Abstract
Purpose:
To investigate the age-dependence of Brillouin spectroscopy of the cornea and to compare normal and keratoconus corneas.
Design:
Retrospective case-control study
Study Population:
Healthy patients and patients suffering from keratoconus seen at the Institut für Refraktive und Ophthalmo-Chirurgie (IROC) between December 2016 and March 2017.
Methods:
Brillouin frequency shifts of patients of 2 different groups were examined with Brillouin spectroscopy perpendicular to the corneal surface. Group 1 consisted of 47 healthy eyes, whereas group 2 included 36 eyes with keratoconus of unclear progression. Beside Brillouin examinations, corneal tomographies were acquired so that correlations and comparisons between geometrical parameters and Brillouin frequency shifts could be evaluated.
Main Outcome Measures:
Corneal Brillouin frequency shifts averaged over full corneal thickness.
Results:
A significant correlation between age and central Brillouin frequency shift was identified (p=0.011) with an increase in Brillouin frequency shift of 4MHz per decade in normal corneas. Keratoconus corneas have a significantly reduced Brillouin frequency shift at the thinnest point compared to normal corneas (5.7072 ± 0.0214 vs. 5.7236 ± 0.0146 GHz, p<0.001). The Brillouin frequency shift at the point of maximum posterior elevation showed best correlation with geometry-derived keratoconus indices. The receiver operating characteristic curve analysis of Brillouin frequency shift showed substantially worse sensitivity and specificity compared to Kmax and thinnest pachymetry for keratoconus detection.
Conclusion:
Non-invasive Brillouin spectroscopy adds clinical information about the biomechanical state of the cornea perpendicular to the surface. An age-dependent stiffening of the cornea has been found and keratoconus corneas are statistically significantly different from normal corneas, but for precise differentiating of keratoconus stages (including normal corneas) the method is currently neither specific nor sensitive enough. Further development including standardized mapping and establishment of new indices may increase the potential of Brillouin spectroscopy substantially.
Introduction
Brillouin spectroscopy introduced in ophthalmology in 19801 has recently been heralded to measure mechanical parameters of the cornea noninvasively2. The detailed information on corneal biomechanics might have a significant impact on diagnosis of corneal diseases and the predictability of corneal surgery such as keratotomies but may also help to interpret the validity of applanation tonometry3.
Brillouin spectroscopy measures the interaction of narrow-banded laser light and phonons in matter. Phonons are wavelets produced by molecular vibrations and occur universally in tissue at room temperature travelling inside matter with a velocity that is related among others to the elastic moduli4. During this interaction, a small portion of the laser light experiences a frequency shift (Brillouin frequency shift) that is proportional to the velocity of the interacting phonons and, therefore, to the square root of the elastic modulus. Measuring the Brillouin frequency shift in a cornea gives a noninvasive access to the so called bulk elastic modulus M of the cornea5. Whether this bulk elastic modulus M, however, gives information about the familiar surface-parallel elastic modulus E (Young`s modulus) is not yet decided6–8.
Keratoconus (KCN) is a chronic disease of the cornea that most probably goes along with an at least localized weakening of the tissue which results in localized bulging outwards9,10. This geometric abnormality is mainly the basis of clinical diagnosis of keratoconus and has been characterized by numerous parameters such as maximal K-reading, corneal coma, astigmatism, other keratoconus indices and the maximum posterior elevation11–14. In contrast, the clinical diagnosis of keratoconus by means of biomechanical minimal-invasive methods, for example with the air puff-analyzers, has so far to be questioned15. Brillouin spectroscopy would be helpful to establish probably shape-independent biomechanical information about a cornea and possibly help to diagnose KCN at an earlier stage.
In this retrospective case-control study, we investigate the Brillouin frequency shift measurements in normal and keratoconus corneas and try to compare and correlate the findings with standard geometrical keratoconus indices obtained from Scheimpflug tomography.
Patients and Methods
Patients
In this retrospective case-control study, two groups of patients were examined: (1) normal eyes to establish a correlation of age and Brillouin frequency shift (n = 47) and (2) patients with KCN irrespective of progression (n= 36). The patients were asked not to use contact lenses during 2 weeks before the examination. The study was performed at the Institut für Refraktive und Ophthalmo-Chirurgie (IROC) in Zürich, Switzerland following approval from the Institutional Review Board (IRB) of Partners HealthCare, the Partners Human Research Committee (PHRC) and the Institutional Review Board of IROC. Informed consent was obtained from every patient before imaging.
