Abstract
Introduction:
The gut microbiome helps to maintain a person’s healthy state while perturbations in its function often leading to the development of inflammatory diseases including multiple sclerosis (MS). Consequently, gut-commensals which restore homeostasis have the potential to become novel therapeutic options for treating MS. MS patients have presented gut microbial dysbiosis with a reduction in bacteria belonging to the Prevotella genus. Notably, increased levels of Prevotella are observed when disease-modifying therapies are used. Additionally, Prevotella histicola, an anaerobic bacterium derived from the human upper GI tract, can suppress disease in mice with experimental autoimmune encephalomyelitis, a preclinical MS mode.
Areas covered:
In this review, the authors compare MS microbiome studies from different geographical regions to identify common gut bacteria. Literature on the potential use of P. histicola as a therapy for MS and the next steps for developing microbial monotherapies in MS is also discussed.
Expert commentary:
Recent findings presenting an inverse correlation between Prevotella and MS disease severity as well as ability of P. histicola to suppress disease in preclinical models suggest that P. histicola might provide an additional treatment option for MS patients. However, rigorous testing in well-designed control trials should be performed to determine the safety and efficacy P. histicola in MS patients.
Keywords: Multiple sclerosis, gut microbiota, Prevotella, Immunomodulation, Prevotella histicola, experimental autoimmune encephalomyelitis (EAE), gut dysbiosis, bacteria as drug (brug), tregs, treatment
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system affecting more than 2.5 million people worldwide and around 600,000 people in the US [1]. MS carries an economic burden of billions of dollars annually in medical care and indirect expenses, including lost wages and productivity [1,2]. Despite the availability of a number of immunomodulatory drugs, current treatment modalities are suboptimal and have substantial adverse and undesired effects [3]. Thus, there is a need to discover and develop novel therapeutic options for patients with MS in order to reduce adverse effects and improve patient outcome.
As is the case in many immune mediated disorders, it is hypothesized that MS patients have gut microbial dysbiosis. Gut microbiota analysis of MS patients have suggested that certain gut bacterial abundances correlate with clinical disease states. This imbalance in the gut microbiota of patients with MS may drive a pro-inflammatory immune response and therefore contribute to the development of autoimmunity. However it is unclear how gut microbiota exactly impact immune responses and contribute to the development of chronic inflammatory disorders.
2. Multiple sclerosis and the gut microbiota
In the last few years, we along with others have demonstrated that MS patients exhibit a distinct gut-microbiota profile, with both depletion and enrichment of certain bacteria compared to healthy individuals [4–10] (Table 1). Majority of bacteria (~90 %) in the adult human gut belong to the Firmicutes or Bacteroidetes phyla, with the remaining belonging to Actinobacteria, Proteobacteria, and a few other phyla are represented at a frequency of less than 5 % [11]. Despite the large amount of heterogeneity in the human microbiome, data summarized from different MS microbiome studies, confirm that certain gut bacteria are consistently found to be depleted or others to be enriched in patients with MS (Table 1) [12]. These studies largely involved patients with relapsing-remitting MS (RRMS) and had reported a decrease in Prevotella levels in patients with MS compared to healthy individuals (Table 1) [4–6,9,10]. MS patients also showed decreased levels of Sutterella, Adlercreutzia, Collinsella, Clostridium, Faecalbacterium, and Parabacteroides (Table 1) [5]. Two studies from the US [5,6], and one from Japan identified a lower levels of Prevotella in MS patients relative to healthy control (HCs) [4]., While these studies assessed microbial signature in stool samples, a study from Italy reported that duodenal biopsies from MS patients with active disease had a lower levels of Prevotella compared to HCs [10]. MS patients in remission showed higher levels of Prevotella compared to MS patients with active disease. Authors also observed that levels of Prevotella negatively correlated with a Th17 response and disease severity. In another autoimmune inflammatory disorder such as rheumatoid arthritis (RA), expansion of Prevotella copri has been linked with development of early onset arthritis and has been suggested that P. copri has ability to induce pro-inflammatory response [13]. However, none of MS microbiome studies had confirmed the finding that the abundance of Prevotella is associated with an active inflammatory state/disease severity. These data suggest that role of Prevotella in MS and RA is not unanimous and it is difficult to say whether discrepancy between RA and MS in regard to abundance of Prevotella is due to difference in disease or due to discrete strains of Prevotella in RA and MS. Based on MS microbiome studies, we can conclude that, a reduction or loss of Prevotella in fecal samples may have significant consequences in the MS immunopathogenesis.
