Abstract
The present study aimed to determine whether grape seed extract (GSE) procyanidin mix, and its active constituent procyanidin B2 3, 3”-di-O-gallate (B2G2) have the potential to target cancer stem cells (CSCs) in prostate cancer (PCa). The CSC populations were isolated and purified based on CD44+-α2β1high surface markers in PCa cell lines LNCaP, C4–2B, 22Rv1, PC3, and DU145, and then subjected to prostasphere formation assays in the absence or presence of GSE or B2G2. Results indicated that at lower doses (<15 μg), the GSE procyanidin mix produced activity in unsorted PCA cells, but not in sorted; however, multiple treatments with low dose GSE over a course of time inhibited sphere formation by sorted PCA CSCs. Importantly, B2G2 demonstrated significant potential to target both unsorted and sorted CSCs at lower doses. As formation of spheroids, under specific in vitro conditions, is a measure of stemness, these results indicated the potential of both GSE and B2G2 to target the self-renewal of CSC in PCa cell lines, though B2G2 was more potent in its efficacy. Subsequent mechanistic studies revealed that both GSE procyanidins and B2G2 strongly decreased the constitutive as well as Jagged1 (Notch1 ligand)-induced activated Notch1 pathway. In totality, these in vitro studies warrant extensive dose-profiling based assessments in in vivo settings to conclusively determine the impact on CSC pool kinetics on the efficacy of both GSE and B2G2 to target PCa growth as well as tumor relapse.
Keywords: Grape seed extract, procyanidins, chemoprevention, prostate cancer, prostate cancer stem cells, B2G2
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy in American men; statistical estimates for year 2018 indicate 164,690 new PCa cases and 29,430 associated deaths in United States alone- despite remarkable advances in screening efforts and treatment strategies.[1] Conventional treatment strategies such as surgery, radiotherapy, chemotherapy are effective in the initial phase of disease; however, during advanced stages of disease, when local therapy may not be advisable, the only treatment options available are hormone ablation therapy or cytotoxic chemotherapy.[2] While hormonal ablation therapy effectively shrinks tumor, in most cases the disease progresses through a castrate-resistant state followed by further differentiation into a lethal malignancy.[2]
In light of this, there is also mounting evidence that prostatic neoplastic lesions are generated and maintained by a small sub-set of undifferentiated cells known as tumor initiating cells (TICs) or cancer stem cells (CSCs) which are inherently resistant to hormone, chemo- and radio-therapies, and their presence could be the major reason for the failure of most of the current therapies against PCa.[3-6] Thus, there is an emerging need to discover and/or develop agents that have the potential to target these ‘initiated’ stem cells and eradicate the pool of CSCs (which otherwise escapes the therapeutic insult). [4] Recently, there is increasing evidence showing that non-toxic naturally occurring dietary compounds have protective efficacy against PCa growth and progression; however, not much is known about their potential to specifically target PCa CSCs.[2] In this regard, several pre-clinical in vitro and in vivo efficacy studies by us and others have demonstrated the strong anti-cancer potential of the non-toxic dietary agent ‘grape seed extract’ (GSE) against PCa.[7-13] GSE is a polyphenolic mixture that contains dimers, trimers and other oligomers (procyanidins) of catechin and epicatechin and their gallate derivatives which together are known as proanthocyanidins. [9] Furthermore, using fractionation based biological activity assays our group recently identified procyanidin B2–3,3’-di-O-gallate (B2G2) as a major bioactive constituent of GSE which caused growth inhibition and induced apoptotic death of human PCa cells. [14-16] However, the efficacy of the parent GSE procyanidin mix and B2G2 towards CSCs in PCa has not yet been established; the present study is an effort in this direction. Importantly, our lab group together with a team of medical oncologists has recently initiated a phase II clinical trial (CT.gov – NCT#: NCT03087903) to investigate GSE effectiveness in a cohort of PCa survivors who have undergone treatment but show signs of rising prostate specific antigen (PSA) after local therapies. Thus, any positive outcomes against prostate CSCs can be of immediate translational significance and can be used to extend the trial in patient cohorts marred by tumor recurrence/tumor relapse with CSC enriched tumors.
For the studies in this communication, we employed a panel of human PCa cell lines which ranged from ‘classical’ cell lines to the new variants that differed in their androgen responsiveness, castration resistance, and metastatic potential.[17] The classical cell lines chosen were PC3, DU145 and LNCaP. Of these cell lines: PC3 and DU145 have their origins from human bone and brain metastatic deposits, respectively, and both cell types do not need androgens for growth, i.e, these are androgen independent (AI); they also lack androgen receptors (AR), prostate specific antigen (PSA), prostate cancer antigen 3 (PCA3/ DD3), 5α-Reductase, and have the potential to generate tumors upon inoculation in immunocompromised (nude) mice.[17] The LNCaP cell line on the other hand, though established from a human lymph node metastatic deposit, demonstrates androgen sensitivity (AS) and also requires androgens for its growth; while it harbors a mutated AR, it also does possess PSA and DD3.[17] However, LNCaP is poorly tumorigenic on its own in nude mice and requires co-inoculation with either mesenchymal/ fibroblast/ stromal cells or matrigel for its immunogenicity.[17] Of the new variants of PCa cell lines available, we further chose a castration resistant variant of LNCaP, C4–2B, derived from the xenografts of castration resistant LNCaP subline-C4 in castrated mice. C4–2B thus represents castration resistant PCa (CRPC) cell line; while it does not require androgen for growth it demonstrates androgen sensitivity due to the presence of AR and is thus categorized as CRPC (AI/ AS) variant. [17] C4–2B is also highly tumorigenic even in the absence of stromal mesenchymal factors in castrated mice and demonstrates metastatic potential. Another selected PCa cell line was 22Rv1; 22Rv1 was originally derived from the AI human PCa xenograft (CWR22R) obtained from serial propagation after castration –induced regression and prolapse of primary xenografts of androgen-dependent CWR22 human PCa cell line.[17] This AI cell line, 22Rv1, from variant CWR22R does not require androgen for growth but is androgen responsive and like C4–2B is categorized as CRPC (AI/ AS).