Before the Brillouin measurements corneal tomographies were generated using a commercial topographer (Vario, Wavelight, Erlangen, Germany) and a commercial Scheimpflug camera (Pentacam HR 70700, Oculus, Wetzlar, Germany) including the determination of the thinnest point, the point of maximum posterior elevation and keratoconus indices. Besides Brillouin spectroscopy, topography and tomography, the patients received a standard examination consisting of autorefractometry and autokeratometry (Humphrey Model 599, Zeiss, Jena, Germany), anterior segment optical coherence tomography (SS-1000, Tomey, Nagoya, Japan), manifest refraction using the fogging technique, unaided (UDVA) and best spectacle-corrected visual acuity (BSCVA), applanation tonometry, endothelial cell count (SP-02, Construzione Strumenti Oftalmici, Scandicci Firenze, Italy) and slit lamp examination.
Group 1
For normal corneas, 5 axial scans were taken in the central cornea within a radius of 1 mm from the photopic entrance pupil center. From subjects having both eyes scanned (n=41), only one eye was randomly assigned and included. Excluded from the normal group were eyes with any pathology or surgery of the anterior segment (including glaucoma), minimal corneal thickness of less than 500 μm or more than 600 μm, maximal K-readings of less than 40D and more than 47D, and an age of less than 18 years.
Group 2
For keratoconus patients, 20 to 40 axial scans were taken at different locations including the point of maximal curvature, the thinnest point and the point of maximum posterior elevation. The KCN was classified according to Amsler-Krumeich16. Excluded from the KCN-groups were eyes with classical pellucid marginal degeneration, previous anterior segment-surgery including corneal crosslinking (CXL), corneal trauma, glaucoma, aphakia, endothelial cell counts less than 2300 cells/mm2, corneas with no distinctive maximum posterior elevation point, corneal scars that may interfere with Scheimpflug photography, history of recurrent erosions, patients younger than 18 years, pregnancy and breast feeding, and neurodermitis. If both eyes of a patient fulfilled the inclusion criteria, one eye was randomly assigned and included.
Brillouin spectroscopy
Data were acquired with a custom-made mobile, clinically viable Brillouin scattering microscopy system representing the prototype of the BOSS (Brillouin optical scanning system, Intelon Optics, Boston, MA). The light source is a single-frequency tunable laser with its output spectrum locked to a near-infrared wavelength of ~780 nm. The laser light is coupled via a polarization-maintaining single-mode fiber to the human interface, in which polarization optics route the laser beam to the eye and direct backscattered light to a single-mode fiber. The spectrometer employs two-stage VIPA (virtually imaged phase arrays) etalons to achieve a free-spectral range (FSR) of ~ 16 GHz, a resolution of ~0.3 GHz, and an extinction efficiency of −65 dB. The optical power at the cornea is 3–5 mW, which is several times lower than the maximum permissible exposure level according to American National Standard Institutes (ANSI) guidelines. With an electron multiplying charge-coupled device (EMCCD, Andor Technology, Belfast, UK) with an integration time of 0.2 s, a shot-noise-limited sensitivity of ~ ±0.008 GHz in Brillouin frequency shift measurement is achieved. During Brillouin frequency shift measurement, the subject sits with their chin and forehead resting in the human interface headrest, directing their gaze towards a fixation target. For corneal scans, an operator adjusts the human interface using a manual joystick to locate the focus at a desired location in front of the cornea while monitoring an eye-tracking video camera based on pupil detection. The coordinates of special locations, like thinnest point or point of the maximum posterior elevation, were taken from Scheimpflug imaging. Axial scanning from anterior surface to aqueous humor in the anterior chamber is accomplished automatically by a motorized stage carrying the objective lens and thus the light focus. Each axial scan comprises 40 points separated by a step size of 35 μm. The EMCCD exposure time for each step is typically 300 ms, resulting in a total axial scan time of about 12 s. Data points of one axial scan were averaged resulting in one data point in the corresponding xy-position with respect to the pupil center. Reconstructed Brillouin frequency shift maps consisted of at least 40 averaged axial scans using linear interpolation with a step size of 0.1 mm. Axial scanning was carried out with a transverse resolution of ~5 μm. The average total measurement time per eye was less than 15 minutes. The schematic setup is illustrated in Figure 1.
