Abstract
Objective:
Mortality from pediatric sepsis has steadily declined over the past several decades, however little is known about morbidity among survivors. We aimed to determine the prevalence of and risk factors for failure to recover to baseline health-related quality of life following community-acquired pediatric sepsis.
Design:
Retrospective cohort study
Setting:
Seattle Children’s Hospital
Patients:
Children aged 1 month to 21 years admitted to the inpatient wards or intensive care units from 2012–2015 who met 2005 consensus sepsis criteria within 4 hours of hospitalization, and were enrolled in the hospital’s Outcomes Assessment Program with baseline, admission, and post-discharge health-related quality of life data available
Interventions:
None
Measurements and Main Results:
We assessed health-related quality of life with the Pediatric Quality of Life Inventory™ for pre-admission baseline, admission, and post-discharge (median 31 days) status. We determined associations between patient and illness characteristics with failure to recover within 4.5 points of baseline at follow-up (the minimum clinically significant difference between two scores). Of 790 patients, 23.8% failed to recover to baseline health-related quality of life at follow-up. Factors associated with failure to recover were septic shock, older age, private insurance, complex chronic disease, immune compromise, central nervous system infection or bacteremia, intensive care unit admission, and longer length of stay. On multivariable analysis controlling for time to follow-up, failure to recover was independently associated with septic shock (relative risk 1.79, 1.24–2.58), older age (RR 1.02/year, 95% CI 1.01–1.05), immune compromise (RR 1.83, 1.40–2.40), and length of stay (RR 1.03/day, 1.01–1.04).
Conclusions:
Nearly one-quarter of children surviving hospitalization for community-acquired sepsis experienced a clinically significant deterioration in health-related quality of life. We identify risk factors for poor outcomes following sepsis and highlight the need for ongoing evaluation and treatment by primary and specialty care providers for pediatric sepsis survivors after hospital discharge.
Keywords: Child, risk factors, quality of life, sepsis, outcome assessment, intensive care units
Introduction
Mortality from pediatric sepsis has declined steadily over the past half-century to a case-fatality rate for severe sepsis in North America of just under 10% (1–4). The prevalence of pediatric sepsis, meanwhile, appears to be increasing (3–5); thus the number of children surviving sepsis is rising dramatically. Little is known, however, about morbidity among survivors or which factors most strongly influence post-discharge outcomes. Recent studies demonstrate that the effects of sepsis can extend well beyond the initial hospitalization, with some children facing long-term disability, hospital readmission, and late mortality (2,6–7).
Increasingly, health-related quality of life (HRQL) has been proposed as an important measure of health outcomes (8–9). HRQL encompasses the impact of health status on physical, mental, emotional, and social functioning (10). While impaired HRQL has been demonstrated in children following critical illness in general (8,11–15), evidence of the impact of sepsis on HRQL is limited. HRQL scores were lower among children surviving meningococcemia (16) and meningitis (17) compared to healthy children, while a study of children surviving septic shock demonstrated no HRQL differences compared to population norms (18). There are no data assessing longitudinal HRQL changes following sepsis among children compared to their own baseline status, and no data assessing post-discharge outcomes across the range of sepsis severity.
The aims of this study were to evaluate the prevalence of HRQL deterioration after hospitalization for community-acquired pediatric sepsis, severe sepsis, and septic shock, and to determine which factors are associated with children failing to recover to their own baseline HRQL after discharge. We hypothesized that failure to recover HRQL status would be associated with pre-existing comorbidities and sepsis severity.
Materials and Methods
This was a retrospective cohort study of patients with community-acquired sepsis admitted to Seattle Children’s Hospital (SCH) who were enrolled in the SCH Outcomes Assessment Program (OAP), which measures baseline, admission, and post-discharge HRQL on a sample of SCH inpatients. It was approved by the SCH Institutional Review Board.
Participants:
We included patients aged 1 month to 21 years who were enrolled in the OAP and were admitted with concern for sepsis to the inpatient wards or ICUs from 01/01/12–12/31/15. Only index admissions for sepsis during the study period were included. Patients were excluded if their diagnosis was ultimately determined to be non-infectious or if they did not have complete HRQL data available for analysis.
Outcomes Assessment Program:
The SCH OAP measures HRQL using the Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL™) (19). OAP staff approach parents within 72 hours of admission to assess their child for baseline (status during the month prior to admission) and admission HRQL. Children ≥8 years old can complete a self-report, from which an average parent-child score is generated. Families are then contacted electronically or via telephone 2–6 weeks following hospital discharge to complete a follow-up PedsQL™. OAP staff attempt to contact families for up to 6 weeks following the initial attempt, and thus follow-up data are typically obtained 2–12 weeks following discharge; 5% of patients in our cohort completed follow-up assessments after 12 weeks. Approximately 80% of families who are approached consent to participate, and approximately 50% of consented families complete all three assessments. Patients without PedsQL™ data from all three time-points were excluded from our analysis.
Sepsis identification:
We identified patients meeting 2005 pediatric consensus sepsis criteria (20–21) during their Emergency Department stay or the first four hours of a direct ICU admission via electronic health record (EHR) query. Patients with sepsis are not eligible for direct admission to the inpatient wards. Patients had to have concern for infection and abnormal age-specific vital signs and laboratory criteria consistent with the consensus criteria. We identified concern for infection by the presence of bacterial, viral, or fungal testing, regardless of result, or administration of antimicrobials. We performed individual chart review for patients meeting sepsis criteria to determine the final diagnosis based on microbiology data and provider documentation. Patients with a final diagnosis that was not infectious in etiology were excluded from analysis.