Table 1:
Comparison of Adult MS Gut Microbiome Studies
| MS Microbiome Study # samples Tissue (Country) (Reference) | Bacteria genera showing lower abundance in MS patients vs. HC | Bacteria genera showing higher abundance in MS patients after treatment |
|---|---|---|
| RRMS (n = 31) HC (n = 36) Fecal (USA) Chen et al.2016 |
Prevotella, Parabacteroides, Adlercreutzia, Collinsella, Lactobacillus, Coprobacillus, | |
| RRMS (n=60) HC (n=43) Fecal (USA) Jangi et al. 2016 |
Butyricimona, Prevotella Parabacteroides | Prevotella Sutterella |
| RRMS (n=20) HC (n=40) Fecal (Japan) Miyake et al. 2015 |
Bacteroides Fecalibacterium Prevotella, Anaerostipes, Clostridium, Sutterella | |
| Treatment Naïve MS (n=64) Fecal (USA) Cekanaviciute et al. 2017 | Parabacteroides, Prevotella | |
| Monozygotic twins (n=32) Fecal (Germany) Berer et al. 2017 | Lower levels of Adlercreutzia in GF mice colonized with fecal samples from MS patients vs GF mice colonized with fecal samples from HCs | |
| RRMS (n=19) HC (n=17) Mucosa (Italy) Cosorich et al. 2017 |
Prevotella Inverse correlation between Prevotella abundance and levels of IL17 producing cells | Prevotella |
2.1. Beneficial role of Prevotella
The characterization of beneficial bacteria and/or small molecules produced by gut bacteria directly or indirectly through metabolism of dietary compounds will help in development of enhanced therapeutic options for MS. In the context of MS, there are several studies that support a beneficial role for Prevotella. MS patients treated with a disease-modifying therapy (DMT) including Copaxone or Interferon-beta had higher levels of Prevotella compared to untreated MS patients [6]. In a separate study, MS patients receiving Interferon-beta treatment showed higher levels of Prevotella copri [10]. Additionally, we have identified a unique strain of the species Prevotella histicola, which modulate immune response in experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and collagen induced arthritis, an animal model of rheumatoid arthritis (RA) [14,15]. Specifically, P. histicola could suppress disease in a dose dependent manner in an HLA-class II transgenic mouse model of EAE, and disease amelioration was associated with substantially reduced or absent CNS inflammation and demyelination [15]. Collectively, animal studies performed utilizing P. histicola support a beneficial role of Prevotella genus in MS patients.
2.2. Inflammation and P. histicola: a “chicken and/or the egg” dilemma
Reduced relative abundance of Prevotella in MS patient combined with ability of P. histicola to suppress inflammatory disease in animal model of MS suggest that lack of Prevotella might be responsible for predisposition to MS. However, we cannot rule out the possibility that host immune changes are what influence the microbiome since treatment with DMTs leads to restoration of Prevotella. Currently we do not have enough information to the confirm it one way or other. Thus there are two possibilities regarding relationship between Prevotella and inflammation in MS, one that Prevotella suppress inflammation or alternatively inflammation can suppress levels of Prevotella. Current observations set us to address the obvious question of the causal relationship of Prevotella with inflammation in MS especially the direction of causality.
2.3. Mechanism of action
The current understanding regarding the role of microbiome in the field of mucosal immunology suggests that the gut microbiota is involved in maintaining the healthy state of a host. It’s believed that gut microbiota regulate the balance between anti-inflammatory regulatory T cells (Tregs) and pro-inflammatory Th1/Th17 cells, thus influencing the immune-homeostasis [16–21]. Alterations in the gut microbiota may result in perturbation of balance between pro- and anti-inflammatory immune cell subsets towards pro-inflammatory cells leading to development of chronic inflammatory diseases. Gut microbiota profiling studies suggest that MS patients have reduced levels of bacteria that can induce immunoregulatory cells and higher abundance of bacteria that induce a pro-inflammatory response [12]. Consistent with this, MS patients had a greater abundance of Akkermansia muciniphila and Acinetobacter calcoaceticus, which can induce pro-inflammatory responses in peripheral blood mononuclear cells. Additionally germfree mice mono-colonized with these gut bacteria develop severe EAE suggesting a pro-inflammatory nature of Akkermansia muciniphila and Acinetobacter calcoaceticus [6,9]. In contrast, gut bacteria showing lower abundance in MS patients, such as Prevotella and Parabacteroides; have ability to induce immunoregulatory cells in human PBMCs [9] and HLA transgenic mice [15]. Since the levels of Prevotella inversely correlate with severity of MS, it can be hypothesized that certain species of Prevotella have immunomodulatory properties and are able to induce immunoregulatory cells. This was indeed true in an EAE model, where oral administration of P. histicola suppressed disease in HLA-class II transgenic mice compared to mice receiving media or control bacteria [15]. Possible mechanisms by which P. histicola may suppress disease are explained below. Thus our animal studies suggest that Prevotella can suppress inflammation.
A current hypothesis in the pathogenesis of MS suggests that peripheral activation of autoreactive T cells with Th1/Th17 phenotypes results in their trafficking into the central nervous system (CNS) where they induce an inflammatory process that leads to increased permeability of the blood-brain barrier and further recruitment of inflammatory cells into CNS [22]. This inflammatory cascade results in demyelination of the CNS, which leads to neurological deficits that are manifested as clinical phenotypes in patients. Therefore, suppression of the initial pro-inflammatory response by immunoregulatory cells (e.g., Tregs, tolerogenic dendritic cells (DCs), regulatory B cells (B-regs), and suppressive macrophages) and/or chemical mediators might ameliorate disease and the associated pathology [23,24]. Oral tolerance studies have suggested that induction of an immune response in the gut can modulate peripheral immune responses [23,24]. Indeed, using a mouse model of MS, administration of P. histicola by oral gavage reduced gut permeability compared to mice treated with media alone or treated with control bacteria, and resulted in disease suppression [15]. This prompted further determination of the ability of immune cells in the gut and periphery to modulate inflammatory immune responses and suppress disease.