MATERIALS & METHODS
Cell lines and reagents
Human PCa cell lines viz., LNCaP, 22Rv1, PC3, and DU145 cells were obtained from American Type Culture Collection (Manassas, VA), while C4–2B cells were purchased from ViroMed Laboratories (Minneapolis, MN). All these cell lines were tested, and authenticated by DNA profiling for polymorphic short tandem repeat markers at University of Colorado cDNA sequencing & Analysis Core. RPMI1640, DMEM/F12, supplement B27, and N2, fetal bovine serum (FBS), penicillin-streptomycin, human recombinant Fibroblast Growth Factor-basic (rhFGF-b), and trypsin were purchased from Invitrogen Corporation (Gaithersburg, MD). Antibodies for cleaved Notch1, total Notch1 and anti-rabbit peroxidase-conjugated secondary antibody were from Cell Signaling (Danvers, MA) and human recombinanat Epidermal Growth Factor (rhEGF) was from Millipore (Billerica, MA). Propidium iodide (PI), dimethylsulfoxide (DMSO), accutase, and β-actin antibody were procured from Sigma-Aldrich (St.Louis, MO). GSE was a gift from Kikkoman Corporation (Noda City, Japan) and its details about composition and characterization are published earlier [14,18], and B2G2 was synthesized by author M.F.W (Director Medicinal Chemistry Core facility, UC Denver) as described previously; notably, our initial efforts led to isolation of B2G2 as the most active constituent in GSE, but its exact amount in GSE could not be determined due to hundreds of compound in GSE and major loss of material during fractionation.[16]
Cell culture and treatments, and Notch RT2 profiler™ PCR array
LNCaP, C4–2B, 22Rv1, PC3, and DU145 cells were cultured in RPMI1640 medium containing 10% FBS and 1% penicillin-streptomycin under standard culture conditions. At 60–65% confluency, cells were treated for 6–72 h with desired doses of GSE (5–150 μg/ml) or B2G2 (25–100 μM) in DMSO or with DMSO alone. Unless specified otherwise, the final concentration of DMSO in the culture medium during different treatments did not exceed 0.1% (v/v). Whole-cell extracts were prepared as described previously. [16] Cell viability, clonogenic assays, immunoblotting, and immunofluorescence procedures were done as described previously. [16,19] In studies with Jagged −1, PCa cells were plated overnight and further incubated in serum-free medium for 24 h; drug treatments were done 30 min after Jagged1 exposure. Human Notch RT2 profiler™ PCR array (SAbiosciences, Valencia, CA) was used (performed on Applied Biosystems® 7500 Cycler) as per manufacturer’s instruction to assess the effect of B2G2 on expression of genes involved in Notch signaling pathway.
Fluorescent-activated cell sorting (FACS), prostaspheres formation assay and Immunofluorescence (IF) staining
FACS was performed using standard protocol, 5–10 million (LNCaP, C4–2B, 22Rv1, PC3 and DU145) adherent cells were collected and washed to remove serum, then suspended in a sorting buffer and labelled with Alexa Fluor @488 mouse/human CD44-cloneIM7 (BioLegend, San Diego) and mouse anti-human α2β1 integrin (Millipore, Billerica, MA), and subsequently stained cells were sorted using a FACS machine (Becton-Dickson, CA). The sorted CD44+ -α2β1high subpopulation was used for the prostaspheres formation assay in serum free DMEM/F12 media supplemented with 20 ng/ml rhEGF, 10 ng/ml rhFGF-b, 2% B27 and 1% N2 supplement. The sorted single cell suspension was plated in 6-well ultra-low attachment plates (Corning) at a density of 3000 cells/well. Cells were either treated once (single treatment) or multiple times (every fourth day) for 14 days with GSE or B2G2, and at the end of the experiment (after 14 days), spheres were examined under a microscope at 40X magnification, and the number and diameter of prostaspheres were counted and measured in four independent fields per well using the Axio Vision microscopy software 4.7(Carl Zeiss, German). In another experiment, where second and third generation prostaspheres were observed, prostaspheres exposed once with 25–50 μM dose of B2G2 (C4–2B and DU145) were collected and dissociated with accutase (Sigma Aldrich, St. Louis) to obtain single-cell suspensions. Live cells were replanted for 14 days without any treatment on ultra-low attachment plate to form second generation prostaspheres. Further, the second generation prostaspheres were dissociated into single cell and used for the third generation prostaspheres culture. IF and confocal based z –stack analysis of prostaspheres was done as described previously by us.[19]
Statistical analysis
Statistical significance of differences between control and treated samples were calculated by one-way ANOVA followed by Bonferroni’s test using Sigma Stat version 2.03 software (Jandel Scientific, San Rafael, CA), and both sided P values of ≤0.05 were considered significant. The representative data in all cases are from at least two different studies each done in triplicate with reproducible results. Fisher’s Exact test was applied to compare degree of colony formation in control vs. GSE group. All microscopic analysis was done using a Zeiss Axioscope 2 microscope (Carl Zeiss Inc, Germany) and photomicrographs were captured by Carl Zeiss AxioCam MrC5 camera.
RESULTS
GSE procyanidins inhibit growth and clonogenic potential of human PCa cells
Trypan Blue dye exclusion assays utilizing doses ranging from 5–150 μg/mL of procyanidins were performed to determine GSE procyanidin effects, as a function of time, on the viability of the selected human PCa cell lines (Fig. S1). Results indicated that the androgen dependent LNCaP cell line and its castration resistant variant, C4–2B, were more sensitive to procyanidins treatment at lower doses [58–95% and 79–99% (P<0.001, for both) growth inhibition of LNCaP cells and C4–2B cells, respectively, after 48 h exposure with 5–15 μg/mL procyanidins]. Importantly, another castration resistant cell line, 22Rv1, also showed significant decrease in cell viability at lower doses [36–83% (P<0.001, 48 h)}. While at these lower doses significant cell death was observed even at time points as early as 12 h in LNCaP (31–81%, P<0.001), C4–2B (44–66%, P<0.001), and 22Rv1 (3–28%, P<0.001) cell lines; the classical AI cell lines PC3 and DU145 were most resistant to procyanidin treatment at lower doses and showed sensitivity (growth inhibition and cell death) only at higher doses (50 μg/ mL and above) and that too after 72 h of procyanidins exposure (Fig. S1).
Since we observed a strong reduction in cell numbers by procyanidins in PCa cell lines, we next assessed the effect of this agent on clonogenicity of human PCa cells by performing a colony formation assay and counting the number of colonies formed (≥ 50 cells) after 10 days of drug exposure (Fig. S1). The assay results indicated that treatment with procyanidins (1.25–5 μg/ml every 48 h) for 10 days significantly inhibited the colony formation by 50–100% (P<0.05–0.001), 34–99% (P<0.001) and 29–60% (P<0.05–0.001) in LNCaP, C4–2B and 22Rv1 cells, respectively, while dose strengths more than 10 μg/ml completely inhibited the colony formation in these cells [100%, P<0.001, (Fig. S1)]. On the other hand, the lower strength doses (up to 15 μg/ml) demonstrated less inhibitory effect against PC3 and DU145 colonies (Fig. S1), and it was only the higher doses of procyanidins (≤50 μg/ml; Fig. S1) which could significantly inhibit [100% (P<0.001)] and [74% (P<0.01)] colony formation in PC3 and DU145 cells, respectively (Fig. S1).
