Abstract
Using optogenetic and chemogenetic manipulations, Chen et al. show that reactivation of fear and reward memory engrams via the dorsal and ventral hippocampus drive context-specific behaviors and bi-directionally control memory strength. The ventral DG and BLA are critical for linking emotional valence to specific contexts.
Keywords: memory, engram, emotion, longitudinal axis, hippocampus, optogenetics
SUMMARY
Emerging evidence indicates that distinct hippocampal domains differentially drive cognition and emotion [1, 2]; dorsal regions encode spatial, temporal, and contextual information [3–5] whereas ventral regions regulate stress responses [6], anxiety-related behaviors [7, 8], and emotional states [8–10]. Although previous studies demonstrate that optically manipulating cells in the dorsal hippocampus can drive the behavioral expression of positive and negative memories, it is unknown whether changes in cellular activity in the ventral hippocampus can drive such behaviors [11–14]. Investigating the extent to which distinct hippocampal memories across the longitudinal axis modulate behavior could aid in the understanding of stress-related psychiatric disorders known to affect emotion, memory, and cognition [15]. Here, we asked if tagging and stimulating cells along the dorsoventral axis of the hippocampus could acutely, chronically, and differentially promote context-specific behaviors. Acute reactivation of both dorsal and ventral hippocampus cells that were previously active during memory formation drove freezing behavior, place avoidance, and place preference. Moreover, chronic stimulation of dorsal or ventral hippocampal fear memories produced a context-specific reduction or enhancement of fear responses, respectively, thus demonstrating bi-directional and context-specific modulation of memories along the longitudinal axis of the hippocampus. Fear memory suppression was associated with a reduction in hippocampal cells active during retrieval, while fear memory enhancement was associated with an increase in basolateral amygdala activity. Together, our data demonstrate that discrete sets of cells throughout the hippocampus provide key nodes sufficient to bi-directionally reprogram both the neural and behavioral expression of memory.
RESULTS
Activity-dependent and Inducible Tagging of Dorsal and Ventral Hippocampus
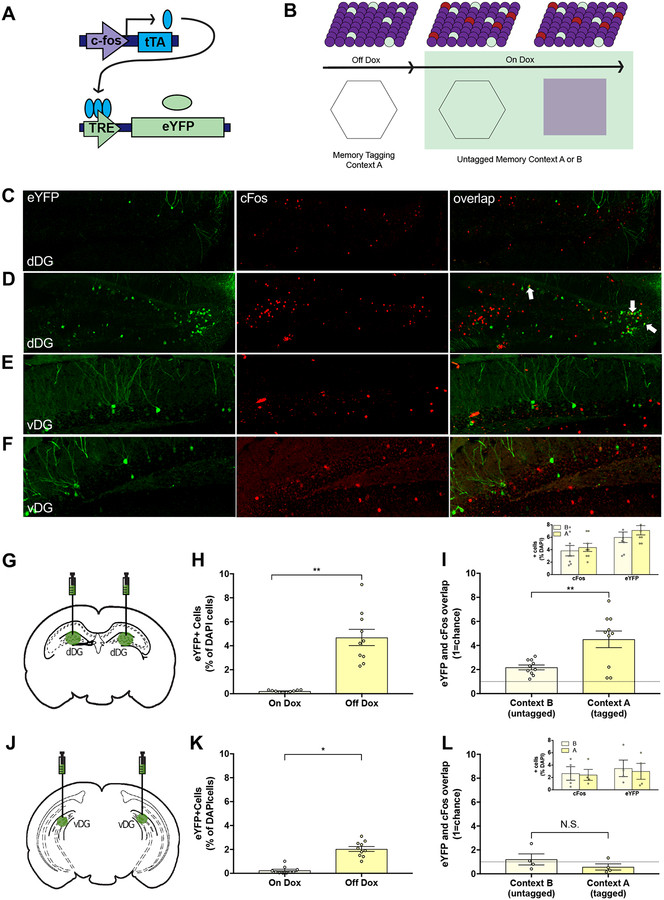
We first infused an inducible, activity-dependent virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-eYFP into the dorsal or ventral dentate gyrus (DG) of adult male mice, which labeled DG neurons expressing the immediate-early gene c-Fos in a doxycycline(Dox)-dependent manner while mice explored a neutral context (Figures 1A–1B) [16]. Exploration of the context while off Dox increased eYFP−expressing (eYFP+) cells in both the dorsal and ventral DG relative to on-Dox controls (Figures 1G–1H, 1J–1K). The following day, mice that explored the same context showed a significant increase in the number of overlapping eYFP+ (i.e. cells labeled by the 1st exposure) and c-Fos+ cells (i.e. cells labeled by the 2nd exposure) in the dorsal, but not ventral DG (relative to chance overlap) consistent with a previously established role of the dorsal DG in context discrimination (Figures 1C–1F, 1I, 1L) [17, 18].
Figure 1. Activity-dependent and inducible expression of enhanced yellow fluorescent protein (eYFP) in dorsal and ventral dentate gyrus (DG).
(A) A virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-eYFP was infused into the dorsal or ventral DG in mice on a doxycycline diet (Dox). (B) Dox was removed prior to placement in Context A and returned to diet following exposure to label active cells with eYFP. The following day, mice were returned to the same or different context and expression of c-Fox was visualized. (C–F) Representative images (20X) of eYFP+ (green), c-Fos+ (red), and overlap of cells in the dorsal (dDG) or ventral (vDG) dentate gyrus. eYFP+, c-Fos+, and overlap in mice exposed to a different (C & E) or the same (D & F) context. (G & H) In the dorsal DG, 4.7% (+/−0.7%) of DAPI+ cells were labeled with eYFP while Off Dox compared to <0.3% of On Dox (t(18)=6.58, p<0.01). (I) Mice exposed to the same context (Context A; tagged) had significantly more overlap between eYFP+ and c-Fos+ granule cells than those exposed to a different context (Context B; untagged) relative to chance (dashed line; n=10/group) (t(18)=3.24, p<0.01). Both Contexts A and B recruited similar amounts of cFos+ and eYFP+ cells (inset). (J & K) In the ventral DG, 2.1% (+/−0.2) of ventral DG granule cells were labeled while Off Dox compared to < 0.3% while On Dox (t(18)=7.96, p<0.01) (top; n=10/group). (L) Re-exposure to the same or different context did not result in significant overlap relative to chance (ns) (bottom; n=4/group). The number of cFos+ and eYFP+ cells was comparable between both Contexts A and B (inset). White arrows indicate cells expressing eYFP and c-Fos.