HRQL assessment:
The PedsQL™ has been used to evaluate more than 750,000 children internationally (19,22–24) and has demonstrated reliability, sensitivity, and validity for the entire spectrum of pediatric ages (23–25). It has been validated in the pediatric inpatient population (26) and the pediatric ICU (27), with established reliability of parent proxy-reporting (23) and use of recall to evaluate change from baseline HRQL status (28–30). There is strong correlation among in-person, telephonic, and electronic modes of administration (28). The PedsQL™ assesses physical, emotional, social, and school functioning. It is scored from 0–100, and the population mean score is 84.1. Physical and psychosocial subscales are also calculated and each scored from 0–100. A change of ≥4.5 points between two scores represents the minimum clinically significant difference (22).
Exposures:
We queried the EHR and local Virtual Pediatric Systems (31) databases to determine demographics, medical history, infection characteristics, and illness severity measures. We used the Pediatric Medical Complexity Algorithm (32) to categorize patients as having non-chronic illness, non-complex chronic illness, or complex chronic illness. We quantified ICU admission severity of illness using the Pediatric Risk of Mortality (PRISM) III score (33), ICU organ failure using highest Pediatric Organ Logistic Dysfunction (PELOD) score (34), and ward acuity using highest Modified Pediatric Early Warning Score (MPEWS) (35).
Outcomes:
The primary outcome was failure to recover to baseline HRQL, defined as a decrease of ≥4.5 PedsQL™ points on the total score from baseline to follow-up assessments, the minimum clinically significant difference between two scores (22). The secondary outcome was the absolute change in total score between baseline and follow-up assessments.
Statistical analyses:
We determined associations between exposures and failure to recover in bivariate analyses, comparing patients with failure to recover to patients with a follow-up score within or above 4.5 points of baseline We conducted a sensitivity analysis using a comparison group of patients who recovered within 4.5 points of baseline but did not demonstrate improvement. We included variables with p≤0.2 on bivariate analyses in a backwards stepwise multivariable generalized linear model in which those with p≤0.05 were maintained in the final model. We determined mean change in score from baseline to follow-up using linear regression, stratified by ICU status to include PRISM, PELOD, and MPEWS scores. We adjusted all multivariable analyses for time to follow-up. We conducted all analyses using Stata/SE 14.2 software (StataCorp LP, College Station, TX).
Results
A total of 790 patients constituted the study cohort (Figure 1). Compared to patients who met screening sepsis criteria but were not enrolled in the OAP or had incomplete PedsQL™ data, patients meeting screening sepsis criteria with complete PedsQL™ data had lower prevalence of complex chronic conditions, septic shock, and ICU admission, with slightly lower MPEWS, PRISM III, and PELOD scores (Table 1).
Figure 1:
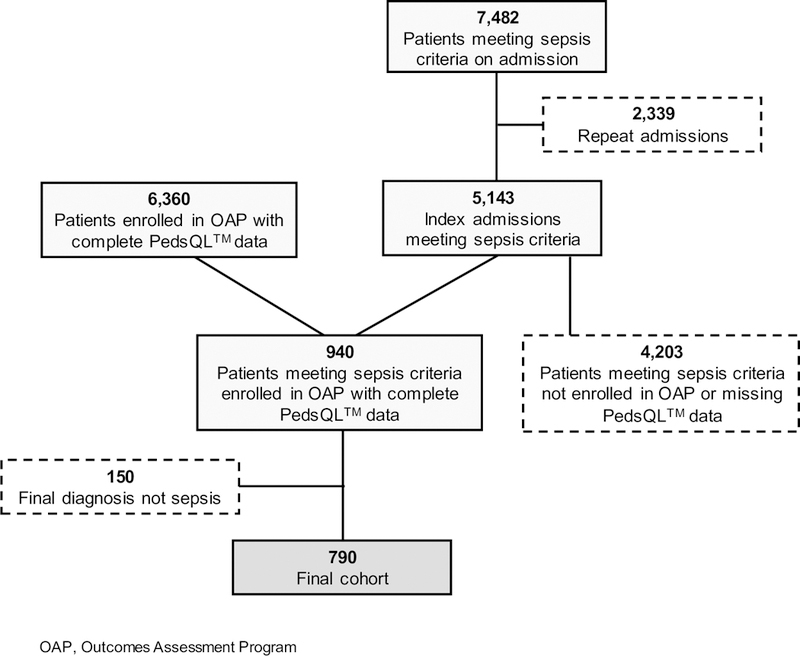
Flowchart of subjects included and excluded from analysis
Table 1:
Characteristics of patients meeting initial screening sepsis criteria who were enrolled in the Outcomes Assessment Program with complete PedsQL™ data compared to patients not enrolled in the Outcomes Assessment Program or with missing PedsQL™ data
| Patient Characteristic | Sepsis with PedsQL™ No. (%) | Sepsis without PedsQL™ No. (%) | p-value |
|---|---|---|---|
| n = 940 | n = 4203 | ||