Differential inhibitory effect of GSE procyanidins and its biologically active constituent against the self-renewal potential of prostate cancer stem cells
It has been widely accepted that human cancer is encompassed of heterogeneous population of cells which include bulk tumor cells as well as CSCs which retain the ability to renew and differentiate.[3,4,20] Similarly, PCa tumors are heterogeneously populated and cell surface markers have been used to identify the CSCs in these tumors. Though no markers are truly stem cells specific, human PCa cells expressing the markers CD44+-α2β1high are now recognized as harboring the tumor initiating capacity (TIC) which is a characteristic of CSCs; these cells are 100–1000 times more tumorigenic than the corresponding non-side population cells.[5,21] Accordingly, employing the FACS technique, we sorted the CD44+-α2β1high expressing CSC enriched population from LNCaP, C4–2B, 22Rv1, PC3, and DU145 cells and subjected these to spheroid formation assay, a stem cell characteristic, in the presence and absence of GSE procyanidins (Fig. 1). Briefly, in the spheroid formation assay, the isolated CD44+-α2β1high enriched PCa cells were subjected to varying concentrations of GSE procyanidins, either as single exposure (Fig. 1A) or multiple dosing regimens, spread over a course of 14 days (Fig. 1B). Results indicated that the CSCs were resistant when treated with the procyanidins (single exposure) only once at the beginning of the spheroid formation assay, and that it required multiple exposures (procyanidin exposure every 4th day after 1st day of treatment) to see a complete inhibition of prostasphere formation. Importantly, the multiple dosing regimens caused a significant reduction in both number and size of generated prostaspheres (Fig. 1B). As observed earlier in viability experiments, the CSCs isolated from LNCaP, C4–2B and 22Rv1 cells were more sensitive to lower procyanidin doses compared to PC3 and DU145 prostaspheres. Specifically, multiple exposure with procyanidin doses (5–15 μg/ml) decreased the prostasphere formation by ~20–100% (P<0.001) in LNCaP and C4–2B CSC enriched cells, while multiple dosing with doses ≥15 μg/ml decreased the prostasphere formation in 22Rv1 CSCs by ~33–100% (P<0.001). In PC3 and DU145 CSCs, the complete inhibition of prostasphere formation was evident only after exposure to significantly higher doses (>75ug/ml) of procyanidins.
Fig. 1. Effect of GSE procyanidins on the formation of CSC enriched prostaspheres in LNCaP, C4–2B, 22Rv1, PC3, and DU145 PCa cells.
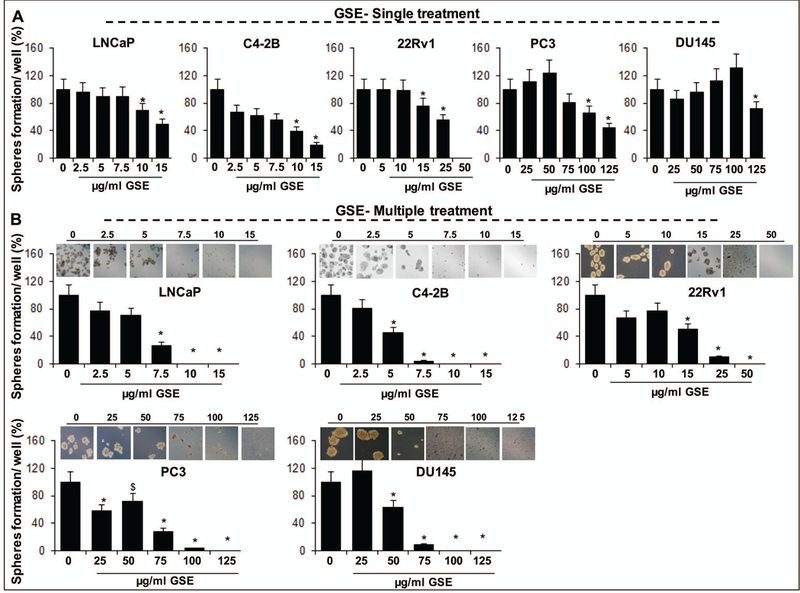
Effect of different concentrations of GSE procyanidins, given as (A) single treatment or (B) multiple treatments, on CSC (CD44+-α2β1high) enriched prostaspheres formation. Treatments were done either as single exposure (only once at the beginning of the spheroid formation assay) or multiple dosing regimens (exposure every 4th day after 1st day of treatment) spread over a course of 14 days. Representative photomicrographs (X400 magnification) of CSC enriched prostaspheres depict a decrease in their number and size by multiple treatments of GSE. In all bar graphs statistical differences are shown w.r.t. respective controls. $, P<0.05; *, P<0.001. C, control; GSE, grape seed extract.
Given that the GSE procyanidins are a complex mix of higher and lower molecular weight procyanidins that may or may not have the individual capacity to target prostate CSCs and could compromise the overall potential of a specific GSE constituent to target CSCs; we next determined whether B2G2, which was recently identified by us as the most effective constituent in GSE for growth inhibition and apoptotic death of human PCa cells[14,16], possesses the inherent potential to target the prostate CSC population. As in the case of GSE procyanidins, CD44+-α2β1high enriched PCa cells were subjected to varying concentrations of B2G2 (25–100 uM) in spheroid formation assay, either as single exposure (Fig. 2A) or multiple dosing regimens, spread (Fig. 2B) over a course of 14 days. Notably, a single exposure of B2G2 (25–100 uM) significantly reduced the number of prostaspheres generated in LNCaP (68–100%, P<0.001), C4–2B (63–100%, P<0.001), 22Rv1 (24–100%, P<0.001), PC3 (27–71%, P<0.001), and DU145 (58–100%, P<0.001) cells, and the % inhibition was comparable to the inhibition observed in the presence of multiple dosing regimen. Not only B2G2 treatment caused a less number of prostasphere formation, the ones formed also had a significantly reduced size. As formation of spheroids under specific in vitro conditions is a measure of stemness, these results indicate that B2G2 was more potent in its efficacy against self-renewal of CSCs in PCa cell lines compared to GSE procyanidin mix, and that there was clearly no additional advantage of multiple dosing of the spheroids with B2G2 treatment; a single dose of B2G2 could suffice to target the self-renewal capacity of CSCs, unlike the procyanidin mix.