Mice with chronic EAE have increased gut permeability, suggesting that intestinal immune compartment may play an important role in regulating peripheral immune responses and CNS inflammation [25]. Specifically, mice treated with P. histicola have an increased frequency of tolerogenic DCs (characterized as CD11c+CD103+) [15] and myeloid suppressors cells (characterized as CD11b+Gr-1+ ) [14], indicating the ability of P. histicola to induce antigen presenting cells (APCs) with tolerogenic capacity. Additionally, mice treated with P. histicola showed a higher frequency of CD4+FoxP3+ Tregs in the gut [14]. The encephalitogenic T cells can traffic to gut and interact with tolerogenic DCs as well as FoxP3+ regulatory T cells. In the gut, pathogenic CD4+ T cells can be converted into FoxP3+ regulatory T cells by action of tolerogenic DCs or Tregs [26]. Alternatively gut resident regulatory FoxP3+ regulatory T cells can suppress pathogenic autoreactive T cells [27]. Thus P. histicola treatment can lead to reduced inflammation and disease due to its ability to induce tolerogenic DCs and Tregs in the gut, which can convert autoreactive T cells into FoxP3+ regulatory T cells. P. histicola induced tolerogenic DCs and/or Foxp3+ Tregs can also directly suppress pathogenic immune cells in the gut. These data indicate that P. histicola can restore intestinal integrity by reducing permeability and promote anti-inflammatory immune responses in the gut.
In the gut, P. histicola itself and/or P. histicola induced dietary metabolites can induce tolerogenic APCs either directly or through their influence on intestinal epithelial cells (IEC), which then induces Tregs. The role of IEC in the generation of immunoregulatory cells is well established [28], however studies in our laboratory are currently underway to determine precise role of intestinal epithelial cells in the induction of immunoregulatory cells in the gut. Higher frequency of tolerogenic DCs and suppressive macrophages have been observed in the periphery, suggesting suppressive cells (i.e., tolerogenic APCs and Tregs) that are generated in the intestine are able to migrate out of the gut. Additionally, APCs isolated from P. histicola-treated mice have a decreased antigen presentation capacity with have a higher ratio of anti-inflammatory to pro-inflammatory cytokines (IL-10 to IL-12 or IL-23). Thus P. histicola can modulate both frequency and function of tolerogenic APCs [15]. Additionally, mice treated with P. histicola showed a higher frequency of CD4+CD25+FoxP3+ Tregs in the periphery and these cells had an enhanced ability to suppress antigen specific T cells [15].
Increased permeability of the blood-brain barrier is linked with increased CNS pathology in MS patients [22]. In mice treated with in P. histicola, the permeability of the blood brain barrier is reduced along with relatively reduced activation of immune cells resulting in fewer inflammatory cells trafficking from periphery to the CNS. Data published from our laboratory showed that mice treated with P. histicola showed a decrease in the permeability of the blood-brain barrier and had decreased proliferation of peripheral antigen-specific T cells. Additionally, CD4 T cells from P. histicola-treated mice also produced decreased levels of pro-inflammatory cytokines IFN-γ and IL-17 [15]. Based on these data, we’ve developed the model that P. histicola or its metabolites interact with intestinal epithelial cells and induce chemical mediators (e.g. TGF-β1, IL-10, and TSLP), which then condition DCs and macrophages to adopt a tolerogenic phenotype. These tolerogenic DCs and macrophages then induce either expansion of natural Treg cell population and/or promote differentiation of naïve T cells into Tregs. Subsequently, these Tregs migrate into the periphery and modulate CNS-specific autoreactive T cells, thus reducing inflammation and disease severity (Figure 1).
Figure 1: P. histicola-induced immunomodulation and disease suppression.
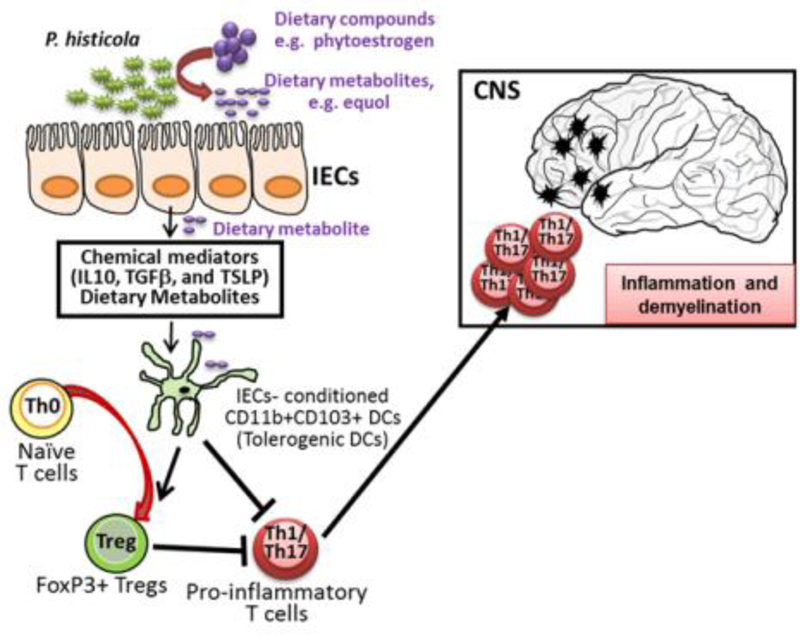
Based on our data, we hypothesize that P. histicola either directly, or through the metabolism of dietary compounds, induces intestinal epithelial cells (IECs) to produce anti-inflammatory cytokines such as IL-10, TGFβ, and TSLP, which condition DCs and/or macrophages to adopt a tolerogenic phenotype characterized by expression of CD11b, CD11C, and CD103. These IEC-conditioned tolerogenic DCs can induce FoxP3+CD4+ Tregs, which can travel to the periphery and suppress CNS-specific autoreactive CD4+ T cells harboring a Th1 or Th17 phenotypes. This cascade of events leads to reduced trafficking of pro-inflammatory cells into the CNS, resulting in reduced inflammation and demyelination.