Figure 1:
Schematic of the Brillouin system. The system is composed of three parts: a light source, a human scanning interface built on a modified slit-lamp platform, and a two-stage VIPA spectrometer using two crossed-axis VIPA etalons. Human interface: PMF: polarization maintaining single-mode fiber; SMF: single mode fiber; λ/2: half waveplate; λ/4: quarter-waveplate; M: mirror; Obj. L: objective lens; PBS: polarizing beam-splitter; S1/S2: optical shutters; Ref: reference materials; Spectrometer: C1/C2: cylindrical lens; A1/A2: achromatic lens; S1/S2: shutters; V1/V2: VIPAs; EMCCD: electron multiplying charge-coupled device;
In 3 healthy and 3 keratoconus subjects the intra-individual repeatability of the Brillouin frequency shift was measured to ±0.01 GHz (range, 5 measurements, central measurement corresponding to the xy-position of the entrance pupil center) close to the theoretical limit of ~ ±0.008 GHz.
Numerical analysis
Brillouin shifts were reported as average ± standard deviation, demographic parameters were presented as average and range. The correlation of central Brillouin frequency shift and age in the normal group as well as keratoconus parameters in the keratoconus group was established by means of the Spearman rank correlation and two-sided significance testing using SPSS (IBM, Armonk, NY). The Bonferroni-correction was used to avoid accidental significances. The comparison of parameters in different groups was performed by means of the Mann-Whitney U-test. Receiver operating characteristic (ROC) curves were accepted to determine the predictive accuracy of corneal parameters (Kmax, thinnest pachymetry) and Brillouin frequency shift at the point of maximum posterior elevation. These curves are obtained by plotting sensitivity versus 1 - specificity. Except the correlation-testing, all other calculations were performed with WinSTAT for Excel (R. Finch Software, 2015). Statistical significance was accepted if p<0.05.
Results
Group 1: Central Brillouin frequency shift in normal corneas
The age of subjects included ranged from 18 to 82 years with an average of 39.0 years and a gender distribution of 22:25 (f:m). The central Brillouin frequency shift Bcentral ranged from 5.6977 to 5.7582 GHz with an average of 5.7242 ± 0.0160 GHz. Age and central Brillouin frequency shift Bcentral were correlated with a correlation coefficient of 0.369 and a two-sided p-value of 0.011 which declares a statistical significance (Figure 2). Regression analysis using linear, polynomial and exponential fits yielded the highest regression coefficient R=0.355 for the linear approach. The slope of the linear regression line is 0.0041 GHz per decade equivalent to 0.07% per decade.
Figure 2:
Brillouin frequency shifts of healthy corneas from patients aged between 18 and 82 years illustrating a significant correlation (p=0.011). Linear regression reveals an increase in Brillouin frequency shift of 4 MHz per decade.
In healthy subjects having both eyes scanned (n=41), the mean Brillouin frequency shifts were nearly identical for right and left eye: 5.7252 ± 0.0167 versus 5.7222 ± 0.0159 GHz within the instrument’s resolution (≤0.01 GHz). The difference between the left and right eyes of each individual subject is narrowly gaussian-like distributed (Figure 3) with an average difference of 0.0030 GHz. The bilateral symmetry between the two eyes of healthy subjects is remarkable, totally in contrast to the large interpersonal variability.
Figure 3:
Difference in Brillouin frequency shifts between right and left eyes in healthy subjects showing a gaussian-like distribution.
Within each axial scan a subtle gradient in Brillouin frequency shift was observed, higher in the anterior stroma decreasing towards posterior stroma (Figure 4, left). A representative axial scan as well as a central reconstructed map of multiple averaged axial scans are depicted in Figure 4.
Figure 4:
(left) A representative axial (depth) scan profile of measured frequency shifts across the cornea and anterior chamber of a healthy subject and (right) a typical Brillouin frequency shift map of a healthy cornea.