| Gender, female | 445 (47.4) | 1980 (47.1) | 0.90 |
| Age, years, median (IQR) | 5.9 (1.9–12.4) | 5.6 (1.4–12.8) | 0.09 |
| Race | <0.001 | ||
| White | 557 (59.3) | 2152 (51.2) | |
| Asian | 96 (10.2) | 449 (10.7) | |
| Other | 248 (26.4) | 1373 (32.7) | |
| Unknown | 39 (4.2) | 227 (5.4) | |
| Hispanic Ethnicity | 170 (18.1) | 726 (17.3) | 0.55 |
| PMCA Category | <0.001 | ||
| No chronic disease | 465 (56.0) | 1724 (43.6) | |
| Non-complex chronic disease | 167 (20.1) | 708 (17.9) | |
| Complex chronic disease | 198 (23.9) | 1523 (38.5) | |
| Sepsis category | <0.001 | ||
| Sepsis | 846 (90.0) | 3537 (84.2) | |
| Severe sepsis | 44 (4.7) | 205 (4.9) | |
| Septic shock | 50 (5.3) | 461 (11.0) | |
| ICU admission | 119 (12.7) | 744 (17.7) | <0.001 |
| MPEWS a,b, median (IQR) | 3 (2–4) | 3 (2–5) | 0.02 |
| PRISM IIIc, median (IQR) | 3 (0–6) | 3 (0–9) | 0.03 |
| PELODa,c, median (IQR) | 4 (2–7) | 5 (3–10) | 0.01 |
| ICU LOS, days, median (IQR) | 2 (1–4) | 2 (1–6) | 0.19 |
| Hospital LOS, days, median (IQR) | 3 (2–6) | 3 (2–6) | 0.17 |
Abbreviations: IQR, interquartile range; PMCA, Pediatric Medical Complexity Algorithm; MPEWS, Modified Pediatric Early Warning Score; PRISM, Pediatric Risk of Mortality; PELOD, Pediatric Organ Logistic Dysfunction; LOS, Length of stay
Highest of admission
Calculated for patients admitted to hospital ward
Calculated for patients admitted to ICU
Failure to recover to baseline HRQL at follow-up occurred in 23.8% of patients (n=188), while 34.9% (n=276) recovered to baseline and 41.5% (n=328) improved to ≥4.5 PedsQL™ points above baseline. Patients with HRQL improvement were more likely to have chronic illnesses and be immunosuppressed compared to patients who recovered within 4.5 points of baseline; they also had longer time to follow-up (Supplemental Table S1). A similar proportion of patients without sepsis who were enrolled in the OAP failed to recover (24.1%), but with earlier median follow-up than the sepsis cohort. By 60 days (±10) from discharge, 20.4% of sepsis patients had failure to recover versus 16.4% of non-sepsis patients, while 52.0% of sepsis patients and 59.0% of non-sepsis patients had HRQL improvement.
The median PedsQL™ scores for the entire sepsis cohort were 88.5 (interquartile range [IQR] 76.4–95.3) at baseline, 51.9 (35.9–71.6) at admission, and 93.6 (79.8–98.7) at follow-up (Figure 2). The median baseline score of sepsis patients who failed to recover to their baseline status after discharge was similar to the overall sepsis cohort (90.6, IQR 80.7–96.1), but fell further at admission to a median score of 42.8 (30.2–61.4) with a median follow-up score of 71.1 (59.8–82.2), a decline of 19.5 points from baseline.
Figure 2:
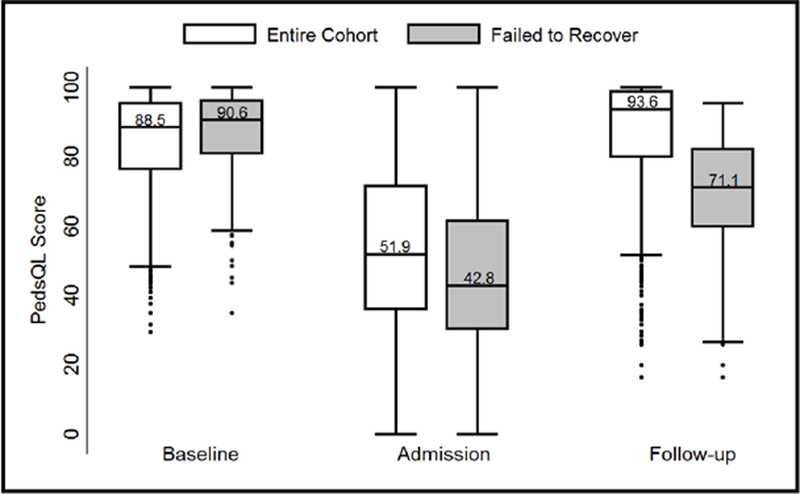
Distribution of PedsQL™ scores at baseline, admission, and follow-up assessments for the entire cohort and for those who failed to recover to baseline health-related quality of life. Box-plots demonstrate median and interquartile range, with whiskers representing upper and lower adjacent values (1.5x the interquartile range) and dots representing outliers.
Among the entire sepsis cohort, there was no significant change from baseline to follow-up scores on either the physical or psychosocial subscales (Figure 3). The group of patients who recovered to baseline experienced median increases of 3.1 points on the physical subscale (IQR 0.0–12.5) and 6.9 points on the psychosocial subscale (0.0–16.7). The group of patients who failed to recover experienced a median physical subscale decline of 18.8 points (−34.4–−9.4) and a median psychosocial subscale decline of 10.2 points (−20.0–−3.3).