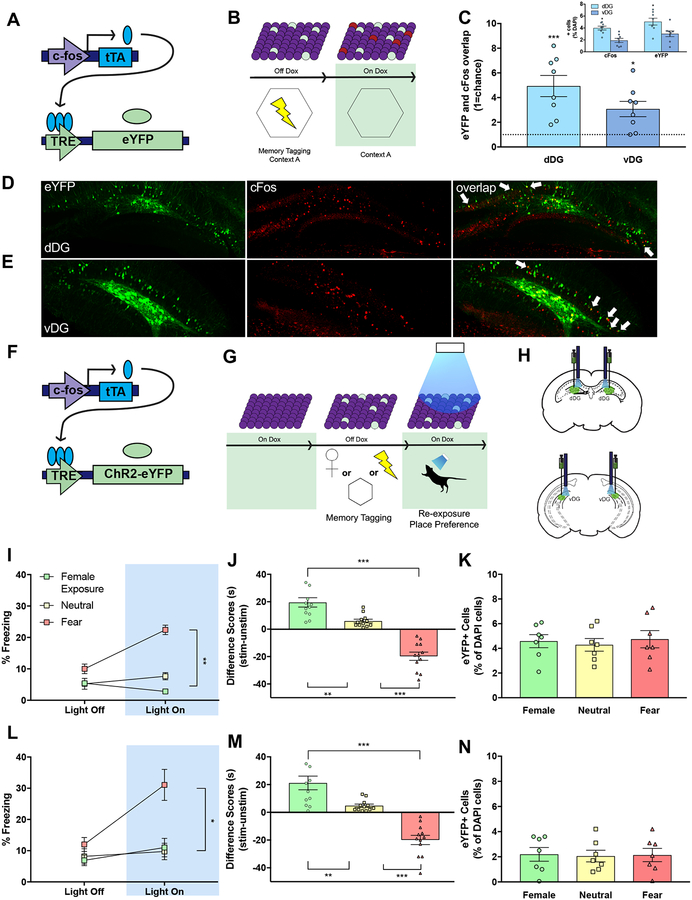
We next examined if DG cells active during contextual fear conditioning were preferentially reactivated during contextual fear retrieval the following day. Following viral injections (as above), mice were taken off Dox and, 48 hours later, were placed into a conditioning chamber where they received four foot shocks following a 180 second baseline period. Following fear acquisition, mice were removed from the context and placed back on a Dox diet. The following day, mice were returned to the conditioning chamber for a five-minute context test session (Figures 2A–2B). Brains were collected 90 minutes following testing (see methods) and processed for eYFP and c-Fos overlap in the dorsal and ventral DG. Here, both the dorsal and ventral DG showed significant increases in re-activated cells, consistent with the notion that the ventral hippocampus processes and relays emotion-related information as well (Figures 2C–2E) [5, 8, 10]. Together, these data demonstrate that the dorsal DG is reactivated following retrieval of both a neutral or aversive context memory whereas cells in the ventral DG show reactivation only in a shock-paired environment.
Figure 2. Acute activation of distinct memories drives discrete behavior across the longitudinal axis of the hippocampus.
(A) A virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-eYFP was infused into the dorsal or ventral DG. (B) While on Dox, mice were fear conditioned in Context A. Dox was then removed prior to exposure to a novel Context B, and overlap of cells in the dDG or vDG was visualized. (C) Overlap between eYFP+ and c-Fos+ neurons was comparable between the dDG and vDG and, in both regions, significantly above chance (dashed line). Inset represents %positive cfos and % positive eYFP labeled cells. There were significantly more cFos+ and eYFP+ in the dDG than in the vDG. (D–E) Representative images (20X) of eYFP+ (green), c-Fos+ (red), and overlap of cells in the dDG or vDG. (F) A virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-ChR2-eYFP was infused into the dorsal or ventral DG. (G) While off Dox, mice were exposed to a female, neutral, or fear memory to label active cells with ChR2, returned to Dox, and administered light stimulation during re-exposure to the training context and an OPP/OPA paradigm. (H) Representative schematic of viral injection and optogenetic stimulation in the dorsal (top) and ventral (bottom) DG. (I, L) Activation of cells (Light On) processing a fear, but not a neutral or a female memory, in both the dorsal (I; female group: n=11; neutral group: n=12; fear group: n=12) and ventral (L; n=12/group) hippocampus drove freezing behavior in a neutral context relative to Light Off epochs. Repeated-measures ANOVA, significant Group × Epoch interaction, dorsal DG [F(2, 32)=41.23, p<0.01] and ventral DG [F(2, 33)=6.48, p<0.01]. (J, M) Light-activation of fear memories drove place avoidance while light-activation of female exposure memories drove place preference in both dorsal (one-way ANOVA, J, dDG [F(2,33)=53.44, p<0.01] and M, vDG [F(2,33)=34.75, p<0.01]). (K, N) In the absence of acute stimulation, fear, neutral, and female memories labeled a comparable amount of eYFP+ neurons in both the dDG (K) and vDG (N). White arrows indicate cells expressing eYFP and c-Fos.
Acute activation of distinct memories drives discrete behavior across the longitudinal axis of the hippocampus
Next, we asked if acute optogenetic activation of tagged cell populations along the longitudinal axis of the hippocampus could drive appetitive and fear-related behaviors. Mice were infused with the same activity-dependent cocktail expressing channelrhodopsin2 (ChR2) into the dorsal or ventral DG and chronically implanted with optic fibers above the injection site (Figures 2F, 2H). Mice were taken off Dox to tag cells processing one of the following experiences, all of which have been shown to recruit similar proportions of DG cells; exploration of a neutral context [12], foot shock exposure in a novel context [11, 12], or exposure to a female conspecific (see Star Methods; hereafter, we refer to these groups as “neutral”, “fear”, or “female exposure” groups, respectively) (Figure 2G). Acute stimulation of a fear memory via either the dorsal or the ventral DG drove freezing behavior and promoted place avoidance (Figures 2I–2J, 2L–2M). Acute stimulation in the female exposure groups promoted place preference, but did not affect fear behavior (Figures 2I–2J, 2L–2M). Importantly, activation of a neutral memory did not affect fear or preference behaviors (Figures 2I–2J, 2L–2M), and all experiences, positive, neutral, and fear, labeled a similar number of cells (2K, 2N). As the dorsal and ventral DG have been implicated in contextual fear acquisition and retrieval [19–22] and artificial reactivation of dorsal DG cells processing a fear memory can lead to behavioral expression of fear [11, 12, 23], our current findings are in broad agreement with these earlier studies showing that the activation of fear-associated cells in the dorsal DG is sufficient to drive multiple behaviors. Together, our data demonstrate that artificial manipulation of dorsal and ventral DG-mediated memories are sufficient to drive fear-related behaviors and promote place avoidance and preference.