Fig. 2. Effect of B2G2 (an active constituent of GSE procyanidins) on the formation of CSC enriched prostaspheres in LNCaP, C4–2B, 22Rv1, PC3, and DU145 PCa cells.
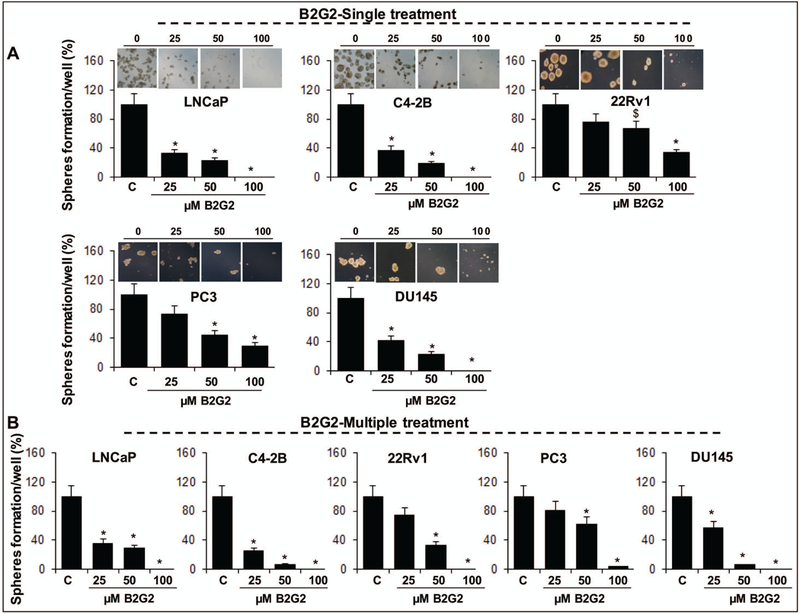
Effect of different concentrations of B2G2 given as (A) single treatment or (B) multiple treatments on CSC enriched prostasphere formation. Treatments were done either as single exposure (only once at the beginning of the spheroid formation assay) or multiple dosing regimens (exposure every 4th day after 1st day of treatment) spread over a course of 14 days. Representative photomicrographs (X400 magnification) of CSC enriched prostaspheres depict a decrease in their number and size by single treatment of B2G2. In all bar graphs statistical differences are shown w.r.t. respective controls. $, P<0.05; *, P<0.001. C, control; B2G2, procyanidin B2 3,3″-di-O-gallate.
B2G2 decreases enrichment of the CD44-α2β1high expressing CSCs in human PCa cells
To determine whether the inhibitory effect of B2G2 on number of prostaspheres formed in spheroid formation assay, involves specific targeting of CD44+-α2β1high enriched PCa cells, we subjected the B2G2 treated unsorted C4–2B and DU145 cells as well as spheroids (from sorted cells after single treatment of 50 μM B2G2) to FACS based cell sorting analysis. Results indicated (Fig. 3A) that CD44+-α2β1high expressing positive cells were significantly decreased in cell culture (~2% in B2G2 treated vs. 9% positive cells in untreated C4–2B controls; and 14% in B2G2 treated vs. 26% positive cells in untreated DU145 controls). Similar reductions in CD44+-α2β1high expressing positive cells were also observed in the prostaspheres after single treatment of 50 μM B2G2 as compared to their respective controls (data not shown). Z stack analysis of prostaspheres after IF staining for CD44 and CSC associated transcription factor SOX-2 indicated that the protein expression of CD44 was higher in the peripheral part of C4–2B and DU145 prostaspheres which was significantly decreased after B2G2 exposure (Fig. 3B); similarly, expression of SOX-2 was also significantly decreased in B2G2 exposed prostaspheres (Fig. 3B). Next, control and B2G2 treated prostaspheres were subjected to 2D differentiation assays (Fig. 3C) in presence of serum containing media to determine whether B2G2 has the potential to induce more differentiated clones. Phase contrast microscopy in 2D assay showed that the spheres had started to adhere to bottom of the plate and showed signs of differentiation early-on in B2G2 treated groups (data not shown). The adhered clones and cellular spread in plate were subjected to IF staining for prostate epithelial cell luminal marker CK18, which showed a higher expression of CK18 in B2G2 treated prostaspheres and their cellular spread (Fig. 3C) indicating increased differentiation by B2G2 compared to controls.
Fig. 3. A) Effect of B2G2 on CD44+-α2β1high expression in human PCa C4–2B and DU145 cells.
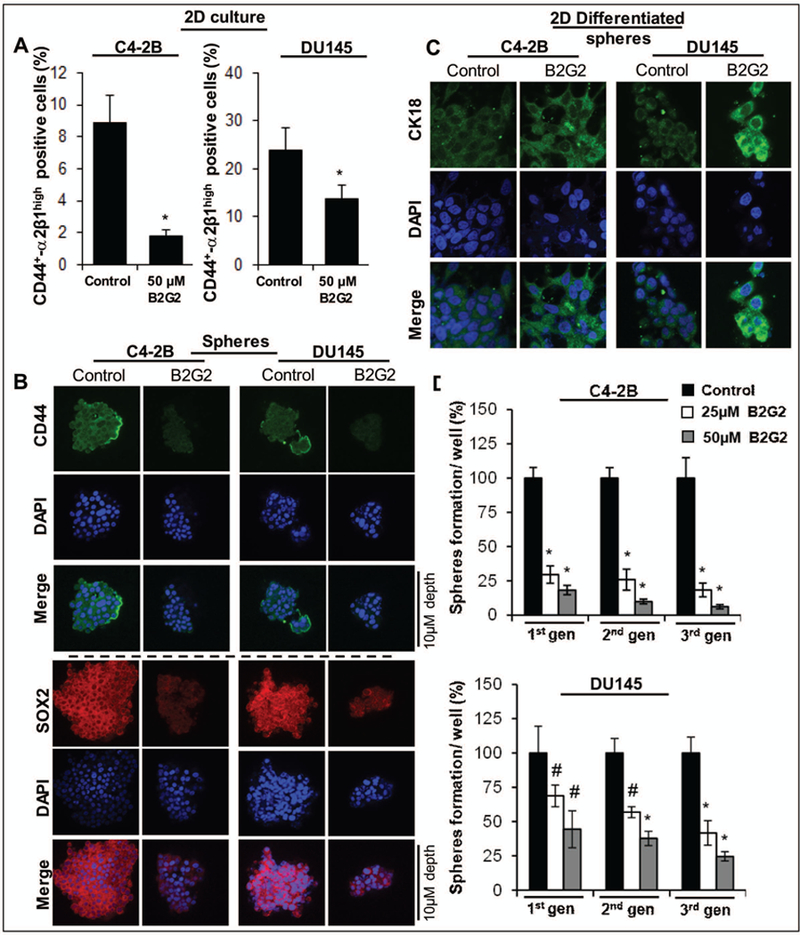
The bar graph shows the percentage of CD44+-α2β1high positive cells in B2G2 treated group vs. their respective DMSO controls. In all bar graphs, statistical differences are shown w.r.t. DMSO controls. B) Effect of B2G2 on protein expression of CSC associated-marker CD44, and -transcription factor SOX-2 in C4–2B and DU145 prostaspheres. Z stack analysis was performed as detailed in ‘Materials and Methods’ section and representative scans (X 600) with individual scan depth of specific prostaspheres are shown. C) Effect of B2G2 on 2D differentiation of CSC enriched prostaspheres. For 2D differentiation, prostaspheres (with or without B2G2 treatment) were allowed to differentiate under adherent culture conditions in the presence of regular culture media (with 10% FBS). Representative IF staining photomicrographs (X600 magnification using confocal microscopy) of 2D differentiated prostaspheres with CK18 and DAPI as nuclear stain are shownx. D) Persistent inhibitory effect of B2G2 on prostasphere formation after sub-culturing in different generations in the absence of B2G2. Equal number of viable C4–2B and DU145 PCa cells from 1st generation prostaspheres (with or without B2G2 treatment) were re-plated for 2nd generation sphere cluster assay and this was process was repeated to generate 3rd generation spheres. The bar graph shows the percentage of prostaspheres formation from cells derived from prostaspheres previously exposed to B2G2 vs. DMSO treated controls. #; P<0.01; *; P<0.001. C, control; B2G2, procyanidin B2 3, 3″-di-O-gallate.