To further understand the mechanism by which P. histicola confers a beneficial effect, we explored whether live P. histicola is required for disease suppression. Notably, we observed greater disease suppression in mice that received live P. histicola relative to those that received heat-inactivated P. histicola. Thus suggesting that interaction of living bacteria with host environment (e.g., mucus, IEC, and dietary compounds) is required for disease suppression. We can speculate that the beneficial effects of bacteria may occur through their metabolism of dietary factors, resulting in the production of small metabolites which harbor immunomodulatory properties. Currently, there are at least two known pathways by which P. histicola produces metabolites: one method is through production of short chain fatty acid (SCFA) from starch/fiber [29,30], and the other method is through the metabolism of phytoestrogens [31,32].
2.3. Diet metabolism and immune regulation by Prevotella
Prevotella have the ability to produce short chain fatty acids (SCFAs) from diet rich in fiber and equol plus enterolactone from diet rich in phytoestrogens. A good amount of daily complex carbohydrate intake in human are indigestible and are fermented in the colon by gut bacteria into SCFAs such as acetic acid, propionic acid, and butyric acid. Although Firmicutes such as Clostridium are considered the major SCFA producers [17,33], Bacteroidetes such as Prevotella have been shown to produce SCFAs from Xylans [29]. SCFAs are known to induce regulatory CD4+CD25+FoxP3+ T cells [34] and suppress inflammatory disease in animal models of MS [35] and inflammatory bowel disease [34]. SCFAs had been shown to promote differentiation of Tregs through their interaction with receptors such as GPR43 [34] or GPR109 [36] as lack of these receptor abolished SCFAs induced anti-inflammatory effect. SCFAs such as butyrate can also induce FoxP3 expression directly through epigenetic modification of FoxP3 promoter or indirectly through enhancing ability of DCs to induce generation of Tregs [37,38]. Thus some of the anti-inflammatory effect of Prevotella can be attributed to their ability to produce SCFAs.
P. histicola can modulate host immunity through metabolism of phytoestrogens. Plant lignans that are present in flaxseed and isoflavones that are present in soy-based foods are a group of compounds termed phytoestrogens and their metabolism leads to the generation of enterolactone and S-equol, both of which have structural similarities to human sterols such as estrogens [39,40]. This structural similarity with natural estrogen enables enterolactone and equol to bind to estrogen receptor (ER) as well as other receptors to elicit biological activities [41,42]. Notably, the end metabolites of lignans and isoflavone which are enterolactone and equol respectively have a higher affinity for ER with thousand fold more estrogenic activity than the parental isoflavones and lignans. Estrogens is known to possess immunomodulatory properties [43–45] and it is hypothesized that enhanced levels of estrogens during pregnancy results in amelioration of MS [46]. Additionally estrogen treatment of mice can reduce the severity of EAE, suggesting it has potential anti-inflammatory and neuroprotective effects [44,45,47,48]. Importantly, phytoestrogen and its metabolites, including equol, have been suggested to regulate immune responses [49].
Certain gut microbia such as the Prevotella species contain enzymes that are critical for the metabolism of lignan [50] and isoflavone [51], including Daidzein reductase (DHNR), Dihydrodaidzein reductase (DHDR), and Tetrahydrodaidzein reductase (THDR). For example, these reductase enzymes provided by gut bacteria such as Prevotella can help convert isoflavone Diadzein to S-equol, a biologically active and potent estrogen mimic. Plant lignan (secoisolariciresinol diglucoside-SDG) and isoflavone (Genistein and Daidzein) can provide health benefits in various diseases [52–54]; however, the use of isoflavone in human clinical trials has produced mixed results [55,56]. This could be due to the fact that, during some disease states there is a loss of the gut flora that is needed for conversion of phytoestrogens into the beneficial metabolites such as enterolactone and equol. Thus, identification of beneficial commensals responsible for the metabolism of phytoestrogens and a better understanding of the underlying mechanisms by which these enable immunomodulatory effects will be necessary to harness their full potential as a therapeutic option. Specifically, understanding the effect of enterolactone and equol on the local mucosal as well as systemic immune response will help in filling this knowledge gap.
2.4. Effect of diet and other gut bacteria on the abundance of Prevotella
The gut microbiome is a community structure with thousands of species of bacteria living together in a small space, particularly in the colon. There is complex interaction between the different bacterial populations to maintain a healthy state. Under homeostatic conditions (i.e., in a healthy individual), the balance among different bacterial populations inhibits the growth of disease-causing pathobionts or lesions which would allow access to the host. Recent developments in the microbiome field suggest that oral intake of certain diet leads to production of small metabolites due to action of gut bacteria. These small metabolites are hypothesized to play an important role in host physiology [57,58]. Similarly, digestion of starch/fiber by gut bacteria leads to production of SCFA, which not only induce proliferation of colonic epithelial cells but can also induce anti-inflammatory Treg cells [34]. Thus, the ability for gut bacteria to metabolize dietary components into small metabolites is crucial for maintaining homeostasis at mucosal surfaces and in the periphery.