Group 2: Brillouin frequency shift in keratoconus corneas
Thirty-six eyes of 36 patients were included in the study. The age ranged from 18 to 63 years with an average of 35.3 years and a gender distribution of 12:24 (f:m). After matching the normal group to the keratoconus group regarding sex (12:24 vs. 12:24) and age (35.4 ± 9.2 years vs. 35.3 ± 11.4 years) the central Brillouin frequency shift Bcentral = 5.7236 ± 0.0146 GHz, which is equivalent to the thinnest point in normal corneas (within the measurement error), could be compared with the Brillouin frequency shift at the thinnest point Bthin = 5.7072 ± 0.0214 GHz of keratoconus corneas. The difference is highly statistically significant (p<0.001). Correlation coefficients and their significances between Brillouin frequency shifts at the points of minimal thickness Bthin or the maximum posterior elevation BMPE and keratoconus parameters (keratoconus index KI, minimal corneal thickness, maximal keratometry Kmax, Amsler-Krumeich stages) are listed in Table 1. This indicates that the bulk modulus at the maximum posterior elevation is better related to specific keratoconus parameters compared to the bulk modulus at the thinnest point. No correlation was found with age, neither with Bthin (p=0.410) nor with BMPE (p=0.682). In Figure 5, a representative keratoconus Brillouin map with corresponding axial curvature map and posterior elevation map is illustrated.
Table 1:
Correlations and their significances of keratoconus parameters and Brillouin frequency shifts.
| Bthin [GHZ] | BMPE[GHz] | |
|---|---|---|
| Kmax [D] | r = −0.20 (p=0.272) | r = −0.43 (p=0.039)* |
| Minimal corneal thickness [μm] | r = 0.34 (p=0.056) | r = 0.53 (p=0.010)* |
| Keratoconus index (KI) | r = −0.11 (p=0.5663) | r = −0.48 (p=0.020)* |
| Amsler-Krumeich (AK) stage | r = −0.16 (p=0.399) | r = −0.47 (p=0.023)* |
Kmax= maximal keratometry, Bthin = Brillouin frequency shift at the thinnest point of the cornea, BMPE = Brillouin frequency shift at the point of maximum posterior elevation (* indicating significance p<0.05).
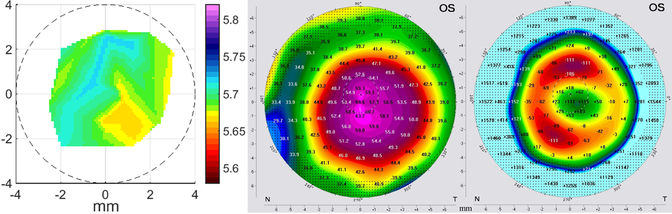
Figure 5:
A representative keratoconus map (left) is depicted with the corresponding axial curvature (middle) and posterior elevation (right, 8mm best-fit-sphere). The Brillouin frequency shift map is centered on the photopic entrance pupil. Curvature and posterior elevation maps are centered on the corneal apex. The pupil is illustrated by the dashed circle, the cross is representing the center of the pupil.
Subclassification according to Amsler-Krumeich within the keratoconus group resulted in average Brillouin frequency shifts at the point of maximum posterior elevation of 5.7113 ± 0.021 GHz for stage 1 and 2 and 5.6970 ± 0.0190 GHz for stage 3 and 4. Compared to the matched healthy group these shifts differ significantly (p=0.009 for stage 1 and 2 resp. p<0.001 for stage 3 and 4). Between both keratoconus groups a significant difference was observed (p=0.016). This is illustrated in Figure 6.
Figure 6:
Brillouin frequency shifts at the point of thinnest pachymetry in normal corneas and the point of maximum posterior elevation in keratoconus corneas. Keratoconus corneas were subdivided into 2 groups by the Amsler-Krumeich (AK) classification, first group AK stage 1 and 2, second group AK stages 3 and 4 (- indicating the average, * indicating significance p<0.05, ** indicating high significance p<0.01). It might be interesting that the outlayer in the AK >2 group had a central keratoconus.