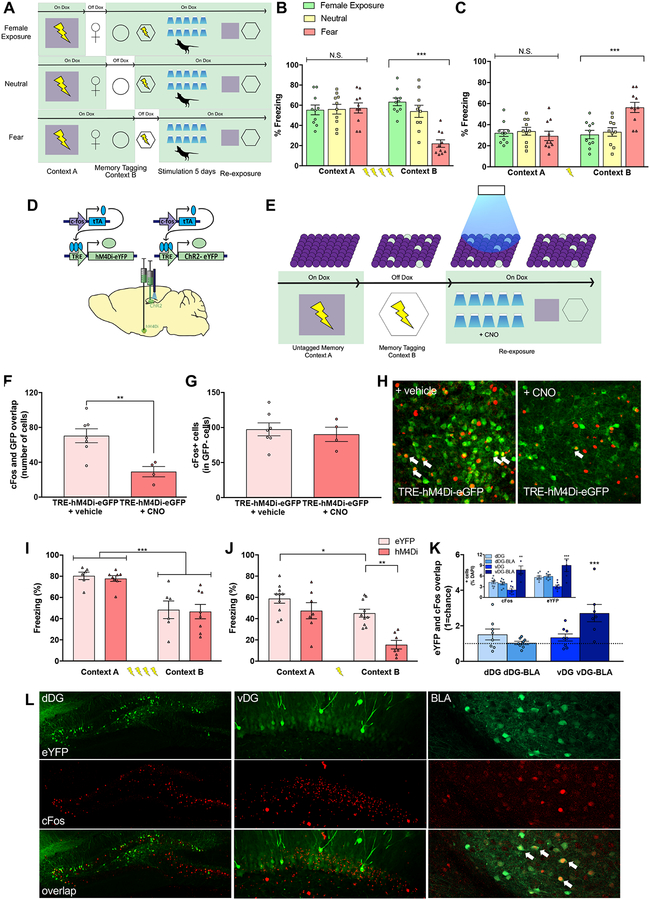
Chronic activation of memories induces long-lasting and bidirectional changes in behavior
Finally, we asked if chronic optical activation of discrete memories could enduringly affect neural activity and behavior. All groups were first fear conditioned in Context A and received subsequent exposures to a female mouse, a neutral context, and were fear conditioned in a novel Context B. Female, neutral, and fear groups were separated based on whether mice were taken off Dox to label a positive, neutral, and fear memory, respectively. After labeling was complete, mice were then placed into a novel context and received chronic optogenetic activation of the dorsal or ventral DG [12, 13] (i.e., ten-minute sessions, twice a day for five days) [14]. Behavioral testing occurred 24-hours later in the absence of light stimulation (Figure 3A). Chronic stimulation of the dorsal DG in a fear group that underwent a four-shock conditioning protocol produced a context-specific suppression of freezing (Figure 3B); conversely, chronic stimulation of the ventral DG in a group that underwent a one-shock conditioning protocol produced a context-specific enhancement of freezing (Figure 3C). These enduring changes in freezing levels following chronic stimulation were specific to reactivation of a fear memory, with mice freezing at equivalent levels in both contexts following stimulation of a neutral memory (Figures 3B–3C). These effects were not observed in eYFP control mice (Figures S1E–S1D, S1G–S1H), nor when a dorsal DG group received a weak one-shock conditioning protocol or when a ventral DG group received a four-shock conditioning (Figures S1D, S1G). These enduring changes in behavior did not result from alterations in locomotion (Figure S2). To investigate whether these behavioral alterations were influenced by contributions from the basolateral amygdala (BLA), a brain region highly interconnected with the HPC that modulates behavioral responses to fear and stress, we utilized Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to attenuate BLA activity during chronic HPC stimulation [15, 24] (Figures 3D–3E). Treatment with clozapine-N-oxide (CNO) significantly reduced cFos activity in hM4Di-expressing cells but not in controls (Figure 3F, 3H). Interestingly, cFos activity was comparable between hM4Di experimental and control groups, suggesting at least three non-mutually exclusive options: CNO may have induced a kind of “rebound” of activity in non-tagged cells through local circuit mechanisms, thus maintaining comparable levels of cFos-positive cells; during a perturbation the BLA maintains an active population that is stable in size; CNO-mediated hM4Di activation non-specifically attenuates a cell’s excitability levels in a manner sufficient to suppress cFos in a subset, but not all, tagged cells (3G). Next, we observed that chemogenetic inhibition of fear-processing BLA cells during chronic stimulation of dorsal DG fear ensembles did not prevent the context-specific suppression of freezing (Figure 3G); notably, however, stimulation-induced enhancement of fear behavior was disrupted when BLA cells were inhibited during chronic stimulation of ventral DG-mediated fear memories. (Figure 3H). Thus, the increase in freezing behavior following chronic ventral DG activation relies, in part, on the reactivation of BLA cells associated with the original fear memory, a mechanism distinct from that underlying the dorsal DG effect. Finally, in the absence of CNO inactivation, animals that underwent chronic stimulation of DG cells processing fear showed chance-level reactivation of the originally tagged population in both the dDG and vDG groups upon being returned to the conditioned context (Figures 3K–3L). However, in the vDG and not dDG group, there was a significant increase in overlap between BLA cells processing the original fear memory and cells active during fear memory recall (Figures 3K–3L)—a finding that perhaps underlies the observed context-specific enhancement of fear memory. Together, these data indicate that chronically reactivating DG cells enduringly alters underlying neural activity in both tagged DG and BLA populations, suggesting that our stimulation protocol is sufficient to functionally reprogram cells processing discrete memories.
Figure 3. Chronic activation of memories induces long-lasting and bidirectional changes in behavior.