In addition to Prevotella, the gut flora of MS patients also has a decreased levels of Parabacteroides and Adlercreutzia [5,9]. Notably, these two strains of bacteria are also capable of metabolizing phytoestrogens such as isoflavone and lignan [31,59]. As the end metabolites of phytoestrogen, such as equol and enterolactone, can induce immunoregulatory cells, the lack of bacteria that metabolize these compounds in a genetically-susceptible individual could disrupt the balance between an anti-inflammatory and a pro-inflammatory environment, which in turn could predispose an individual to develop an inflammatory disease such as MS and chronic inflammatory arthritis. Thus, these microbiome studies reinforce the importance of a plant-based diet and influences our physiological healthy status.
2.5. Inflammation and microbial dysbiosis
Lack of beneficial gut bacteria (symbionts) due to events such as stress, infection, antibiotics, or illness can perturb gut homeostasis. This might result in a temporary pro-inflammatory environment that would allow the growth of pathobionts. In an animal model of colitis, inflammation promoted the growth of certain gut bacteria belonging to the Enterococcus family due to their ability to utilize free oxygen generated by the inflammatory environment [60]. In MS patients there is an elevated Th17 population in the small intestine with concurrent higher levels of Streptococcus and lower levels of Prevotella [10]. This suggests the possibility of an inverse correlation between Prevotella and Streptococcus that contributes to the elevated levels of proinflammatory cytokines such as IL-17 [10]. However, currently we do not fully understand the interplay between these factors and how that affects disease development. For example, it is unclear whether there is first a decrease in the levels of Prevotella, which leads to higher levels of IL-17 and Streptococcus or if higher levels of IL-17 suppress the levels of Prevotella. Future mechanistic studies are warranted that may provide insights into the relationship between symbionts and pathobionts, particularly with regard to their effects on disease.
2.6. P. histicola and prebiotics
P. histicola can mediate its immunomodulatory effect through metabolism of dietary compounds, including the production of enterolactone, equol and SCFAs. Therefore, a potential synergetic therapy for patients with MS could be to provide dietary compounds, such as phytoestrogen, along with P. histicola with the aim being to restore the gut microbiota composition to pre-disease state. The combined use of prebiotics (either dietary or chemical-based) and bacteria (probiotics/brugs) is termed synbiotics and could be a potential non-complex therapeutic option for patients with MS. Thus, in order to optimize the effect of a Brug such as P. histicola, the dietary preference of individual patient needs to be ascertained and optimized. Further studies are needed to determine whether Brugs alone (i.e., P. histicola) will have an adequate effect on disease, or if patients will also need prebiotics, especially phytoestrogens such as diazine and genistein, to provide substrates for optimal therapeutic benefit.
2.7. Small intestine vs colon
There has been a substantial amount of attention placed on the fact that the effects of probiotics are mediated through the largest microbial mass in the human body (i.e., the stool). However the significance of colonic vs. small intestine microbiota in maintaining mucosal immune homeostasis is not well understood. It is clear that colonic bacteria produce copious amounts of SCFAs that exert local effects on the gut to protect the colonic mucosa from microbiota, and that these are suppressed upon treatment with broad spectrum antibiotics [15]. Further, the deficit of Prevotella in the duodenum of MS patients, and the fact that these mucophilic bacteria are expanded in the small intestine and are in close association with the epithelium, suggests that a close relationship exists between these two components and likely impacts the gut environment. The bacterial density in the small intestine (101-103 CFU/ML) is many logs lower than that in the colon (1012-1013 CFU/ML), providing a less competitive environment in which bacteria colonize and expand. The fact that P. histicola prefers to inhabit the small intestine and that it must be alive to suppress MS suggests that the beneficial effects of P. histicola involve its active interaction with food and/or mucosal substrates. In general, the colon has a much more impermeable mucosal barrier as it must act as a containment compartment for resident bacteria; in contrast the small intestine acts aa a conduit for bacteria movement. Thus, the luminal barrier of the small intestine is much more permeable and has a much greater surface area than that of the colon. The preference displayed by P. histicola to colonize the small intestine confers an advantage to this type of therapeutic approach, as the effects of P. histicola are likely exerted through its interactions along the length of the small intestine, thus avoiding competition from large bacterial population in the colon.
3.0. Challenges in translating P. histicola treatment to the clinic
P. histicola makes an ideal candidate to translate into clinics as a potential therapeutic option due to its ability to thrive in the gut combined and suppress autoimmunity in two different disease models (MS and RA). However, as with any drug, there are a number of factors that need considerations before it can be used in the clinic. Drug development involves five steps, which include: 1) discovery and development; 2) preclinical research to determine the efficacy and potential toxic effects of a drug in an animal model; 3) implementation of a clinical trial to determine the safety and efficacy of a drug in humans; 4) submission of the results from the clinical trial to the FDA for approval and, if approved, progression of the drug to market; 5) post-market safety monitoring of the drug. The use of P. histicola as a potential treatment option for MS is currently being investigated and is in the later stages of step 2 of the drug development pathway. The efficacy of P. histicola in treating MS has been shown in two different animal models and safety data analysis of its safety is currently underway. Notably, P. histicola is part of the normal human gut microbiota as it was isolated from human subjects [14,15]. Currently used outcome measures such as annual relapse rate, Expanded Disease Severity Scale (EDSS) and MRI imaging can be employed to measure the efficacy of P. histicola in modulating disease in patients with MS.