The ROC curves of Kmax, thinnest pachymetry and Brillouin frequency shift are depicted in Figure 7. The Brillouin frequency shift curve shows a reduced potential to detect keratoconus compared to Kmax and thinnest pachymetry.
Figure 7:
Comparison of receiver operating characteristic (ROC) curves of the single parameters Kmax (green line), thinnest pachymetry (red line) and Brillouin frequency shift (blue line). The Brillouin frequency shift curve shows a reduced potential to detect keratoconus compared to Kmax and thinnest pachymetry.
Discussion
The main findings of this study are:
The Brillouin frequency shift (and consequently the bulk modulus) of normal corneas increases with age with an average rate of approximately 4 MHz per decade.
Ectatic areas of keratoconus corneas have a highly significantly smaller Brillouin frequency shift than central normal corneas.
At the point of maximum posterior elevation Brillouin frequency shift correlates significantly with geometrical keratoconus indices.
The stiffness of the human cornea increasing with age reflects our daily experience as corneal surgeons: younger corneas are softer compared to corneas of elderly patients. Since a century it is known that ocular rigidity increases with age17,18. Aging of living connective tissue generally goes along with increased stiffness (or decreased elasticity) and this effect has been seen in sclera19, tendon20, skin21, and cornea22. Elsheikh et al. found a substantial increase of the tangential elastic modulus in 80-year-old corneas compared to 40-year-old corneas23 corresponding to an increase of approximately 15% per decade. The age-related increase Brillouin frequency shift measured here was 0.07% per decade, which is at least two orders of magnitude less compared to surface-parallel elastic modulus. Does the Brillouin frequency shift reflect biomechanical parameters that are different from the familiar biomechanical transverse moduli measured by extensiometry? Reiss et al. state that neither Young`s elastic modulus (E) nor the shear modulus (G) can be determined from measurements of the surface-perpendicular Brillouin frequency shift6. Also, Lepert et al. believe that at the current state of knowledge it is not appropriate to convert the measured Brillouin frequency shifts into mechanical moduli7. In contrast, Scarcelli et al. claim that the bulk modulus determined from the Brillouin frequency shift in the cornea is related to conventional Young`s (or shear) moduli through a log-log linear relationship24, however, this was shown only for the surface-perpendicular compression modulus in porcine crystalline lens nuclei5. The lens nucleus may be considered an isotropic matter and to transfer this technique to cornea may not be correct because the cornea is highly anisotropic. The lamellar ultrastructure of the cornea demands different elastic moduli regarding surface-parallel in contrast to surface-perpendicular measurements. Recently, we could show that the Brillouin frequency shift of the rabbit cornea (and, therefore, the bulk modulus) is independent of surface-parallel stress up to an intraocular pressure of 75 mmHg8. To prevent misinterpretations, we report here about Brillouin frequency shift rather than about Brillouin bulk modulus.
Kohlhaas and coworkers found a 2.7-fold increase between the anterior half of human corneas compared to the posterior half using tangential extensiometry25. Also Brillouin spectroscopy finds a higher value in the anterior stroma compared to posterior stroma, however, only with a minor difference. Again, Brillouin spectroscopy is measuring the surface-perpendicular bulk modulus which may not be confused with the surface-parallel elastic modulus.
The difference in Brillouin frequency shift between fellow eyes was tested in 41 patients. The average difference was comparable to the measurement error of the device and, is therefore, negligible. This is in substantial contrast to the much higher interindividual variability (Figure 2) and may indicate that the bulk modulus represents a personal parameter, characterizing both eyes simultaneously in heathy individuals.
Scarcelli et al. found in ex vivo-experiments that keratoconus corneas show a smaller bulk modulus compared to normal corneas26. In the present study the difference between normal and KCN-corneas is highly significant, however, the absolute difference of the Brillouin frequency shifts is only in the order of the standard deviation of the two groups. Therefore, one single measurement of Brillouin frequency shift is neither specific nor sensitive enough to allow a diagnostic discrimination between KCN and normal cornea. This is underlined by the ROC curves that demonstrate the weaker potential of Brillouin frequency shift compared to Kmax and thinnest pachymetry. On the other hand, parameters like thickness or curvature in one single point alone is generally not a good measure and it is the overall geometric distribution of values that defines diagnosis. The next generation of clinical Brillouin spectrometers will have a scanner and eye tracker system to facilitate reproducible Brillouin maps of the cornea. As mentioned before, the current Brillouin spectrometers employ the measurements in surface-perpendicular direction, however, the clinically relevant elastic modulus is determined in a surface parallel plane. Therefore, the measurement direction of a clinically useful Brillouin spectrometer will have to be also surface-parallel.