(A) A virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-ChR2-eYFP was infused into the dorsal and ventral DG. All groups were first fear conditioned in context A, followed by fear conditioning in context B a day later, during which the fear group was off Dox. A separate group was exposed to a neutral context to label active cells with ChR2 and returned to being on Dox. Memories were reactivated twice a day for 5 days in a novel context. (B) Upon re-exposure to Contexts A and B, chronic reactivation of cells processing a four-shock fear memory in the dorsal DG resulted in reduced freezing in the tagged context (e.g. Context B) compared to reactivation of cells processing female and neutral memories (n=10/group. Repeated measures ANOVA, Group × Context, [F(2, 27)=12.67, p<0.01]). Newman-Keules posthoc test revealed significantly lower levels of freezing in Context B compared to Context A only in fear group (p<0.001). (C) When returned to contexts A and B, chronic reactivation of cells processing a single-shock fear memory in the ventral DG resulted in increased freezing in the tagged context (e.g. Context B) compared to reactivation of cells processing female and neutral memories (n=10/group: Repeated measures ANOVA, Group × Context, [F(2, 27)=10.88, p<0.01]). Posthoc revealed significantly higher levels of freezing in Context B compared to Context A only in fear group (p<0.001). (D,E) A virus cocktail of AAV9-c-Fos-tTA and AAV9-TRE-ChR2-eYFP (ChR2) was infused into the dorsal or ventral hippocampus and AAV9-c-Fos-tTA and AAV9-TRE-hM4Di-eYFP (hM4Di) or AAV9-TRE-eYFP (eYFP) was infused into the basolateral amygdala (BLA). Mice were fear conditioned in Context A while on Dox and in Context B while off Dox to label DG and BLA cells associated with Context B fear memory with ChR2 and hM4Di or eYFP, respectively. Following tagging, mice underwent a total of 10 sessions over 5 days of optical stimulation while in a novel environment; all mice received 1 mg/kg CNO (i.p.) 30 minutes prior to each session. Finally, mice were placed back into Context A and Context B and their levels of freezing were assessed (in the absence of either light activation or CNO injection). (F) Treatment with CNO significantly reduced cFos and GFP overlap when compared to treatment with a vehicle control (Student’s t-test, p = 0.006). (G) cFos expression in GFP− cells was comparable between CNO- and vehicle-treated brains following CNO infusion. (H) Representative images of cFos (red) and eGFP (green) overlap in the BLA following CNO treatment. (I) Chronic reactivation of cells processing a strong four-shock fear conditioning memory in the dorsal DG led to a context-specific reduction of freezing behavior, an effect not affected by hM4Di-expression in the BLA. (eYFP: n=6; hM4Di: n=8; Repeated-Measures ANOVA, Main effect of Context [F(1,12)=41.93, p<0.001] without a main effect of Group or Context × Group interaction (ns). (J) Chronic reactivation of cells processing a weak single-shock fear conditioning memory in the ventral DG led to a context-specific increase in freezing behavior. This effect was ablated by chemogenetic inactivation of the BLA (eYFP: n = 10; hM4Di: n = 7; Repeated-Measures ANOVA, Main effect of Context [F(1,15) = 23.92, p < 0.001], Main effect of Group [F(1,15) = 15.47, p < 0.01]). Posthoc revealed significantly lower levels of freezing between the eYFP and hM4Di group in Context A (p < 0.001). (K) Following chronic activation of dDG fear memories, there was no significant change in overlap between eYFP+ and cFos+ cells in either the dDG or BLA (denoted here as dDG-BLA). However, after chronic activation of vDG fear memories, there was a significant amount of overlap between eYFP+ and c-Fos+ cells above chance (dashed line) in the BLA (denoted by vDG-BLA), but not in the vDG. Inset represents % positive cFos+ and eYFP+ cells. cFos+ and eYFP+ cells were significantly greater in the BLA after chronic vDG activation compared to all other groups. (L) Representative images of eYFP (green), c-Fos (red), and overlap in the dDG, vDG, and BLA. White arrows indicate cells expressing eYFP and c-Fos. See also Figures S1 and S2.
DISCUSSION
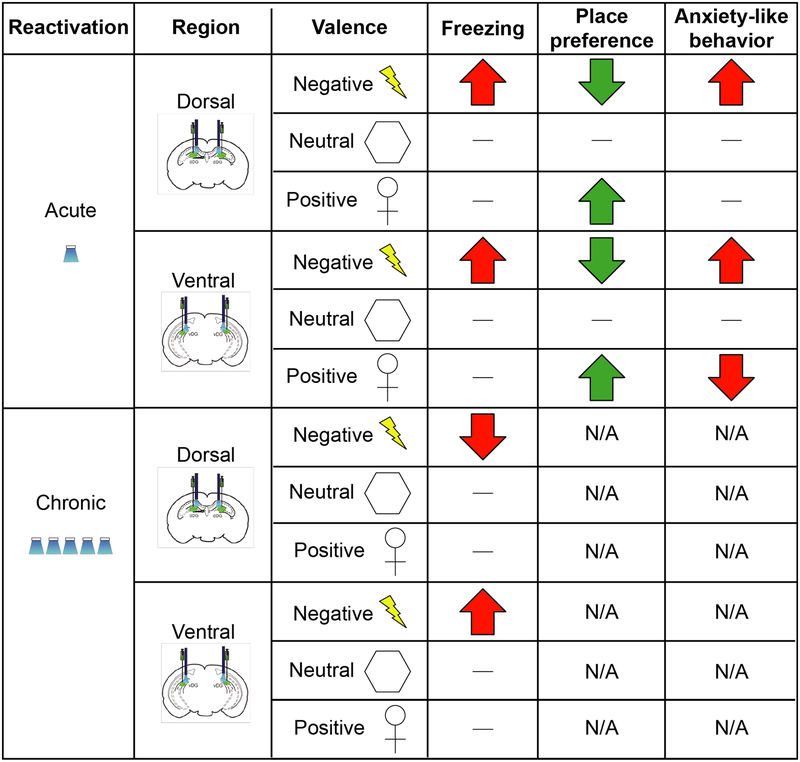
The hippocampus mediates key aspects of episodic memories, including spatial, temporal, and contextual elements of experience that can also be imbued with emotion and salience. The dorsal regions are thought to process the former cognitive aspects of experience whereas ventral regions process the latter emotional components of learning and memory [2, 25]. Here we asked if artificially reactivating cells previously active during the formation of positive, neutral, or negative memories across the longitudinal axis of the hippocampus would differentially modulate behavioral outputs. Our results demonstrate that acute reactivation of tagged cells in the dorsal and ventral dentate gyrus was sufficient to drive freezing, place avoidance, and place preference depending on the aversive or rewarding nature of the tagged memory. Moreover, chronic reactivation of fear-associated cells in the dorsal or ventral hippocampus lead to distinct, lasting behavioral changes in behavior and neuronal activity, namely, chronic reactivation of dorsal DG cells resulted in a context-specific reduction in freezing as well as disengaged the originally tagged group of cells when exposed to the conditioned context, whereas chronic reactivation of ventral DG cells resulted in a context-specific enhancement of freezing as well as above-chance levels of overlap in the basolateral amygdala. Additionally, inhibition of BLA activity during chronic stimulation in the ventral, but not in the dorsal, DG blocked context-specific changes in freezing behavior, which we speculate occurs by blocking potentiation between monosynaptic connectivity in the ventral DG and basolateral amygdala. A graphical summary of our behavioral results can be found in Figure 4. Together, our data point to a differential contextual and emotional encoding of memory along the dorsoventral axis of the hippocampus.