Furthermore, to determine whether B2G2 has a persistent inhibitory effect on the sphere forming ability of the CSCs, even in its absence, in the subsequent generations, the primary (1st generation) C4–2B and DU145 prostaspheres from control and B2G2 (25–50 μM) treatments were cultured again for two additional passages without B2G2 (Fig. 3D). Briefly, first generation prostaspheres (generated in the presence of 25 and 50 μM B2G2 for 14 days) were collected, dissociated and equal number of live cells were seeded (1000 cells/well) for fresh spheroid formation assays in the absence of B2G2, and allowed to form second generation prostaspheres (Fig. 3D). This procedure, using the live cells from 2nd generation prostaspheres was repeated again to determine whether the effect of B2G2 on the sphere forming ability of CSCs persists in next generations (Fig. 3D). As shown in Figure 3D, the prostaspheres generated in subsequent generations by cells isolated from B2G2 treated groups in previous generation were less compared to those generated from untreated controls [C4–2B: (70–82%, P<0.001, B2G2 dose 25 μM); (82–94%, P<0.001, B2G2 dose 50 μM), and DU145: (32–59%, P<0.001, B2G2 dose 25 μM);-(66–76%, P<0.001, B2G2 dose 50 μM)], suggesting a reduced self-renewal capacity of stem/progenitor population in C4–2B and DU145 cells by B2G2 in next generations (Fig. 3D).
Given that unlike GSE treatment, a single dosing regimen of B2G2 was as effective as multiple exposures with GSE procyanidins against prostaspheres, we decided to investigate the characteristics of the PCa cells unaffected by a single exposure of GSE but responded to multiple exposures. We used C4–2B cells to delineate the mechanism (Fig. 4) involved in a single dose “GSE inefficacy”, as in viability assays these PCa cells had shown increased sensitivity to GSE treatment at early time points, but similar inhibitory effects were not observed under spheroid assay propagation. As a first investigative step (Fig. 4A), we treated unsorted C42B cells with different ‘single’ doses of GSE procyanidins (as done earlier) and counted remaining live and dead cells at different time points. Interestingly, we observed that exposure with lower doses of GSE for 6 h caused significant induction of cell death, 20% (2.5μg/ml, P<0.001) and 74% (5 μg/ml, P<0.001, Fig. 4A). Similarly, GSE treatment (2.5–5 μg/ml) for 12 h also significantly inhibited cell growth and induced cell death as compared to the control cells (Fig. 4A). Interestingly, 2.5 μg/ml GSE for 24 −48 h showed almost the same number of live cells (9.0×105, P<0.001) as the control cells (8.4×105) and simultaneously dead cells were significantly fewer as compared to the 6–12 h treatment (Fig. 4A, right panel). Furthermore, 5 μg/ml GSE for 48 h showed significantly lower number of dead cells as compared to the 6–24 h treatment of GSE (Fig. 4A, right panel). In summary, it meant that the left over C4–2B cells after 24 h exposure of lower doses of GSE (2.5–5 μg/ml, Fig. 4A) relapse (start regrowth/proliferate), while higher doses of GSE (10 μg/ml and above) completely inhibited the cells growth (data not shown). Thus, there is a possibility that in case of unsorted cells, the lower doses of GSE target only the bulk tumor cells not the CSCs; plausibly, the left-over cells are the CSCs rich population and these cells start growing back after 24–48 h of 2.5 μg/ml GSE treatment (the number of live cells are approx. similar to the DMSO controls). However, higher doses of GSE (10 μg/ml and above) target both sorted CSCs and bulk cancer cells (both sorted and unsorted) via the apoptotic pathway as GSE is known to induce apoptosis in various cancer cells including PCa cells. To confirm this possibility, we determined the stemness of the left over unsorted PCa cells after GSE treatment (Fig. 4B). Results indicated that, GSE (5 μg/ml) treatment for 6–12 h significantly increased the CD44+-α2β1high positive (5–3%, P<0.05) population as compared to 2% in controls; however, at later time-points, both control and GSE treated groups showed almost comparable percentage (2%) of CD44+-α2β1high positive cells (Fig. 4B). To determine whether the stemness capacity of the left over cells after single exposure of GSE (2.5–5 μg/ml) was increased, we performed spheroid assays with the left over cells. Results indicated that left over cells after 6 h GSE treatment formed significantly more spheres (~1.5 fold increase) as compared to the controls (Fig. 4C-D); while sphere formation capacity in left over cells after 48 h of GSE treatment was comparatively less (~10–20% decrease) as compared to the left over cells after 6 h of GSE treatment. These results are very informative and indicate that the cells which relapse after a single (48 h) of GSE exposure are not enriched in CSCs as compared to the 6 h GSE-treated cells (Fig. 4C-D), explaining the need for multiple dosing regimen (Fig. 4D) with GSE procyanidins to target both CSCs and bulk tumor cells.