Figure 3:
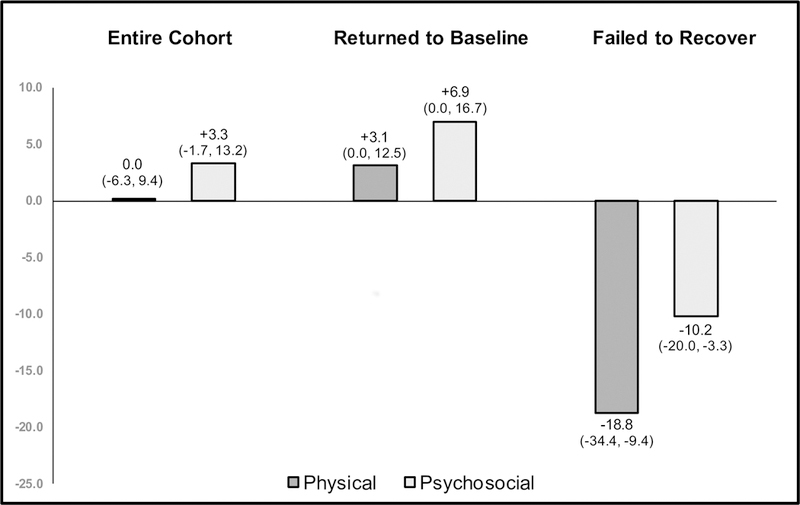
Median change in PedsQL™ by subscale for the entire cohort and stratified by those who returned to baseline health-related quality of life and those who failed to recover. Interquartile ranges are represented in parentheses.
We evaluated factors associated with failure to recover by comparing patients with sepsis who did and did not recover across four categories of potential risk factors: demographics, medical history, infection characteristics, and illness severity (Table 2). Demographic factors associated with failure to recover included older age and private insurance. Age ≥12 years was associated most strongly with failure to recover compared to other age categories, with 30.1% failing to recover to baseline. Older patients were more likely to have complex chronic conditions, immune compromise, and septic shock (p<0.001). Patients with private insurance were older than those with public insurance (p=0.005), but did not differ on other measures.
Table 2:
Bivariate analyses of associations between risk factors and failure to recover to baseline health-related quality of life
| Risk Factor | Failed to recover, No. (%) 188 (23.8) | Returned to baseline, No. (%) 602 (76.2) | p-value | |
|---|---|---|---|---|
| Demographics | Male Gender | 107 (25.4) | 314 (74.6) | 0.25 |
| Age, years, median (IQR) | 7.5 (2.6–13.0) | 4.8 (1.5–10.7) | 0.002 | |
| Race | 0.32 | |||
| White | 121 (25.9) | 346 (74.1) | ||
| Asian | 19 (22.6) | 65 (77.4) | ||
| Other | 48 (20.0) | 191 (80.0) | ||
| Hispanic Ethnicity | 30 (21.0) | 113 (79.0) | 0.62 | |
| English Language | 172 (24.4) | 533 (75.6) | 0.25 | |
| Parent Education | 0.34 | |||
| College degree | 94 (26.1) | 266 (73.9) | ||
| High school graduate | 77 (21.9) | 275 (78.1) | ||
| Some high school | 14 (20.0) | 56 (80.0) | ||
| Public Insurance | 114 (26.6) | 314 (73.4) | 0.04 | |
| History | PMCA Category | 0.005 | ||
| No chronic disease | 103 (23.1) | 343 (76.9) | ||
| Non-complex chronic disease | 26 (16.7) | 130 (83.3) | ||
| Complex chronic disease | 59 (31.4) | 129 (68.6) | ||
| Immune Compromised | 46 (40.7) | 67 (59.3) | <0.001 | |
| Indwelling Device Present | 11 (25.0) | 33 (75.0) | 0.85 | |
| Infection Characteristics | Source of Infection | <0.001 | ||
| Blood | 17 (58.6) | 12 (41.4) | ||
| CNS | 9 (47.4) | 10 (52.6) | ||
| Lung | 42 (25.9) | 120 (74.1) | ||
| Intra-abdominal/GI | 42 (22.1) | 148 (77.9) | ||
| Bone/joint | 8 (20.5) | 31 (79.5) | ||
| Soft tissue | 17 (19.8) | 69 (80.2) | ||
| Urinary | 13 (22.0) | 46 (78.0) | ||
| Other/Non-focal | 40 (20.1) | 166 (79.9) | ||
| Positive Culture | 111 (24.3) | 346 (75.7) | 0.70 | |
| Culture Category | 0.81 | |||
| Bacterial/fungal | 66 (25.2) | 196 (74.8) | ||
| Viral | 45 (23.1) | 150 (76.9) | ||
| Culture negative | 77 (23.1) | 256 (76.9) | ||
| Illness Severity | Sepsis Category | <0.001 | ||
| Sepsis | 156 (21.8) | 559 (78.2) | ||
| Severe sepsis | 10 (29.4) | 24 (70.6) | ||
| Septic shock | 22 (53.7) | 19 (46.3) | ||
| ICU Admission | 35 (38.5) | 56 (61.5) | <0.001 | |
| MPEWS a,b, median (IQR) | 3 (2–4) | 3 (2–4) | 0.39 | |
| PRISM IIIc, median (IQR) | 3 (0–6) | 3 (0–5.5) | 0.96 | |
| PELODa,c, median (IQR) | 4 (2–8) | 3 (2–7) | 0.53 | |
| ICU LOS, median (IQR) | 2 (1–3) | 2 (1–4.5) | 0.57 | |
| Hospital LOS, median (IQR) | 4 (2–8) | 3 (2–5) | 0.001 | |
| Weeks to Follow-up, median (IQR) | 4.0 (2.7–5.9) | 4.6 (3.1–6.9) | 0.02 | |
Abbreviations: IQR, interquartile range; PMCA, Pediatric Medical Complexity Algorithm; MPEWS, Modified Pediatric Early Warning Score; PRISM, Pediatric Risk of Mortality; PELOD, Pediatric Organ Logistic Dysfunction; LOS, Length of stay
Highest of admission
Calculated for patients admitted to hospital ward
Calculated for patients admitted to ICU
Components of medical history associated with failure to recover included chronic condition category and immune status. One-third of patients with complex chronic disease did not recover to their baseline HRQL compared to 23.1% of those with no chronic disease and 16.7% of those with non-complex chronic disease (p=0.005). Failure to recover occurred in 40.7% of immunocompromised patients versus 21.0% of non-immunocompromised patients (p<0.001). Patients with complex chronic disease were more likely to be immunocompromised (p<0.001); neither chronic condition category nor immune status were associated with sepsis category.