Figure 4. Graphical summary of behavioral results.
Dash indicates no change. “N/A” indicates not measured.
In line with previously published results, acute optogenetic reactivation of negative memory traces in both the dorsal and ventral DG was sufficient to drive fear-related behavior [4, 5, 6]. These data support previous studies also demonstrating a role for the ventral hippocampus in the encoding and retrieval of memories, and resonates with its proposed role in modulating anxiety-like behavior through the activity of discrete populations of “anxiety cells” [8, 19]. Interestingly, we observed a modest increase in anxiety-like behavior when reactivating cells processing fear memories via both the dorsal and ventral hippocampus but without significantly increasing freezing responses (Figure S2). We speculate that the environmental contingencies present in a given arena or task can dictate the capacity of tagged cells to drive a given behavioral output, suggesting that these cells are not hardwired or pre-determined to promote a behavioral response. For instance, in a traditional conditioning chamber, activating hippocampus cells processing fear can default to a passive defensive behavior, including freezing, while enlarging an environment to an open field now “switches” these cells’ capacity such that they drive active anxiogenic responses [15] (Figure S2). Future experiments can expand on these findings by varying the mnemonic demands (or lack thereof) of a given task or arena and titrate the capacity of tagged DG cells to promote varying behavioral outputs contingent on both an animal’s state and the structure of its environment. Together, these data indicate that the entire hippocampal structure, not just the dorsal hippocampus, is sufficient to activate freezing behavior in a context-specific manner, perhaps by parsing information into mnemonic categories that are subsequently encoded by discrete cell populations whose behavioral relevance is contingent on environmental demands.
In support of this hypothesis, chronic reactivation of cells processing a fear memory in the dorsal DG reduced context-associated freezing, and these changes in behavior were independent of BLA involvement. On the other hand, chronic reactivation of cells processing a fear memory in the ventral hippocampus significantly increased freezing, and these behavioral effects were blocked by inhibition of BLA activity. These results could be explained by differences in connectivity between the dorsal and ventral hippocampus: while the dorsal hippocampus is extensively connected to areas such as the retrosplenial cortex and anterior cingulate cortex, areas critical for navigation and visuospatial processing, the ventral hippocampus sends and receives projections from the amygdala, prefrontal cortex, and bed nucleus of the stria terminalis, all of which are strongly implicated in processing emotion [2]. Of note, while our virus spread was confirmed histologically in each subject to ensure that injections reached dorsal or ventral hippocampus, a putative role for the intermediate hippocampus and its functional role in partially modulating these behaviors remains unresolved. Nonetheless, it has recently been shown that the CA1 of the ventral hippocampus stores neurons responsible for processing social memory, and it is widely believed that the ventral hippocampus is an important node for modulating such multi-modal information while also regulating stress responses [19, 26–28]. We speculate that our manipulation in the ventral hippocampus may have increased freezing behavior by strengthening monosynaptic inputs to the amygdala or hypothalamus. This hypothesis is reinforced by our data demonstrating increased eYFP and c-Fos overlap in the BLA following chronic reactivation of fear memories in the vDG. Thus, it is likely that while the dorsal hippocampus encodes and recalls contextual components of memories, the ventral hippocampus accesses circuitry necessary for the storage and recall of context-guided emotional components associated with specific experiences.
Chronic stimulation of fear memories in either the dorsal or ventral DG lead to context-specific reductions or enhancements, respectively, in fear behavior. The dorsal, and not ventral, DG showed a greater proportion of overlap between cells active during the encoding and retrieval of a neutral context (Figure 1), but the ventral DG became engaged when an emotional event occurred in that context, and as such the cells in the ventral hippocampus were now reactivated upon re-exposure, as well as in the dorsal hippocampus. These data are in agreement with a number of studies that report reactivation of DG cells during fear memory retrieval and demonstrate significant ventral DG reactivation during retrieval of a fearful, but not a neutral, context [20, 29].
We next demonstrated that chronic reactivation of cells active during fear acquisition in both the dorsal and ventral DG produced lasting changes in fear behavior. The direction of behavioral change depended upon the longitudinal axis of the hippocampus: chronic stimulation of cells in the dorsal DG resulted in a context-specific reduction in freezing behavior, whereas chronic reactivation within the ventral DG resulted in a context-specific increase. We propose that these opposing behavioral outcomes are partly a result of differential cellular mechanisms recruited by our stimulation parameters. For example, optogenetically inducing long-term depression (LTD) or long term potentiation (LTP) at auditory inputs to the amygdala was sufficient to inactivate or reactivate a fear memory, respectively [30]. To induce LTP or LTD in the former study, the authors utilized separate optical parameters while, in our study, the same optical parameters (see methods) were used to chronically reactivate cells within the dorsal and ventral DG—any difference in behavioral outcome from our stimulation protocol can be attributed, at least in part, to the anatomical region of reactivation, given that the main outputs for the dorsal and ventral hippocampus project to distinct anatomical regions. For example, the ventral hippocampus has monosynaptic projects to the amygdala nuclei, hypothalamus, prefrontal cortex, and nucleus accumbens, which dovetails with its functional role in modulating myriad information embedded with emotion and salience [10, 31]. Our overlap data (Figure 2) demonstrate that chronic reactivation resulted in a significant reduction in overlap of cells active during fear acquisition and those active during fear retrieval for both the dorsal and ventral hippocampus. Thus, in addition to producing changes in behavior, our manipulation drove cellular changes within the neural substrates underlying memory recall.