Fig. 4: Sequential delineation of GSE effects on cancer stem cells and bulk tumor population in C4–2B PCa cells.
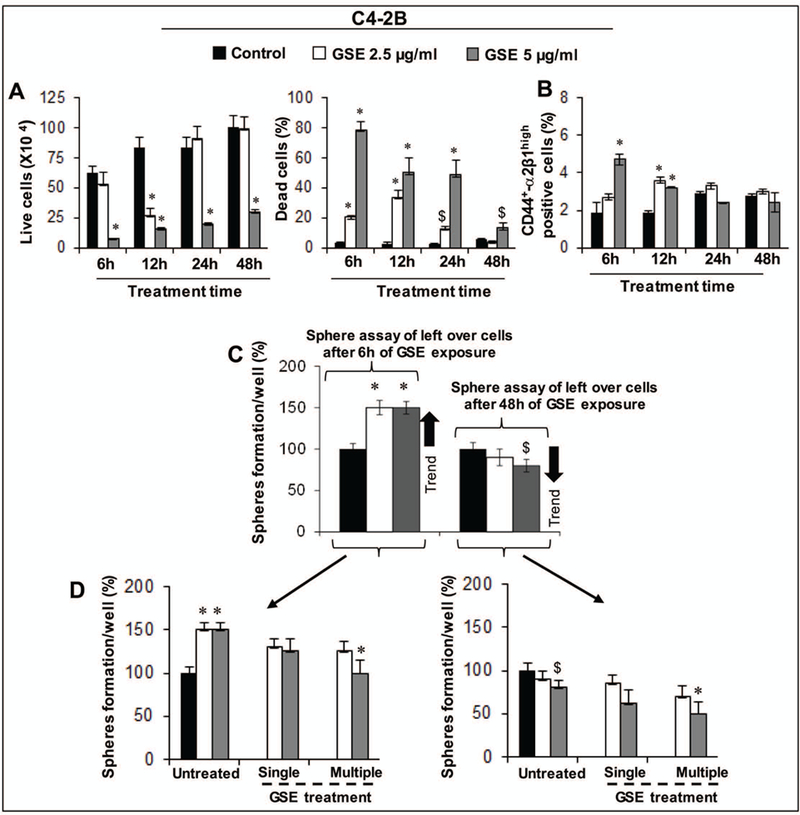
A) Time and dose- dependent effects of GSE (2.5–5 μg/ml) on the growth and death of C4–2B cells. B) The bar graph shows the percentage of CD44+- α2β1high positive C4–2B cells after treatment with GSE procyanidins (2.5–5 μg/ml) for 6–48 h or with DMSO control. C) Potential of C4–2B cells, which were viable after 6–48h of GSE procyanidins exposure, to form prostaspheres. D) Sphere cluster assay in ‘C’ was carried in the absence or presence of single or multiple GSE exposures. D-left panel depicts fate of spheres generated after initial 6h GSE procyanidin exposure, and D-right panel depicts fate of spheres generated after initial 48h GSE procyanidin exposure. Treatments were done either as single exposure (only once at the beginning of the spheroid formation assay) or multiple dosing regimens (exposure every 4th day after 1st day of treatment) spread over a course of 14 days. $, P<0.05; *, P<0.001 for differences with respective untreated group. C, control; GSE, grape seed extract.
GSE and B2G2 down-regulate Notch1 expression in prostate cancer cells
Next, we investigated the mechanisms that may contribute to the differential effect of GSE and B2G2 on prostate CSCs. The Notch1 pathway is an important controller of stem cell self-renewal process and its expression is increased in high grade prostate tumors in humans as compared to normal epithelial cells in adjacent areas of the same tissue. [22−24] Subsequent mechanistic studies revealed that both GSE procyanidins and B2G2 strongly decreased the constitutive as well as Jagged1 (Notch1 ligand)-induced generation of cleaved Notch in PCa cells; indicating inhibitory effect of the agents on activated Notch1 pathway (Fig. 5). Specifically, in the cell culture studies, short exposure (6–12hrs) with lower doses of GSE procyanidins (≥5ug/mL) had a more significant effect on decreasing constitutively activated Notch1 pathway (decreased cleaved-Notch 1 levels vs. no change in total Notch 1 levels) in LNCaP, C4–2B, and 22Rv1 cells compared to the effect observed in PC3 and DU145 cells, where increased exposure duration (12–72hrs) with higher doses of GSE procyanidins (≥50ug/mL) was required to observe similar inhibitory effects (Fig. 5A, left panel). Similarly, lower doses of B2G2 (≥ 25–100uM) decreased cleaved-Notch1 levels in LNCaP and C42B cells after 6–12 hrs of exposure, while higher exposure times and doses were required to observe similar effects in 22Rv1, PC3 and DU145 cells (Fig. 5A, right panel). Next, to determine whether these effects could also be observed when Notch1 pathway is activated by exogenous addition of Notch ligand Jagged 1, we performed cell culture studies (using C4–2B and DU145 PCa cells) in the presence of Jagged1 alone or in the presence of Jagged1 and GSE procyanidins or B2G2 under serum starved conditions (no FBS) (Fig. 5B). Interestingly, both agents were able to significantly decrease the Jagged1 induced formation of cleaved-Notch1; notably even expression of total Notch 1 levels were also decreased by GSE procyanidins and B2G2 in DU145 cells. However, the decrease in total Notch1 levels was only observed in C42B cells after exposure to B2G2 and not GSE procyanidins, indicating that the GSE caused decrease in (Jagged1 induced) cleaved Notch1 levels is not due to a decrease in total Notch1 levels (Fig. 5B). In line with the decrease in cleaved Notch1 levels, the protein expression of HES-1 molecule (effector transcription factor downstream of Notch signaling)[22−24] was also significantly decreased in both GSE and B2G2 treated PCa cells (Fig. 5C). Taken together all these cell culture studies indicate that both GSE procyanidins and its active constituent B2G2 have inhibitory effects against Notch1 signaling pathway (Fig. 5); however, it does not address the question why B2G2 is more effective than the GSE procyanidin mix against prostaspheres. These results were in line with the results observed earlier in viability and sphere assays indicating that increased capacity of lower dose GSE procyanidins and B2G2 to target the Notch pathway in only some of the PCa cells could be partly responsible for the increased sensitivity of these specific PCa cell lines to be targeted by lower doses of these agents. The implications of these effects are important, since the modulation of Notch signaling among stem cells and progenitor cells in PCa results in the expansion of CSC pool together with an increase in proliferative cell population.[22,23] Next, we analyzed the expression of cleaved Notch1 (after single exposure of B2G2 50 μM) in C4–2B and DU145 prostaspheres and found that cleaved Notch1 expression was significantly reduced in the treated spheres as compared to untreated controls (data not shown).