Another approach to measure ocular biomechanics is the air puff-induced deformation of the corneal shape. Although sclera, IOP and other extraocular tissues are contributing to the deformation behavior of the cornea27, parameters were determined that allow an assessment of corneal biomechanics in vivo. Recently, Vinciguerra and others were able to distinguish keratoconus corneas from healthy corneas using the Corvis ST device28, 29 with a sensitivity of 94.1% and a specificity of 100%. Whereas the deformation-based analysis of the cornea does not yield spatially resolved data, Brillouin spectroscopy offers the spatial resolution at different points of the cornea. On the other hand, the current technique of Brillouin spectroscopy is not capable to separate early keratoconus corneas from normal corneas by 1 measurement value because of a significant overlap (Figure 6).
Clinically accepted keratoconus parameters like minimal thickness or Kmax correlate with the Brillouin frequency shift and the most significant correlation was obtained for Brillouin frequency shifts measured over the area around the maximum posterior elevation. Brillouin frequency shifts at the point of minimal thickness or, even worse, the point of Kmax correlated much weaker indicating that near the point of maximum posterior elevation the cornea is softest. This is supported by the fact that in keratoconus the epithelium thickness modulates corneal thickness as well as anterior curvature30. The epithelium is thinner in th0e center of the ectasia and thicker around the ectasia with the potential of masking the stromal point bulging forward. Since corneal endothelium consists of a single layer of cells31, the posterior surface measured by OCT (and less reliable with Scheimpflug photography) indicates the locus of maximal protrusion and consequently the weakest area of the cornea at best.
Beside the limitation arising from surface-perpendicular instead of surface-parallel measurements, the are other limitations that need to be addressed: (1) the tomographic shape of the cornea need to be known prior to Brillouin spectroscopy in order to find the thinnest point or the point of maximum posterior elevation (2) the total measurement time of 15 minutes per cornea is too long for a clinical routine (3) the measurement location on the cornea need to be captured that requires an active eye tracker, (4) the spectral frequency resolution of 0.008 GHz is too high compared to the total measurement range of the 0.1 GHz, because this implies only 12 categories of Brillouin frequency shift. In addition, only corneas with a distinctive maximum in posterior elevation were included which might act a potential bias.
In summary, the non-invasive measurement of the Brillouin frequency shift in cornea adds clinical information about the surface-perpendicular biomechanical state of a cornea. Although the response of keratoconus corneas is statistically different from normal corneas, a differential diagnosis is currently not possible because of the lack of specificity and sensitivity.
Acknowledgements/Disclosure
Funding/financial support: The project was funded by the U.S. National Institutes of Health (R01EY025454, P41EB015903) and the Harvard Catalyst Incubator program (UL1-RR025758).
Financial disclosures: Dr. Yun, Dr. T. Seiler and Dr. P. Shao are scientific consultants of Intelon Inc, Boston, MA. Dr. T. Seiler is also a scientific consultant of Avedro Inc, Waltham, MA. No conflicting relationship exists for any other author.
