BK
bradykinin
- CD31
platelet endothelial cell adhesion molecule 31
- CLI
critical limb ischemia
- eNOS
endothelial nitric oxide synthase
- HF
heart failure
- H2S
hydrogen sulfide
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- NO
nitric oxide
- PAD
peripheral artery disease
- SNP
sodium nitroprusside
- SP
substance P
- SS
sulfane sulfur
Footnotes
Disclosures
The authors have nothing to disclose.
JVS-D-18-00230R2, Effects of A Novel Hydrogen Sulfide Prodrug in a Porcine Model of Acute Limb Ischemia
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114: 688–99. [DOI] [PubMed] [Google Scholar]
- 2.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000; 31: S1–S296. [PubMed] [Google Scholar]
- 3.Mangiafico RA, Mangiafico M. Medical treatment of critical limb ischemia: current state and future directions. Curr Vasc Pharmacol 2011; 9: 658–676. [DOI] [PubMed] [Google Scholar]
- 4.Howell MA, Colgan MP, Seeger RW, Ramsey DE, Sumner DS. Relationship of severity of lower limb peripheral vascular disease to mortality and morbidity: a six-year follow-up study. J Vasc Surg 1989; 9: 691–696. [DOI] [PubMed] [Google Scholar]
- 5.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischemia: Still poor outcomes and lack of guideline adherence." Eur Heart J 2015; 36: 932–938. [DOI] [PubMed] [Google Scholar]
- 6.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health and Risk Manag 2007; 3: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991; 87: 119–128. [DOI] [PubMed] [Google Scholar]
- 8.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992; 326: 381–386. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110: 738–743. [DOI] [PubMed] [Google Scholar]
- 10.Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci U S A 2007; 104: 17907–17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemici B, Wallace JL. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol 2015; 555: 169–193. [DOI] [PubMed] [Google Scholar]
- 12.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 2014; 111: 3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 2007; 104: 15560–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polhemus DJ, Li Z, Pattillo CB, Gojon G, Giordano T, Krum H. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther 2015; 33: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013; 127: 1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840–844. [DOI] [PubMed] [Google Scholar]
- 17.Dopheide JF, Rubrech J, Trumpp A, Geissler P, Zeller GC, Bock K, et al. Leukocyte-platelet aggregates-a phenotypic characterization of different stages of peripheral arterial disease. Platelets 2016; 27: 658–667. [DOI] [PubMed] [Google Scholar]
- 18.Ho TK, Rajkumar V, Black CM, Abraham DJ, Baker DM. Increased angiogenic response but deficient arteriolization and abnormal microvessel ultrastructure in critical leg ischemia. Br J Surg 2006; 93:1368–1376. [DOI] [PubMed] [Google Scholar]
- 19.Polhemus DJ, Bradley JM, Islam KN, Brewster LP, Calvert JW, Tao YX, et al. Therapeutic potential of sustained-release sodium nitrite for critical limb ischemia in the setting of metabolic syndrome. Am J of Physiol Heart Circ Physiol 2015; 309: H82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, Hart JL. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch Pharmacol 2014; 387: 67–74. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J of Physiol Heart Circ Physiol 2002; 283: H474–480. [DOI] [PubMed] [Google Scholar]
- 22.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997; 237: 527–531. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Hu Q, Ma F, Zhu YZ. Vasorelaxant effect of a new hydrogen sulfide-nitric oxide conjugated donor in isolated rat aortic rings through cGMP pathway. Oxid Med Cell Longev 2016; 2016: 7075682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, Lefer DJ. Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI). J Am Heart Assoc 2015; 4: e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, et al. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 2010; 122: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnarumma E, Ali MJ, Rushing AM, Scarborough AL, Bradley JM, Organ CL, et al. Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability. J Am Heart Assoc 2016; 5: e003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashida R, Kondo K, Morita S, Unno K, Shintani S, Shimizu Y, et al. Diallyl trisulfide augments ischemia-induced angiogenesis via an endothelial nitric oxide synthase-dependent mechanism. Circ J 2017; 81: 870–878. [DOI] [PubMed] [Google Scholar]
- 28.Langston JW, Toombs CF. Defining the minimally effective dose and schedule for parenteral hydrogen sulfide: long-term benefits in a rat model of hindlimb ischemia. Med Gas Res 2015; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson PW, Jimenez N, Ruffino J, Sohn AM, Weinstein AL, Krijgh DD, et al. Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J Vasc Surg 2011; 53: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson PW, Singh SP, Weinstein AL, Nagineni V, Rafii DC, Kadouch D, et al. Therapeutic metabolic inhibition: hydrogen sulfide significantly mitigates skeletal muscle ischemia reperfusion injury in vitro and in vivo. Plast Reconstr Surg 2010; 126: 1890–1898. [DOI] [PubMed] [Google Scholar]
- 31.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 2010; 12:1065–1077. [DOI] [PubMed] [Google Scholar]
- 32.Bir SC, Kolluru GK, McCarthy P. Shen X, Pardue S, Pattillo CB, et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1a and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 2012; 1: e004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, et al. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 2009; 120: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 2012; 302: H2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley JM, Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, Tao YX, et al. Sustained release nitrite therapy results in myocardial protection in a porcine model of metabolic syndrome with peripheral vascular disease. Am J Physiol Heart Circ Physiol 2015; 309: H305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr LA, Shimizu Y, Lambert JP, Nicholson CK, Calvert JW. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide 2015; 46: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long CA, Timmins LH, Koutakis P, Goodchild TT, Lefer DJ, Pipinos II, et al. An endovascular model of ischemic myopathy from peripheral arterial disease. J Vasc Surg 2017; 66: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA 2006; 295: 547–553. [DOI] [PubMed] [Google Scholar]
- 39.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease. J Vasc Surg 2007; 45: S5–S67. [DOI] [PubMed] [Google Scholar]
- 40.Ouriel K Peripheral arterial disease. Lancet 2001; 358: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 41.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003; 108: 2093–2098. [DOI] [PubMed] [Google Scholar]
- 42.Froehlich JB, Mukherjee D, Avezum A, Budaj A, Kline-Rogers EM, López-Sendón J, et al. Association of peripheral artery disease with treatment and outcomes in acute coronary syndromes. The Global Registry of Acute Coronary Events (GRACE). Am Heart J 2006; 151: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 43.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: Evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci USA 2008; 105: 11430–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taddei S, Ghiadoni L, Virdis A, Versari D, Salvetti A. Mechanisms of endothelial dysfunction: clinical significance and preventive non-pharmacological therapeutic strategies. Curr Pharm Des 2003; 9: 2385–2402. [DOI] [PubMed] [Google Scholar]
- 45.Kimura H The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014; 41: 4–10. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, et al. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 2010; 17:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boger RH, Bode-Boger SM, Thiele W, Junker W, Alexander K, Frolich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation 1997; 95: 2068–2074. [DOI] [PubMed] [Google Scholar]
- 48.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma Nitrite Response and Arterial Reactivity Differentiate Vascular Health and Performance. Nitric Oxide. 2009; 20(4): 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolluru GK, Bir SC, Yuan S, Shen X, Pardue S, Wang R, Kevil CG. Cystathionine γ-lyase regulates arteriogenesis through NO-dependent monocyte recruitment. Cardiovasc Res. 2015. September 1;107(4):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 2009; 105: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]