Fig. 5. Effect of GSE procyanidins and active constituent B2G2 on constitutively active and Jagged-1-induced cleaved Notch1 expression in human PCa cell lines.
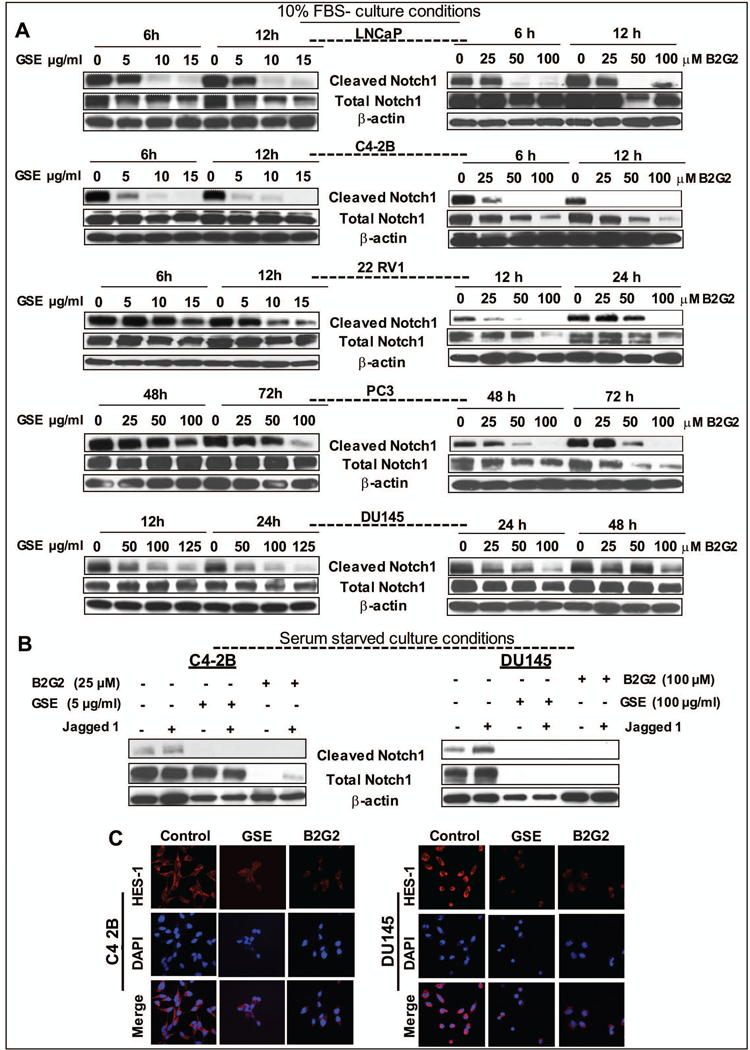
A) Both GSE procyanidins and B2G2 cause a dose-dependent decrease in the expression of constitutively active cleaved-Notch1 protein levels in LNCaP, C4–2B, 22Rv1, PC3, and DU145 PCa cells (under 10% FBS culture conditions). B) Both GSE procyanidins and B2G2 inhibit Jagged1 (Notch1 ligand)-induced cleaved Notch1 levels in C4–2B and DU145 cells (under serum free culture conditions). GSE procyanidins or B2G2 were added 30 min after Jagged1 treatment. At the end of the treatments, both adherent and non-adherent cells were harvested and total cell lysates were prepared. Lysates were subjected to SDS-PAGE followed by Western immunoblotting. Equal protein loading was confirmed by stripping and re-probing the membranes with β-actin. C) Decrease in the protein expression of HES-1 after exposure of C4–2B and DU145 cells with either GSE procyanidins or B2G2. Representative IF staining photomicrographs (X600 magnification using confocal microscopy) with HES-1 and DAPI as nuclear stain are shown. C4–2B cells were treated with 5μg/mL GSE or 25μM B2G2 while DU145 cells were treated with 100μg/mL GSE or 100μM B2G2 for 24 hrs. C control; GSE, grape seed extract; B2G2, procyanidin B2 3,3″-di-O-gallate.
Moreover, in RT2 PCR gene array analysis (Fig. 6), we found that B2G2 treatment was also able to differentially alter the gene expression levels of several molecules regulating the Notch signaling pathway in the prostaspheres of both the cell lines C4–2B and DU145 cells (Fig. 6); however, the inhibitory effects on the activation of this pathway could not be related to any transcriptional downregulation of associated genes and seemed more likely due to its interference in signal transduction cascades activating the Notch pathway. Together, our results are significant as they show that both GSE procyanidins and B2G2 could interfere with kinetics of CSC pool expansion via targeting Notch1 regulatory signals, which eventually could help control PCa growth and progression.
Fig. 6. Effect of B2G2 on gene expression of Notch signaling pathway members in CSC enriched prostaspheres.
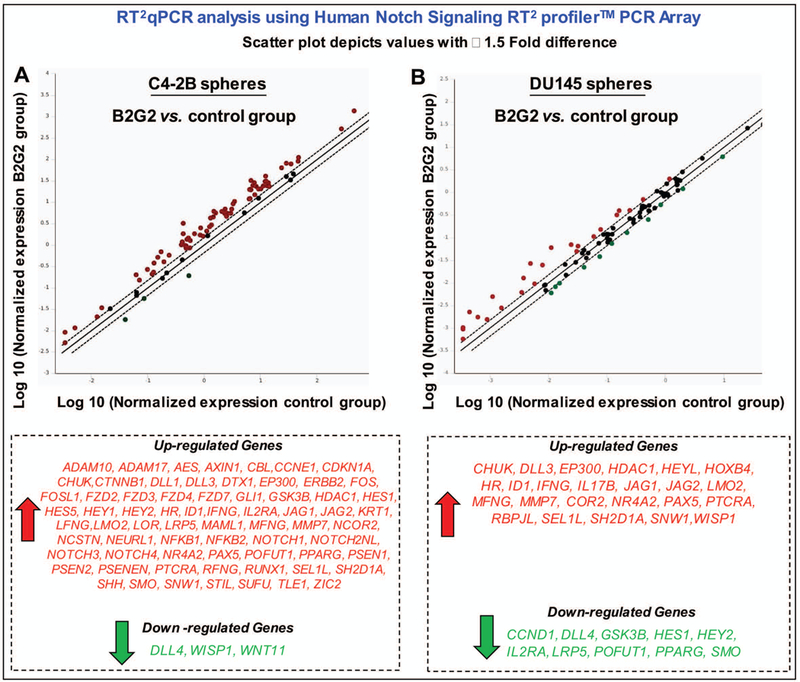
Effect of B2G2 on A) C4–2B, and B) DU145 prostaspheres. Trizol extraction method was used to extract total RNA from C4–2B and DU145 prostaspheres treated with 50 μM B2G2 or DMSO alone. Reverse transcription was performed using 2–3 μg of RNA and First strand system for RT-PCR (Qiagen), and subjected to RT2qPCR analysis using Human Notch Signaling RT2 profiler™ PCR Array (Qiagen) to assess the effect of B2G2 on expression of genes involved in Notch signaling pathway. The relative quantification of gene expression between control and B2G2 treated samples was achieved by normalization against endogenous GAPDH and β-Actin using the ΔΔCT method of quantification and the data was analyzed using the software provided by the manufacturer. C, Control; GSE, grape seed extract.B2G2, procyanidin B2 3,3″-di-O-gallate.