Source of infection was the only infection characteristic associated with outcome, with failure to recover occurring in 58.6% of patients with primary bacteremia and 47.4% of patients with central nervous system infections (p<0.001). Neither the presence of a positive culture nor whether the sepsis was bacterial, fungal, or viral conferred increased risk.
The category of sepsis at admission was strongly associated with outcome. Failure to recover to baseline HRQL occurred in 53.7% of patients with septic shock, 29.4% of patients with severe sepsis without shock, and 21.8% of patients with sepsis alone (p<0.001). ICU admission was also associated with failure to recover, with 38.5% of ICU patients failing to recover versus 21.9% of those without an ICU stay (p<0.001). Finally, longer hospital length of stay (LOS) was associated with failure to recover, with a threshold of ≥7 days most strongly associated; 44.4% of those with LOS ≥7 days failed to return to baseline compared to 20.5% with LOS <7 days (p<0.001).
Time to follow-up ranged from 14–137 days after hospital discharge (median 31 days, IQR 21–47). The proportion of patients who failed to recover decreased over time up to eight weeks post-discharge, but this trend did not continue among patients with later follow-up assessments (Figure 4). Shorter time to follow-up was associated with a higher likelihood of failure to recover on bivariate analysis.
Figure 4:
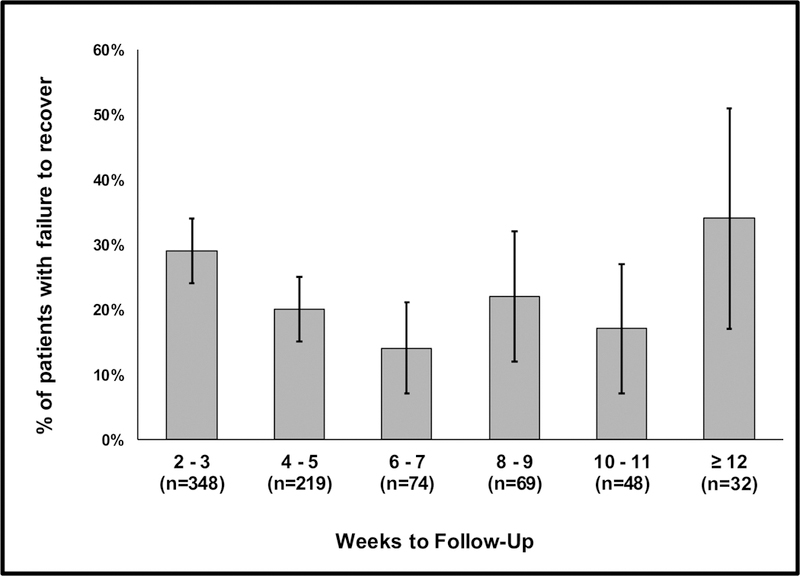
Prevalence of failure to recover to baseline HRQL as a function of time to follow-up (error bars represent 95% confidence intervals). The number of patients sampled during each follow-up time-period is indicated. Shorter time to follow-up was associated with failure to recover on bivariate (p=0.02) but not multivariable analysis.
After controlling for time to follow-up, multivariable analysis demonstrated that older age, immune compromise, septic shock, and longer hospital LOS were independently associated with increased risk of failure to recover to baseline (Table 3). Immune compromise conferred the highest relative risk of 1.83 compared to those without immune compromise (95% CI 1.40–2.40), followed by septic shock with a relative risk of 1.79 compared to sepsis (95% CI 1.24–2.58). Risk of failure to recover increased by 2% per year of age (95% CI 1.01–1.05), and by 3% with each additional hospital day (95% CI 1.01–1.04). Time to follow-up was no longer significantly associated with failure to recover on multivariable analysis. Sensitivity analysis using a comparison group of only patients who recovered within 4.5 points of baseline demonstrated similar bivariate and multivariable results (Supplemental Table S2).