P. histicola could either be used alone or in combination with existing therapeutic options to treat MS. Two studies have already demonstrated that treatments with disease modifying therapies (DMT) are associated with increase in levels of Prevotella [6,61]. In a study by Jangi et., al both Copaxone and Interferon-beta (IFN-β) were grouped together as DMTs whereas study by Castillo-Alvarez et. al., only analyzed effect IFN-β on gut microbiota [61]. Thus there is direct association between Interferon-beta and abundance of Prevotella (copri), however it’s not clear whether Copaxone can also mediate its effect by increasing levels of Prevotella. Future studies investigating a direct effect of Copaxone alone on Prevotella abundance are needed. Studies are currently ongoing in our laboratory to determine if combining P. histicola and first-line therapies such as Copaxone or IFN-β have enhanced beneficial effect. Two drugs working through different pathways might show cumulative effects in suppressing disease; therefore combining P. histicola with medication that do not induce levels of Prevotella might be a better option. The majority of drugs are associated with some side effects, and one benefit to the use of P. histicola is its sensitivity to antibiotic treatment. Thus, patients that show an adverse reaction to P. histicola can be treated with the antibiotic, metronidazole, to kill and deplete the bacteria and thus eliminate side effects from this treatment. Interestingly, we have observed that P. histicola can suppress EAE disease in the absence of gut microbiota [15]. It should be cautioned that there is a probability of developing antibiotic resistance due to overuse of antibiotics; therefore antibiotic use should be monitored carefully to avoid development of antibiotic resistant strains of P. histicola.
Diet is a major factor that influences our gut microbiome. The increased abundance of Prevotella in MS patients that have received DMT suggests maintaining an appropriate balance of the microbiome is important for positive disease outcomes. Moving forward, it will be interesting to identify diets that may be associated with an increased abundance of Prevotella. As Prevotella can metabolize phytoestrogen and fiber, a vegetarian diet that is rich in these types of foods could help to colonize and expand Prevotella and other beneficial bacteria. Thus, a clinical trial investigating the effects of certain diets on the expansion and/or colonization of Prevotella and other strains of bacteria will be beneficial to patients with MS and other autoimmune disorders.
4. Expert commentary
4.1. P. histicola as a therapeutic agent in the clinic
MS patients have decreased levels of Prevotella [4–6,10] and multiple reports suggest that levels of Prevotella are elevated following treatment with DMT [6,10]. These observations combined with the ability of a human-derived strain of Prevotella, P. histicola, to suppress disease in an animal model of MS [15], strongly enforces P. histicola as a potential therapeutic option to treat MS. Many bacteria are purported to have beneficial effects but have limited proof of being able to specifically modify the course of disease (e.g., probiotics). Because of this, we coined the term “Brug” to imply a bacterium that has drug-like effects. Based on our current studies, we propose P. histicola could be used as a therapeutic option for MS or other immune disorders. The dose, frequency and duration of P. histicola treatment need to be standardized in MS patients. However based on our studies in preclinical model of MS and RA, we can predict a dose of around one billion CFU of brug every other day for first six months to a year and then once a week as a maintenance therapy [14,15]. P. histicola could be used as a therapy on its own, or in combination with other MS disease-modifying drugs. Currently, studies are underway in our laboratory to determine whether administration of P. histicola, either alone or in combination with MS drugs such as Copaxone or Betaseron (IFNβ), is effective in suppressing disease in a mouse model of EAE. While the relationship between DMT and Prevotella is not completely understood, studies such as ours will enable enhanced understanding of the interaction of existing therapeutic option of MS.
However, gut microbiome research in MS is still in early stages because majority of MS microbiome studies so far have been cross-sectional and have performed16s rRNA metagenomic sequencing. Longitudinal studies which involve analysis of gut microbiome along with identification of microbial signatures and metabolic pathways being modulated will help in determining the direction of causality between gut bacteria, metabolites and associated inflammatory processes. As gut microbiome is a community structure, utilizing system biology approach to build a model for defining the relationship among different gut bacteria will be useful in identifying the gut bacteria functionally linked with disease positively or negatively. Studies which integrate data derived from metagenomic, metabolomics, inflammatory markers, and disease state will help in narrowing down the bacterial communities linked with healthy state vs. those associated with a disease state. Recent studies highlight that host and microbiome together form a holobionts which work in unison to optimize host physiological and immune responses. Thus understanding the interactions between host and gut microbiota as well as among the gut bacteria during health and diseases states is required. This information will provide the required data required to manipulate the gut microbiota through either diet or directly replenishing the beneficial bacterial population to assist us in correcting a disease state. There is paucity of data regarding other members of microbiome such as fungal, viral, and phage which need to be explored such that they can also be utilized to modulate gut bacteria and cumulatively correct pro-inflammatory responses and restoring the normal gut homeostasis and achieving optimal healthy status. Creating an environment to diversify as well as promote growth of beneficial microbiome might be used as a non-tradition therapeutic intervention. Reduced asthma in individuals living in farmlands or correcting asthma by introducing children to farmlands/green space is already being promoted in the field of allergy. Thus altering the environment will enable us to improve therapy for MS. One of the major challenges in the field of gut microbiome is to define the normal gut microbiota. Currently it is unknown whether a given gut bacterial signature is beneficial or harmful as the interaction among multiple bacteria with its environment might determine the final outcome. Specifically, Akkermansia muciniphila had been shown to be having beneficial effect in diabetes whereas in MS it has been shown to be abundant in active disease state. The discrepancy might be due to the ability of same bacterium to produce a metabolite which can be protective in diabetes but promote inflammation in MS. Thus the P. histicola is a promising therapeutic candidate for treating MS patients but there are still number of steps needs to be taken before it can be used in clinic.