Other Acknowledgements: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaughan JM, Randall JT. Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature. 1980;284:489–491 [DOI] [PubMed] [Google Scholar]
- 2.Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Invest Ophthalmol Vis Sci. 2012;53:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155 [DOI] [PubMed] [Google Scholar]
- 4.Brillouin L Diffusion de la lumière et des rayons X par un corps transparent homogène-Influence de l’agitation thermique. Ann. Phys 1922;9:88–122 [Google Scholar]
- 5.Scarcelli G, Kim P, Yun SH. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys J. 2011;101:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiß S, Burau G, Stachs O et al. Spatially resolved Brillouin spectroscopy to determine the rheological properties of the eye lens. Biomed Opt Express. 2011;2:2144–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepert G, Gouveia RM, Connon CJ, Paterson C. Assessing corneal biomechanics with Brillouin spectro-microscopy. Faraday Discuss. 2016;187:415–428 [DOI] [PubMed] [Google Scholar]
- 8.Seiler TG, Shao P, Frueh BE, Yun SH, Seiler T. The influence of hydration on different mechanical moduli of the cornea. Graefes Arch Clin Exp Ophthalmol. 2018;;256:1653–1660 [DOI] [PubMed] [Google Scholar]
- 9.Horner JF. Zur Behandlung des Keratoconus. Klin Monbl Augenheilkd. 1869;5:24–26. [Google Scholar]
- 10.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–441 [DOI] [PubMed] [Google Scholar]
- 11.Belin MW, Ambrósio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013;61:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda N, Klyce SD, Smolek MK. Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. 1995;113:870–874 [DOI] [PubMed] [Google Scholar]
- 13.Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11:371–379 [DOI] [PubMed] [Google Scholar]
- 14.Randleman JB, Dupps WJ Jr, Santhiago MR, et al. Screening for Keratoconus and Related Ectatic Corneal Disorders. Cornea. 2015;34:e20–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vellara HR, Patel DV. Biomechanical properties of the keratoconic cornea: a review. Clin Exp Optom. 2015;98:31–38 [DOI] [PubMed] [Google Scholar]
- 16.Amsler M Kératocõne classique et kératocône fruste; arguments unitaires. Ophthalmologica. 1946;111:96–101 [DOI] [PubMed] [Google Scholar]
- 17.Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024 [Google Scholar]
- 18.Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Tsilimbaris MK. Ocular rigidity in living human eyes. Invest Ophthalmol Vis Sci. 2005;46:409–414 [DOI] [PubMed] [Google Scholar]
- 19.Friberg TR, Lace JW. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988;47:429–436 [DOI] [PubMed] [Google Scholar]
- 20.Gillis C, Pool RR, Meagher DM, Stover SM, Reiser K, Willits N. Effect of maturation and aging on the histomorphometric and biochemical characteristics of equine superficial digital flexor tendon. Am J Vet Res. 1997;58:425–430 [PubMed] [Google Scholar]
- 21.Biniek K, Kaczvinsky J, Matts P, Dauskardt RH. Understanding age-induced alterations to the biomechanical barrier function of human stratum corneum. J Dermatol Sci. 2015;80:94–101. [DOI] [PubMed] [Google Scholar]
- 22.Malik NS, Moss SJ, Ahmed N, Furth AJ, Wall RS, Meek KM. Ageing of the human corneal stroma: structural and biochemical changes. Biochim Biophys Acta. 1992;1138:222–228 [DOI] [PubMed] [Google Scholar]
- 23.Elsheikh A, Geraghty B, Rama P, Campanelli M, Meek KM. Characterization of age-related variation in corneal biomechanical properties. J R Soc Interface. 2010;7:1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarcelli G, Kling S, Quijano E, Pineda R, Marcos S, Yun SH. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest Ophthalmol Vis Sci. 2013;54:1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283 [DOI] [PubMed] [Google Scholar]
- 26.Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest Ophthalmol Vis Sci. 2014;55:4490–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kling S, Marcos S. Contributing factors to corneal deformation in air puff measurements. Invest Ophthalmol Vis Sci. 2013;54:5078–5085 [DOI] [PubMed] [Google Scholar]
- 28.Vinciguerra R, Ambrósio R Jr, Elsheikh A, et al. Detection of Keratoconus with a New Biomechanical Index. J Refract Surg. 2016;32:803–810 [DOI] [PubMed] [Google Scholar]
- 29.Wang YM, Chan TCY, Yu M, Jhanji V. Comparison of Corneal Dynamic and Tomographic Analysis in Normal, Forme Fruste Keratoconic, and Keratoconic Eyes. J Refract Surg. 2017;33:632–638 [DOI] [PubMed] [Google Scholar]
- 30.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604–610 [DOI] [PubMed] [Google Scholar]
- 31.Maurice DM. The cornea and the sclera, in: Davson H (Ed.), The Eye, 3rd Ed. Vol 1b, Orlando, Academic Press, 1984:1–158 [Google Scholar]