Table 3:
Multivariable analysis of association between risk factors and failure to recover to baseline health-related quality of life
| Risk Factor | Relative Risk | 95% CI | p-value |
|---|---|---|---|
| Age, years | 1.02 / year | 1.01 – 1.05 | 0.04 |
| Immune Status | <0.001 | ||
| Non-compromised | Ref | ||
| Compromised | 1.83 | 1.40 – 2.40 | |
| Sepsis category | 0.006 | ||
| Sepsis | Ref | ||
| Severe sepsis | 1.38 | 0.82 – 2.31 | |
| Septic shock | 1.79 | 1.24 – 2.58 | |
| Hospital LOS | 1.03 / day | 1.01 – 1.04 | 0.002 |
| Weeks to follow-up | 0.96 / week | 0.92 – 1.01 | 0.09 |
Abbreviations: CI, Confidence Interval; LOS, Length of stay
Decrease in PedsQL™ score from baseline to follow-up among ward patients was associated with immune compromise and longer hospital LOS (Supplemental Table S3). Among ICU patients, a decrease in score was associated with older age, immune compromise, soft tissue infection, and longer hospital LOS (Supplemental Table S4).
Discussion
In this study, we provide the first description of the trajectory of children’s HRQL status before, during, and after a hospitalization for sepsis. Previous studies have demonstrated lower HRQL among pediatric ICU survivors (8,14–15) and survivors of meningococcemia (16) and meningitis (17) compared to population norms, but there are no data evaluating HRQL after pediatric sepsis in general, or comparing individual patient HRQL outcomes to their own baseline status. The majority of patients in this four-year cohort of children hospitalized with community-acquired sepsis fully recovered to their baseline status after hospital discharge or even improved, consistent with prior studies of ill and injured children (30,36) and patients without sepsis at our institution. Nearly one-quarter of patients, however, experienced a clinically significant decline in HRQL from their pre-admission baseline to their post-discharge follow-up. The median persistent declination in this group was 19.5 points below baseline, representing a decline of over four times the minimum clinically significant difference and a 22% decline from the median baseline score. Evaluating how this group differs from the patients who recovered fully or improved may help identify targets for interventions to enhance post-discharge outcomes for at-risk children.
We identified multiple factors associated with HRQL deterioration spanning all four domains of focus: demographics, pre-existing health status, infection characteristics, and illness severity. Older age was the demographic factor most strongly associated with failure to recover. Older age has previously been demonstrated to be a risk factor for poor HRQL compared to population norms among pediatric ICU patients (15). This may suggest greater resilience among younger children recovering from illness, less morbidity from illnesses requiring hospitalization for younger patients (e.g., bronchiolitis), or that rapidly developing infants and toddlers may manifest impact on HRQL by lack of developmentally expected improvement rather than decline.
Chronic comorbidities were associated with failure to recover only on bivariate analysis, and were in fact associated with slight improvements in follow-up score in multivariable linear regression models. Previous studies have demonstrated that patients with chronic health conditions are at risk for poor outcomes after critical illness (2,4,9,15,37). In our cohort, many patients with chronic comorbidities were also immunocompromised from cancer, rheumatologic conditions, or organ transplants. Immune compromise was strongly associated with poor outcome across all analyses. When controlling for immunocompromised status, the remaining chronic health conditions no longer conferred excess risk for HRQL decline, suggesting that conditions associated with immune compromise may place patients at highest risk of adverse outcomes. Notably, however, patients with chronic illness and immune compromise were also more likely to experience HRQL improvement, perhaps due to better control of underlying conditions among these patients while hospitalized.
Our finding that bacterial infection was not associated with increased risk of adverse outcomes highlights the impact of viral and culture-negative sepsis, with nearly one-quarter of these patients failing to recover to baseline. Existing literature in adult populations on the association of culture-negative sepsis with outcomes is mixed (38,39). When a specific infection was identified in our cohort, however, the source did matter; prevalence of failure to recover ranged from under 20% for soft tissue infections to nearly 50% for central nervous system infections and nearly 60% for bacteremia. The range of outcomes even within these subcategories is apparent when stratifying by ICU status, where soft tissue infections in the ICU population were associated with an average decline of 17 points between baseline and follow-up, presumably reflecting the impact of severe mucocutaneous and necrotizing soft tissue infections.
PRISM III, PELOD, and MPEWS scores were not associated with outcome, likely reflecting the low illness severity in our cohort. When evaluating illness severity by sepsis category, however, severe sepsis and septic shock were highly correlated with failure to recover to baseline. Other indicators of illness severity such as ICU admission and hospital LOS were also associated with adverse outcome; longer LOS was, in fact, the only factor other than immune compromise that was associated with failure to recover across all analyses.
While most of these factors are not modifiable, they do allow identification of patients at highest risk for poor HRQL outcome. Using the linear regression modeling results, we can estimate how a given patient with multiple risk factors may do after discharge. For example, a previously healthy 12-year-old admitted to the ICU with meningitis requiring a 20-day hospital stay would be predicted to have a decline in PedsQL™ score by 38.3 points (95% CI 21.8–54.9) from baseline at 28 days after discharge.
These findings also support the use of HRQL as an outcome measure following sepsis, distinguishing between patients with varying levels of sepsis severity, chronic conditions, and prolonged hospitalizations. Mortality has been the most common outcome measure used in interventional sepsis trials over the past 30 years, but as mortality continues to decline we are in need of an outcome measure that is clinically meaningful and captures additional patient-centered effects. Historically, pediatric critical care interventional trials have not reported HRQL as an outcome measure, and there has been no consensus on when or how to measure HRQL nor data describing how clinical factors or interventions may affect HRQL (40).
We have found that persistent declines in HRQL occur in a large proportion of patients surviving sepsis, suggesting that it may be a more sensitive outcome measure than mortality to evaluate differences between groups and epidemiologic trends. Failure to recover to baseline HRQL may also be a more patient-centered and clinically meaningful outcome measure than physiologic parameters such as duration of shock or mechanical ventilation (9,40).