4.2. Five-year view
Currently majority of MS drugs are either small molecules or biologics but we hypothesize that in the next five years microbial and human cell-based therapies will be as effective, if not more so, than currently prescribed small molecule and biological-based therapies. Inteferon-1β was the first biologic approved for treatment of MS in the early 1990s, followed soon by other small molecules such as Copaxone and Mitoxantrone. Later Natalizumab and Rituximab were approved for MS. The Human Microbiome Project (HMP), a NIH initiative to determine the role of the microbiome in health and disease, has helped to identify and characterize a number of gut bacteria with health promoting properties. Successful use of fecal microbiome transplantation therapy for severe Clostridium difficile colitis has galvanized interest in gut microbiota based therapies for inflammatory conditions. P. histicola is one such human-gut bacterium that appears attractive Brug, which together with other bacteria or stem cell-based therapies could become frontline therapeutic options for treating MS and other immune mediated diseases. Given the rapidity with which novel therapies such as chimeric antigen receptor (CAR) T-cell are being adapted for immune cell therapeutics, we expect that there will similarly be a strong impetus for the clinical development of microbial therapies for the treatment and prevention of MS. Many patients are already exploring self-treatment with probiotics and dietary manipulations. However several refinement steps need to be taken before P. histicola can be prescribed as a treatment option in the clinic. Specifically, large scale toxicological and efficacy studies need to be performed in MS patients and on healthy individuals to ensure that treatment with P. histicola is a safe and effective therapeutic option. We foresee a time when there will be a whole variety of microbial derived options being explored in therapeutics of human disease. These could include monotherapies with potent single living bacteria (Brugs), administration of bacterial byproducts or components, manipulation of the background microbiome by alterations of diet, addition of prebiotics or targeted removal of bacteria and finally blocking or enhancing the hoist responses to the microbiome. Thus various clinical studies and experimental evidence suggest that Prevotella could be taken from bench to bedside in the form of Brug.
Key issues.
MS patients have gut dysbiosis with depletion and enrichment of certain gut bacteria
The majority of MS microbiome studies show that MS patients are depleted of, or have a lower abundance of, Prevotella
MS patients on disease-modifying therapies have higher levels of Prevotella compared to those that are untreated
Prevotella histicola has been isolated from the human gut and shows disease-suppressing abilities in a preclinical animal model of MS and in an animal model of rheumatoid arthritis
P. histicola suppresses disease by restoring gut permeability and by inducing immunoregulatory cells
P. histicola induces immunoregulatory cells by metabolizing phytoestrogens into equol and enterolactone, which share structural similarities to estrogen and regulate immune responses.
Due to its ability to indirectly modulate immune responses, P. histicola is a potential cell-based therapeutic agent for the treatment of MS
Rigorous testing to determine the safety and efficacy of P. histicola is needed before its use can be translated into the clinic.
Acknowledgments
Funding
This paper was not funded.
Declaration of interest
A.M. receives research support from the National Multiple Sclerosis Society (RG 5138A1/1) and the NIH (R01AI137075). A.M. and J.M. have a patent on the use of Prevotella histicola for treatment of autoimmune condition (Licensed to Evelo Biosciences) and receive royalties from Mayo Clinic (paid by Evelo Biosciences). J.M. also serves as a consultant for Evelo Biosciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Campbell JD, Ghushchyan V, Brett McQueen R et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: National US estimates. Mult Scler Relat Disord, 3, 227–36 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Jones E, Pike J, Marshall T, Ye X. Quantifying the relationship between increased disability and health care resource utilization, quality of life, work productivity, health care costs in patients with multiple sclerosis in the US. BMC Health Serv Res, 16, 294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saidha S, Eckstein C, Calabresi PA. New and emerging disease modifying therapies for multiple sclerosis. Ann N Y Acad Sci, 1247, 117–37 (2012). [DOI] [PubMed] [Google Scholar]
- 4.*.Miyake S, Kim S, Suda W et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One, 10, e0137429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This MS microbiome studies shows that RRMS patients have lower levels of Prevotella compared to healthy controls.
- 5.*.Chen J, Chia N, Kalari KR et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep, 6, 28484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This MS microbiome studies shows that RRMS patients have lower levels of Prevotella compared to healthy controls.
- 6.*.Jangi S, Gandhi R, Cox LM et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun, 7, 12015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This MS microbiome studies shows that RRMS patients have lower levels of Prevotella compared to healthy controls. This study also shows that disease modifying therapies (Copaxone and/or interferonβ−1b) restored levels of Prevotella in MS patients compared to untreated groups.
- 7.Tremlett H, Fadrosh DW, Faruqi AA et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol, 23, 1308–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berer K, Gerdes LA, Cekanaviciute E et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cekanaviciute E, Yoo BB, Runia TF et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.**.Cosorich I, Dalla-Costa G, Sorini C et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv, 3, e1700492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that disease modifying therapies (Copaxone and/or interferonβ−1b) restored levels of Prevotella in MS patients compared to untreated groups. This study also shows that MS patients with high disease activity and higher Th17 cell frequencies had decreased levels of Prevotella strains in small intestine tissues compared to healthy controls and MS patients with no disease activity. Thus this study provides direct evidence for inverse correlation between high disease activity and Th17 cells with lower levels of Prevotella in the small intestine.