Finally, our data demonstrate that both dorsal and ventral DG cells tagged during fear acquisition are re-activated (above chance) during fear retrieval and that chronic reactivation of tagged cells results in a significant reduction in the number of cells that overlap. We speculate that chronic reactivation of dorsal DG cells lead to either extinction-like effects or to a permanent suppress of the memory itself. Fittingly, extinction learning is thought to represent new learning (i.e., repeated exposure to a conditioned stimulus) that competes with and actively inhibits the original fear memory [32, 33]. Within the basolateral amygdala, a discrete populations of cells have been shown to be active during fear retrieval (i.e., ‘fear cells’) while a discrete population of non-overlapping cells is active during retrieval of a fear extinction memory (i.e., ‘extinction cells) [34, 35]. We propose that the context specific reduction in freezing behavior following chronic stimulation of the dorsal hippocampus is not affected by inhibition of BLA cells active during fear acquisition because, presumably, the DREADDS are not expressed in those BLA cells responsible for the reduction in freezing behavior. However, BLA inactivation during chronic stimulation of the ventral DG was sufficient to block the stimulation-induced enhancement in freezing behavior presumably because it is precisely those cells that retain the fear memory that are actively inhibited and thus are not amenable to any changes induced by our stimulation protocol.
In conclusion, our results demonstrate distinct roles of the dorsal and ventral DG in the neuronal and behavioral expression of memory formation and retrieval. Our study provides insight into the functional distinctions of the hippocampus along its dorsoventral axis as well as the interaction between context specificity and emotional regulation in the brain. Together, investigating these neurobiological phenomena may contribute to more specific and improved interventions to treat stress-related neuropsychiatric disorders by pointing to engrams as a therapeutic node.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by Steve Ramirez (dvsteve@bu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Subjects.
Wildtype male C57BL/6 mice (2–3 months of age; Charles River Labs) were housed in groups of 2–5 mice per cage. The animal facilities (vivarium and behavioral testing rooms) were maintained on a 12:12-hour light cycle (lights on at 0700). Mice were placed on a diet containing 40 mg/kg doxycycline (Dox) for a minimum of one week before receiving surgery with access to food and water ad libitum. Mice recovered for ten days after surgery. Dox-containing diet was replaced with standard mouse chow (ad libitum) 48 hours prior to behavioral tagging to open a time window of activity-dependent labelling [12, 14]. All subjects were treated in accord with protocol 17–008 approved by the Institutional Animal Care and Use Committee at Boston University.
METHODS DETAILS
Stereotaxic injection and optical fiber implant.
Stereotaxic injections and optical fiber implants follow methods previously reported [11, 12, 14]. All surgeries were performed under stereotaxic guidance and subsequent coordinates are given relative to Bregma (in mm). Mice were mounted into a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) and anesthetized with 3% isoflurane during induction and lowered to 1–2% to maintain anesthesia (oxygen 1L/min) throughout the surgery. Ophthalmic ointment was applied to both eyes to prevent corneal desiccation. Hair was removed with a hair removal cream and the surgical site was cleaned with ethanol and betadine. Following this, an incision was made to expose the skull. Bilateral craniotomies involved drilling windows through the skull above the injection sites using a 0.5 mm diameter drill bit. Coordinates were −2.2 anteroposterior (AP), ±1.3 mediolateral (ML), and −2.0 dorsoventral (DV) for dorsal dentate gyrus (DG) [12]; −3.60 AP, ± 2.5 ML, and −2.6 DV for the ventral DG; and −1.7 AP, ±3.4 ML, and −4.2 DV [36] for basolateral amygdala (BLA). All mice were injected with a volume of 0.2 (dorsal and ventral DG) or 0.3 (BLA) μl of AAV9 cocktail per site at a control rate of 0.7 μl min−1 using a mineral oil-filled 33-gage beveled needle attached to a 10 μl Hamilton microsyringe (701LT; Hamilton) in a microsyringe pump (UMP3; WPI). The needle remained at the target site for five minutes post-injection before removal. For DG targets, a bilateral optic fiber implant (200 μm core diameter; Doric Lenses) were chronically implanted above the injection site (−1.6 DV for dorsal DG; −2.6 for ventral DG). Jewelry screws secured to the skull acted as anchors. Layers of adhesive cement (C&B Metabond) followed by dental cement (A-M Systems) were spread over the surgical site. Mice received 0.1 mL of 0.3 mg/ml buprenorphine (intraperitoneally) following surgery and placed on a heating pad during recovery. Histological assessment verified viral targeting and fiber placement. Data from off-target injections were not included in analyses.
Optogenetic Methods.
Optic fiber implants were plugged into a patch cord connected to a 473 nm blue laser diode controlled by automated software (Doric Lenses). Laser output was tested at the beginning of every experiment to ensure that at least 15 mW of power was delivered at the end of the patch cord (Doric lenses). For acute reactivation studies, mice received 3-min optical stimulation (15 ms pulse width, 20 Hz). For chronic reactivation studies, mice were placed into a neutral context and received a 10-min session with light delivery (15 ms pulse width, 20 Hz) over morning and afternoon session for five consecutive days, as previously reported [6].
Behavioral tagging.
Dox diet was replaced with standard lab chow (ad libitum) 48-hours prior to behavioral tagging. Female exposure [13, 14, 37]: One female mouse (PD 30–40) was placed into a clean home cage with a clear cage top, which was used as the interaction chamber. The experimental male mouse was then placed into the chamber and allowed to interact freely for one hour. Neutral exposure [12]: Mice were placed into a conditioning chamber for one hour. Fear exposure [11, 12]: Mice were placed into a conditioning chamber and received fear conditioning (see below) over a 500-second training session (including exposure to four 0.5 mA foot shocks). Following behavioral tagging, the male mouse was returned to their home cage with access to Dox diet.
Behavioral assays.
All behavior assays were conducted during the light cycle of the day (0700–1900). Mice were handled for 3–5 days, 2 min per day, before all behavioral experiments. Behavioral assays include fear conditioning [11, 12, 14], optogenetic place preference/avoidance (OPP/OPA) [13], female exposure [13, 14], and open field [14] (see below).
Fear Conditioning.