Our data highlight the importance of comparing HRQL outcomes to patients’ own baseline status rather than to population norms as has previously been reported. While the proportion of patients with a follow-up PedsQL™ score ≥4.5 points below the population mean of 84.1 points (24.9%) was similar to the proportion of patients with a follow-up score ≥4.5 points below their individual baseline score (23.8%), there was only moderate overlap between the sets of patients identified by these two methods. Thirty-four percent of the patients who were below the population mean at follow-up were not significantly below their individual baseline score, and only 69% of the patients who had a persistent clinically significant decline from their individual baseline score would have been identified as being below the population norm.
Finally, HRQL assessment is also applicable to patients across the range of illness severity. Previous evaluations of outcomes among children surviving sepsis have all focused on patients with severe sepsis or septic shock. To our knowledge, there are no data evaluating outcomes in non-ICU patients. The responsiveness, wide applicability, patient-centeredness, and potential for improved sensitivity all support the implementation of HRQL outcome assessment in future sepsis research.
There were several limitations to this study. Only a subset of inpatients were sampled for HRQL assessment, and our cohort had lower illness severity and fewer comorbidities than the general population of patients with sepsis admitted to our institution. Reluctance of research assistants to approach families of the sickest patients and lower consent rates from these families likely contributed to difficulty enrolling the most acutely ill patients. This incomplete sampling may limit generalizability to more severely ill populations, though most likely biases our results towards the null given the association between higher illness severity and worse HRQL outcomes.
An additional limitation was that our assessment of patients’ baseline HRQL status was based on recall, which is inherently subject to bias. The direction of recall bias in this situation is unknown and likely variable; some families may recall their child to have had a better prior HRQL in comparison to the acute illness than they actually did, while others may be influenced by the acute illness or a potential prodrome such that they recall their child’s baseline status to be worse than it actually was. The finding that many patients had an improvement in HRQL following discharge supports the possibility that recall was biased towards lower HRQL, though improvement could also be due to normal variation, improvement in HRQL after treatment for an underlying condition, or developmental progression of physical, social, and cognitive functioning. While several studies have demonstrated construct validity of the PedsQL™ for evaluating baseline HRQL based on recall (28–30), this has not been prospectively studied and a tendency towards positive recall of baseline HRQL in our cohort would bias our results towards a higher proportion of patients with failure to recover.
A final limitation was that follow-up was performed at variable time-points with overrepresentation of earlier time-points, introducing the potential for sampling-time bias and preventing direct comparison across patients at equivalent intervals between discharge and follow-up. Our data suggest an improvement in HRQL over time, though this was not statistically significant on multivariable analysis. Further research is needed to better evaluate how HRQL varies over time in order to more precisely describe its use as an outcome measure, and to determine which factors contribute to persistent HRQL declines.
Despite these limitations, this study contributes substantially to our understanding of the ongoing burden of sepsis experienced by both ICU and non-ICU patients after hospital discharge. Our cohort of 790 patients represents one of the largest studies of HRQL outcomes in hospitalized children and to our knowledge is the largest evaluation of morbidity following pediatric sepsis. This study complements the ongoing multi-center Life After Pediatric Sepsis Evaluation (R01HD073362) by assessing outcomes across the spectrum of sepsis severity including non-ICU patients.
Conclusion
HRQL impairment is common among children surviving sepsis, with nearly one-quarter of children experiencing a clinically significant deterioration in HRQL from their baseline status at the time of post-discharge follow-up. Failure to recover to baseline HRQL is associated with illness severity but importantly also occurs frequently in children with sepsis who do not require ICU care, a population for which outcomes have not previously been characterized. Identification of factors associated with HRQL deterioration is essential to guide interventions to improve long-term outcomes for at-risk children. Our data identify risk factors for adverse outcomes following sepsis and imply the need for ongoing evaluation and treatment by outpatient providers for all survivors of pediatric sepsis for at least the first three months after hospital discharge, with particular attention to teenagers, those hospitalized for more than a week, immunocompromised patients, and those who experienced septic shock.
Supplementary Material
Table S1: Characteristics of patients who recovered to within 4.5 PedsQL™ points of baseline compared to patients who improved to >4.5 PedsQL™ points between baseline and follow-up
Table S2: Multivariable analysis of association between risk factors and failure to recover to baseline health-related quality of life, using a comparison group of only patients who recovered within 4.5 PedsQL™ points of baseline but did not demonstrate improvement
Table S3: Linear regression of change in PedsQL™ score from baseline to follow-up among non-ICU patients
Table S4: Linear regression of change in PedsQL™ score from baseline to follow-up among ICU patients
Acknowledgments
Financial support: No external funding for this manuscript
Copyright form disclosure: Dr. Zimmerman’s institution received funding from the National Institutes of Health (NIH)/National Institute of Child Health and Human Development and Immunexpress, Seattle, WA; he received funding from Elsevier Publishing and the Society of Critical Care Medicine (travel reimbursement to attend board meetings); and he received support for article research from the NIH.