- 11.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature, 486, 207–14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.**.Shahi SK, Freedman SN, Mangalam AK. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes the majority of MS microbiome studies and discusses possible mechanisms through which gut bacteria might modulate the disease.
- 13.Scher JU, Sczesnak A, Longman RS et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife, 2, e01202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**.Marietta EV, Murray JA, Luckey DH et al. Human Gut-Derived Prevotella histicola Suppresses Inflammatory Arthritis in Humanized Mice. Arthritis Rheumatol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes the majority of MS microbiome studies and discusses possible mechanisms through which gut bacteria might modulate the disease.
- 15.*.Mangalam A, Shahi SK, Luckey D et al. Human Gut-Derived Commensal Bacteria Suppress CNS Inflammatory and Demyelinating Disease. Cell Rep, 20, 1269–77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that P. histicola can suppress disease in animal models of MS and RA. Additionally, this study indicates that P. histicola suppresses disease through induction of immunoregulatory cells and suppression of pro-inflammatory immune response.
- 16.*.Yissachar N, Zhou Y, Ung L et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell, 168, 1135–48 e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that P. histicola can suppress disease in animal models of MS and RA. Additionally, this study indicates that P. histicola suppresses disease through induction of immunoregulatory cells and suppression of pro-inflammatory immune response.
- 17.Atarashi K, Tanoue T, Oshima K et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 500, 232–6 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell, 157, 121–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KS, Hong SW, Han D et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science, 351, 858–63 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol, 16, 295–309 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Begum-Haque S, Telesford KM et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes, 5, 552–61 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Denic A, Wootla B, Pirko I, Mangalam A. Pathophysiology of Experimental Autoimmune Encephalomyelitis. In: Multiple Sclerosis- A Mechanistic View Minagar, A (Ed. (Elsevier, 2015) 249–80. [Google Scholar]
- 23.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science, 265, 1237–40 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev, 206, 132–48 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Nouri M, Bredberg A, Westrom B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One, 9, e106335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issazadeh-Navikas S, Teimer R, Bockermann R. Influence of dietary components on regulatory T cells. Mol Med, 18, 95–110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berer K, Boziki M, Krishnamoorthy G. Selective accumulation of pro-inflammatory T cells in the intestine contributes to the resistance to autoimmune demyelinating disease. PLoS One, 9, e87876 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rescigno M Dendritic cell-epithelial cell crosstalk in the gut. Immunol Rev, 260, 118–28 (2014). [DOI] [PubMed] [Google Scholar]
- 29.De Filippo C, Cavalieri D, Di Paola M et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A, 107, 14691–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovatcheva-Datchary P, Nilsson A, Akrami R et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab, 22, 971–82 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Tsuchihashi R, Sakamoto S, Kodera M, Nohara T, Kinjo J. Microbial metabolism of soy isoflavones by human intestinal bacterial strains. J Nat Med, 62, 456–60 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Schogor AL, Huws SA, Santos GT et al. Ruminal Prevotella spp. may play an important role in the conversion of plant lignans into human health beneficial antioxidants. PLoS One, 9, e87949 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atarashi K, Tanoue T, Shima T et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 331, 337–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith PM, Howitt MR, Panikov N et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haghikia A, Jorg S, Duscha A et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity, 43, 817–29 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol, 13, 869–74 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Arpaia N, Campbell C, Fan X et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature, 504, 451–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A, 111, 2247–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. The Journal of nutrition, 133 Suppl 3, 956S–64S (2003). [DOI] [PubMed] [Google Scholar]
- 40.Sirotkin AV, Harrath AH. Phytoestrogens and their effects. Eur J Pharmacol, 741, 230–6 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Branca F, Lorenzetti S. Health effects of phytoestrogens. Forum Nutr, 100–11 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Rietjens IM, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laffont S, Garnier L, Lelu K, Guery JC. Estrogen-mediated protection of experimental autoimmune encephalomyelitis: Lessons from the dissection of estrogen receptor-signaling in vivo. Biomed J, 38, 194–205 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci, 1089, 343–72 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Lang TJ. Estrogen as an immunomodulator. Clin Immunol, 113, 224–30 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med, 339, 285–91 (1998). [DOI] [PubMed] [Google Scholar]
- 47.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol, 33, 105–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res, 175, 239–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masilamani M, Wei J, Sampson HA. Regulation of the immune response by soybean isoflavones. Immunol Res, 54, 95–110 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe, 12, 140–7 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Rafii F The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites, 5, 56–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes, 6, 271–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takagi A, Kano M, Kaga C. Possibility of breast cancer prevention: use of soy isoflavones and fermented soy beverage produced using probiotics. Int J Mol Sci, 16, 10907–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J. Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr, 103, 929–38 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update, 16, 745–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu AH, Spicer D, Garcia A et al. Double-Blind Randomized 12-Month Soy Intervention Had No Effects on Breast MRI Fibroglandular Tissue Density or Mammographic Density. Cancer Prev Res (Phila), 8, 942–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitfield-Cargile CM, Cohen ND, Chapkin RS et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes, 7, 246–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu W, Sun M, Chen F et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol, 10, 946–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol, 58, 1221–7 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Hughes ER, Winter MG, Duerkop BA et al. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe, 21, 208–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo-Alvarez F, Perez-Matute P, Oteo JA, Marzo-Sola ME. The influence of interferon beta-1b on gut microbiota composition in patients with multiple sclerosis. Neurologia, (2018). [DOI] [PubMed] [Google Scholar]