Fear conditioning occurred in one of four mouse conditioning chambers (Coulbourn Instruments, Whitehall, PA, USA) with metal-panel side walls, Plexiglas front and rear walls, and a stainless-steel grid floor composed of 16 grid bars. The grid floor was connected to a precision animal shocker (Coulbourn Instruments, Whitehall, PA, USA) set to deliver a 2-second 0.5 mA foot shock unconditioned stimulus (US). A ceiling-mounted video camera recorded activity and fed into a computer running FreezeFrame3 software (Actimetrics, Wilmette, IL, USA). The software controlled stimuli presentations and recorded videos from four chambers simultaneously. The program determined movement as changes in pixel luminance over a set period. Freezing was defined as a bout of 0.75-sec or longer without changes in pixel luminance and verified by an experimenter blind to treatment groups. Context alterations included changes to spatial, olfactory, tactile, and lighting cues. The conditioning chamber with room lights on was designated as Context A. Context B involved modifications to the conditioning chamber, including vertical black and white strips spaced ~ 3 cm apart obscuring the front and rear walls, an opaque Plexiglas A-frame insert, 1 mL of almond extract in a plastic container positioned below the grid floor, and room lights at 50% illumination. Context C was a custom-build chamber (with Plexiglass walls and floor with horizontal black and white stripes) measuring 17.8 × 16.5 × 14 cm with 1 mL of orange extract in a plastic container position below the grid floor. The chambers were cleaned with 70% ethanol solution prior to animal placement.
For acute reactivation experiments, contextual fear conditioning occurred in one distinct context (i.e., Context A). In chronic reactivation experiments, contextual fear conditioning occurred in two distinct contexts (i.e. Context A and Context B). Briefly, mice were placed into the conditioning context for a 500-second acquisition session, including a 180-second baseline period followed by one (Low Fear) or four (High Fear) 0.5 mA, 2-second foot shock USs (interstimulus interval [ISI] equals 80-sec). Acute reactivation studies involved placement into a novel context and involved 3-minute ‘light off’ and 3-minute ‘light on’ periods. Contextual fear testing for chronic reactivation studies involved placing mice into the conditioning context and assessing freezing over a 5-minute stimulus-free session. Testing occurred in Context A and Context B twenty-four hours apart.
Fear conditioning data are collected using FreezeFrame3 software (Actimetrics, Wilmette IL) with the bout length set at 0.75-sec and the freezing threshold initially set as described in the program instructions. We quantified the expression of fear by assessing bouts of freezing behavior, defined as the absence of movement except that needed for respiration. Freezing behavior in FreezeFrame3 was defined as changes in pixel luminance falling below a threshold. An experimenter blind to treatment groups adjusted the threshold so that freezing behavior involves the absence of all movement except those needed for respiration as previously described. Freezing behavior was scored as the percentage of time spent freezing during a given bout of time. Statistical analyses involved repeated-measures ANOVA with the between subjects the between-subjects factor of Group and the within-subjects factor of Epoch (i.e., light off/light on), Trials, or Context as a within-subjects variables.
Place preference/avoidance.
The testing chamber consisted of a custom-built rectangular box with a fiber optic holder (38 × 23.5 × 42 cm). Red tape divided the chamber down the middle, creating two halves, each with unique designs on each wall. Right and left sides for stimulation were randomized. Day 1 was used to assess baseline levels, during which the mouse was given 10 minutes to freely explore the arena. The following day, mice received light stimulation (15 ms pulses at 20-Hz) upon entry in the designated side of the chamber (counterbalanced across groups) over a 10-minute test period. Once the mouse entered the stimulated side, a TTL signal from the EthoVision software via a Noldus USB-IO Box triggered a stimulus generator (STG-4008, Multi-channel Systems). A video camera (Activeon CX LCD Action Camera) recorded each session and an experimenter blind to treatment conditions scored the amount of time on each side. Statistical analyses involved a one-way ANOVA comparing group difference scores [time (in seconds) on stimulated side minus time on unstimulated side].
Female Exposure.
One female mouse (PD 30–40) was placed into a clean home cage with a clear cage top, which was used as the interaction chamber. The experimental male mouse was then placed into the chamber and allowed to interact freely for five minutes. Interaction was defined as movements initiated by the male towards the female, including sniffing and physical contact [10–11].
Open Field.
Mice were tested in an open field as previously described [14]. Briefly, mice were placed into a 45 cm × 45 cm metal chamber with Plexiglass walls (Omnitech Electronics, Columbus, OH) and allowed to freely explore for 12 minutes. An automated video-tracking system (Ethovision by Noldus) tracked mice during exploration. The tracking software defined the center as a square of 32 cm × 32 cm in the middle of the arena. Total distance traveled, and time spent in the center was quantified and analyzed between groups.
Immunohistochemistry.
Immunohistochemistry follows protocols previously reported [11, 12, 14]. Mice were overdosed with 3% isoflurane and perfused transcardially with cold (4° C) phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Brains were extracted and stored overnight in PFA at 4°C. Fifty μm coronal sections were collected in serial order using a vibratome and collected in cold PBS. Sections were blocked for 1 hour at room temperature in PBST and 5% normal goat serum (NGS) on a shaker. Sections were transferred to wells containing primary antibodies (1:1000 rabbit anti-c-Fos [SySy]; 1:5000 chicken anti-GFP [Invitrogen]) and allowed to incubate on a shaker overnight at 4°C. Sections were then washed in PBST for 10-min (x3), followed by 2-hour incubation with secondary antibody (1:200 Alexa 555 anti-rabbit [Invitrogen]; 1:200 Alexa 488 anti-chicken [Invitrogen]). Following three additional 10-min washes in PBST, sections were mounted onto micro slides (VWR International, LLC). Vectashield Hart Set Mounting Medium with DAPI (Vector Laboratories, Inc) was applied, slides were cover slipped, and allowed to dry overnight.
Cell counting.
The number of eYFP- or c-Fos-immunoreactive neurons in the dentate gyrus were counted to measure the number of active cells during defined behavioral tasks in 3–5 coronal slices (spaced 160 mm from each other) per mouse. Only animals that had accurate bilateral injections in the dentate gyrus were selected for counting. Fluorescence images were acquired using a microscope (Zeise LSM800, Germany) with a 20X objective. All animals were sacrificed 90 minutes post-assay or optical stimulation for immunohistochemical analyses. The number of eYFP-positive, c-Fos-positive, and DAPI-positive cells in a set region of interest (0.5 mm^2 per brain area analyzed) were quantified with ImageJ (https://imagej.nih.gov/ij/) and averaged within each animal. To calculate the percentage of active cells we counted the number of eYFP-positive cells and divided by the total number of DAPI-positive cells. Statistical chance was calculated by multiplying the observed percentage of eYFP-single-positive cells by the observed percentage of c-Fos-single-positive cells; overlaps over chance were calculated as observed overlap divided by chance overlap: the percentage of double-labeled neurons ([eYFP and cFos]/DAPI) were analyzed against overlap expected by chance ([eYFP/DAPI] × [cFos/DAP]) using paired t tests.