References
- 1.Balamuth F, Weiss SL, Neuman MI, et al. Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014;15:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics 2009;123:849–857. [DOI] [PubMed] [Google Scholar]
- 3.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013;14:686–693. [DOI] [PubMed] [Google Scholar]
- 4.Ruth A, McCracken CE, Fortenberry JD, et al. Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014;15:828–838. [DOI] [PubMed] [Google Scholar]
- 5.de Souza DC, Barreira ER, Faria LS. The epidemiology of sepsis in childhood. Shock 2017;47:2–5. [DOI] [PubMed] [Google Scholar]
- 6.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med 2013;14:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015;191:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlon NP, Breatnach C, O’Hare BP, et al. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med 2009;10:41–44. [DOI] [PubMed] [Google Scholar]
- 9.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med 2015;41:1235–1246. [DOI] [PubMed] [Google Scholar]
- 10.Healthy People 2020 Foundation Health Measure Report 2010. Health-related quality of life and well-being https://www.healthypeople.gov/2020/about/foundation-health-measures/Health-Related-Quality-of-Life-and-Well-Being. Accessed June 1, 2017.
- 11.Namachivayam P, Taylor A, Montague T, et al. Long-stay children in intensive care: long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr Crit Care Med 2012;13:520–528. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahim S, Singh S, Hutchinson JS, et al. Adaptive behavior, functional outcomes, and quality of life outcomes of children requiring urgent ICU admission. Pediatr Crit Care Med 2013;14:10–18. [DOI] [PubMed] [Google Scholar]
- 13.Polic B, Mestrovic J, Markic J, et al. Long-term quality of life of patients treated in paediatric intensive care unit. Eur J Pediatr 2013;172:85–90. [DOI] [PubMed] [Google Scholar]
- 14.Colville GA, Pierce CM. Children’s self-reported quality of life after intensive care treatment. Pediatr Crit Care Med 2013;14:e85–e92. [DOI] [PubMed] [Google Scholar]
- 15.Morrison AL, Gillis J, O’Connell AJ, et al. Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med 2002;3:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Buysse CM, Raat H, Hazelzet JA, et al. Surviving meningococcal septic shock: health consequences and quality of life in children and their parents up to 2 years after pediatric intensive care unit discharge. Crit Care Med 2008;36:596–602. [DOI] [PubMed] [Google Scholar]
- 17.Edmond K, Dieye Y, Griffiths UK, et al. Prospective cohort study of disabling sequelae and quality of life in children with bacterial meningitis in urban Senegal. Pediatr Infect Dis J 2010;29:1023–1029. [DOI] [PubMed] [Google Scholar]
- 18.Bronner MB, Knoester H, Sol JJ, et al. An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med 2009;10:636–642. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–812. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 21.Gebara BM. Values for systolic blood pressure. Pediatric Crit Care Med 2005;6:500; author reply 500–501. [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–341. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Limbers CA, Neighbors K, et al. The PedsQL™ Infant Scales: feasibility, internal consistency, reliability, and validity in healthy and ill infants. Qual Life Res 2011;20:45–55. [DOI] [PubMed] [Google Scholar]
- 25.Limbers CA, Newman DA, Varni JW. Factorial invariance of child self-report across race/ethnicity groups: a multigroup confirmatory factor analysis approach utilizing the PedsQL 4.0 Generic Core Scales. Ann Epidemiol 2009;19:575–581. [DOI] [PubMed] [Google Scholar]
- 26.Desai AD, Zhou C, Stanford S, et al. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 Generic Core Scales in the pediatric inpatient setting. JAMA Pediat 2014;168:1114–1121. [DOI] [PubMed] [Google Scholar]
- 27.Aspesberro F, Fesinmeyer MD, Zhou C, et al. Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatr Crit Care Med 2016;17:e272–e279. [DOI] [PubMed] [Google Scholar]
- 28.Kruse S, Schneeberg A, Brussoni M. Construct validity and impact of mode of administration of the PedsQL™ among a pediatric injury population. Health Qual Life Outcomes 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivara FP, Vavilala MS, Durbin D, et al. Persistence of disability 24 to 36 months after pediatric traumatic brain injury: a cohort study. J Neurotrauma 2012;29:2499–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and health-related quality of life after pediatric inpatient surgery. J Pain 2015;16(12):1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virtual Pediatric Systems, LLC. www.myvps.org.
- 32.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014;133:e1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 1996;24:743–752. [DOI] [PubMed] [Google Scholar]
- 34.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making 1999;19:399–410. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen KR, Migita R, Batra M, et al. Identifying high-risk children in the emergency department [published online February 10, 2015]. J Intensive Care Med doi: 10.1177/0885066615571893 [DOI] [PubMed]
- 36.Zonfrillo MR, Durbin DR, Koepsell TD, et al. Prevalence of and risk factors for poor functioning after isolated mild traumatic brain injury in children. J Neurotrauma 2014;31:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med 2012;40:2196–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013;17:R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Sakhuja A, Kumar G, et al. Culture-negative severe sepsis: nationwide trends and outcomes. Chest 2016;150:1251–1259. [DOI] [PubMed] [Google Scholar]
- 40.Menon K, McNally JD, Zimmerman JJ, et al. Primary outcome measures in pediatric septic shock trials: a systematic review. Pediatr Crit Care Med 2017;18:e146–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Characteristics of patients who recovered to within 4.5 PedsQL™ points of baseline compared to patients who improved to >4.5 PedsQL™ points between baseline and follow-up
Table S2: Multivariable analysis of association between risk factors and failure to recover to baseline health-related quality of life, using a comparison group of only patients who recovered within 4.5 PedsQL™ points of baseline but did not demonstrate improvement
Table S3: Linear regression of change in PedsQL™ score from baseline to follow-up among non-ICU patients
Table S4: Linear regression of change in PedsQL™ score from baseline to follow-up among ICU patients