QUANTIFICATION AND STATISTICAL ANALYSIS
Sampling strategy.
Subjects were randomly assigned to groups. No statistical methods were used to determine sample size; the number of subjects per group were based on those in previously published studies and are reported in figure captions.
Image Integrity.
Acquired image files (.czi) were opened in ImageJ. Processing of images in Figure 1 involved maximizing intensity, removing outlier noise, and adjusting contrast of images.
Data Analysis.
Data were analyzed using Prism (GraphPad Software, La Jolla, CA) and Statistica 13 data analysis software (TIBCO Software, Inc., Palo Alto, CA). Data were analyzed using paired t-tests (two factors), unpaired t-tests, one-way or two-way ANOVAs with repeated measures ANOVAs (more than two factors), where appropriate. Post-hoc analyses (Newman-Keuls) were used to characterize treatment and interaction effects, when statistically significant (alpha set at p< 0.05, two-tailed). Statistical analyses are reported in figure captions.
DATA AND SOFTWARE AVAILABILITY
For full behavioral datasets and cell counts, please contact the corresponding author Dr. Steve Ramirez (dvsteve@bu.edu).
Supplementary Material
Acute activation of dorsal and ventral HPC engrams drives reward and aversion
The ventral DG is preferentially reactivated in emotionally salient contexts
Chronic activation of HPCl engrams decreases or increases context-specific freezing
Memory enhancement is disrupted when BLA cells processing fear are silenced
ACKNOWLEDGEMENTS
We would like to thank Joshua Sanes and his lab at the Center for Brain Science, Harvard University, for providing laboratory space within which the initial behavioral experiments were conducted, the Center for Brain Science Neuroengineering core for providing technical support, and the Society of Fellows at Harvard University. We would also like to thank Susumu Tonegawa and his lab for providing the activity-dependent virus cocktail. This work was supported by an NIH Early Independence Award (DP5 OD023106–01), a Young Investigator Grant from the Brain and Behavior Research Foundation, a Ludwig Family Foundation grant, and the McKnight Foundation Memory and Cognitive Disorders award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Poppenk J, Evensmoen HR, Moscovitch M, and Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in cognitive sciences 17, 230–240. [DOI] [PubMed] [Google Scholar]
- 2.Fanselow MS, and Dong HW (2010). Are The Dorsal and Ventral Hippocampus functionally distinct structures? Neuron 65, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser MB, Moser EI, Forrest E, Andersen P, and Morris RG (1995). Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America 92, 9697–9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung MW, Wiener SI, and McNaughton BL (1994). Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 14, 7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, and Eichenbaum H (2013). Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman JP, Ostrander MM, Mueller NK, and Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in neuro-psychopharmacology & biological psychiatry 29, 1201–1213. [DOI] [PubMed] [Google Scholar]
- 7.Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, Richards BA, and Kim JC (2017). Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 42, 1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, et al. (2018). Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron 97, 670–683.e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn JJ, Loya F, Ma QD, and Fanselow MS (2005). Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus 15, 665–674. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Krabbe S, Grundemann J, Botta P, Fadok JP, Osakada F, Saur D, Grewe BF, Schnitzer MJ, Callaway EM, et al. (2016). Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell 167, 961–972.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, and Tonegawa S (2012). Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, and Tonegawa S (2013). Creating a false memory in the hippocampus. Science 341, 387–391. [DOI] [PubMed] [Google Scholar]
- 13.Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, and Tonegawa S (2014). Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez S, Liu X, MacDonald CJ, Moffa A, Zhou J, Redondo RL, and Tonegawa S (2015). Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roozendaal B, McEwen BS, and Chattarji S (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10, 423–433. [DOI] [PubMed] [Google Scholar]
- 16.Reijmers LG, Perkins BL, Matsuo N, and Mayford M (2007). Localization of a stable neural correlate of associative memory. Science 317, 1230–1233. [DOI] [PubMed] [Google Scholar]
- 17.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, and Tonegawa S (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99. [DOI] [PubMed] [Google Scholar]
- 18.Leutgeb JK, Leutgeb S, Moser MB, and Moser EI (2007). Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966. [DOI] [PubMed] [Google Scholar]
- 19.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, and Hen R (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, and Hen R (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier BE, Lacagnina AF, Ayoub A, Shue F, Zemelman BV, Krasne FB, and Drew MR (2017). Dentate Gyrus Contributes to Retrieval as well as Encoding: Evidence from Context Fear Conditioning, Recall, and Extinction. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 6359–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madronal N, Delgado-Garcia JM, Fernandez-Guizan A, Chatterjee J, Kohn M, Mattucci C, Jain A, Tsetsenis T, Illarionova A, Grinevich V, et al. (2016). Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nature communications 7, 10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan TJ, Roy DS, Pignatelli M, Arons A, and Tonegawa S (2015). Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roth BL (2016). DREADDs for Neuroscientists. Neuron 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strange BA, Witter MP, Lein ES, and Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15, 655–669. [DOI] [PubMed] [Google Scholar]
- 26.Okuyama T, Kitamura T, Roy DS, Itohara S, and Tonegawa S (2016). Ventral CA1 neurons store social memory. Science 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kheirbek MA, and Hen R (2011). Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, and Hen R (2018). Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng W, Mayford M, and Gage FH (2013). Selection of distinct populations of dentate granule cells in response to inputs as a mechanism for pattern separation in mice. eLife 2, e00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, and Malinow R (2014). Engineering a memory with LTD and LTP. Nature 511, 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, and Klausberger T (2015). Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 348, 560–563. [DOI] [PubMed] [Google Scholar]
- 32.Bouton ME, Trask S, and Carranza-Jasso R (2016). Learning to inhibit the response during instrumental (operant) extinction. Journal of experimental psychology. Animal learning and cognition 42, 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maren S (2011). Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron 70, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, and Luthi A (2008). Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606. [DOI] [PubMed] [Google Scholar]
- 35.Pare D, and Duvarci S (2012). Amygdala microcircuits mediating fear expression and extinction. Current opinion in neurobiology 22, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, and Luthi A (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437. [DOI] [PubMed] [Google Scholar]
- 37.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, and Axel R (2011). Driving opposing behaviors with ensembles of piriform neurons. Cell 146, 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For full behavioral datasets and cell counts, please contact the corresponding author Dr. Steve Ramirez (dvsteve@bu.edu).