Discussion
One dietary supplement that has attracted attention in recent years is GSE [25]; it is widely consumed for its cardiovascular protective effects and benefits against metabolic syndrome-associated complications.[9,26-31] GSE also exerts free radical scavenging, anti-oxidant, anti-bacterial, anti-inflammatory, anti-viral, anti-allergic, and vasodilatory actions.[9,32,33] Clinically, GSE is prescribed for microcirculatory disorders including treatment of venous insufficiency in Europe. [32] Importantly, GSE is safe for long-term human consumption and has been categorized as GRAS ‘generally regarded as safe’ by FDA.[9,34] Most notably, in recently published vitamin and life-style cohort studies [35,36] analyzing the biological outcomes of several dietary supplements’ consumption for approximately 5–10 years, GSE stood out as the one associated with reduced risk for PCa [35] and hematological malignancies.[36]
Thus, the focus of the present study was to determine whether the anti-PCa efficacy of the parent GSE procyanidin mix and its biologically active constituent B2G2 differed in their potential to target PCa cells and their CSC population. Interestingly, while both compounds have shown comparable potential to target the bulk PCa cells under in vitro conditions; the androgen dependent LNCaP cell line and the AI/AS CRPC variants were more sensitive to both GSE procyanidins and B2G2 treatment at lower doses while the classical AI cell lines PC3 and DU145 showed sensitivity (growth inhibition and cell death) only at higher doses. An important observation was that at lower doses, GSE could preferentially target the bulk population and not the CSCs which results in increased fraction of CSC population. Given that the bulk cells are easily targeted, we see early cell death with GSE exposure. However, the remaining CSC population self-renew/ multiply after sometime which results in an increase in cell numbers. These results were corroborated in the CSC based spheroid assays where lower doses of GSE could not decrease the prostaspheres generated by CSC enriched population, and multiple dosing was required to elicit the anti-CSC effect. However, B2G2 demonstrated significant potential to target both bulk and CSCs at lower doses too, which resulted in decrease in the number/size of prostaspheres by a single dose of B2G2. Overall, the results indicated that the biologically active constituent ‘B2G2’ of the GSE procyanidin mix had more potent efficacy against PCa CSCs; while on the other hand in the GSE procyanidin mix, the overall potential to significantly target CSCs is relatively compromised. Thus, based on the above outcomes, one of the strategies to combat tumor cells efficiently, is a treatment approach that targets both CSC and non-CSC population; on the other hand, one has to be cautious when any drug (including chemopreventive agent) shows potent efficacy against non-CSC bulk tumor cells; there is a possibility of enriching the CSC population which could be more resistant to following drug treatments and result in tumor relapse. Notably, while lower doses of GSE procyanidin mix (unlike the higher doses) did cause such CSC enrichments; however, continued treatments over a course of time with lower doses seemed to push the CSC division towards an asymmetrical self-renewal (in which CSC generates one CSC and one 1st generation progenitor cell) or probably a symmetric commitment (in which CSC generates two 1st generation progenitor cells) which eventually results in GSE procyanidin mix to target these cells and result in a decrease in total cell numbers (both CSC and non-CSC cells).
Furthermore, mechanistic studies revealed that both agents strongly decreased the constitutive as well as Jagged1 (Notch1 ligand)-induced protein levels of cleaved Notch1 and its transcriptional target HES-1 in PCa cells. Recently, our studies have shown that the apoptotic and survival pathways mediated by the transcription factors NF-κB and STAT3 are significantly inhibited by B2G2 treatment.[16] Thus, there is a possibility that NF-κB and STAT3 pathways were regulated by cleaved Notch1 signaling as it is strongly inhibited in PCa cells by the B2G2 treatment. Given that CSCs are the major players behind the recurrence and are inherently resistant to chemo- and radio- therapy and possess the self-renewable capacity[3,4,37], the possibility to target both differentiated cells and CSCs in the tumor mass would help us to treat this deadly malignancy via tumor mass reduction and prevention of relapse. In totality, the results of the present study are highly significant and provide a strong rationale for further detailed preclinical investigation of these agents against PCa tumor recurrence driven by CSCs enrichment caused by current therapeutic regimens.
Supplementary Material
GSE procyanidins (A) inhibit growth, and (B) induce death in LNCaP, C4–2B, 22Rv1, PC3, and DU145 PCa cells, in both dose- and time dependent manner. Cells (1.05 × 105) were plated in 60 mm dishes, treated with DMSO (control) or different concentrations of GSE procyanidins, and after 12, 24, 48 or 72 h, cells were harvested and counted as detailed in ‘Materials and Methods’ section. C) GSE procyanidins inhibit the clonogenic potential of human PCa cells. LNCaP, C4–2B, 22Rv1, PC3, and DU145 cells (∼1 × 103) were plated in 6-well plates, after 24 h cells were treated with DMSO or different doses of procyanidins. Fresh media with DMSO or respective GSE dose was added every 48 h till 10 days after which the colonies were stained with crystal violet and true colonies (≥ 50 cells) were counted. The data shown are mean of 3 wells (in different plates-treated similarly). In all bar graphs statistical differences are shown w.r.t. respective DMSO controls. $, P<0.05; #, P<0.01; *, P<0.001. C, control; GSE, grape seed extract.
Acknowledgement:
This work was supported by the R01 grants CA91883 (to CA) and CA140368 and CA195708 (to RA) from the National Cancer Institute, and the generous support by the Diane D. Writer Foundation, and the Barbara and Richard Gardner Fund for Prostate Cancer Research. This work was also supported by UCCSG P30CA046934 for supporting the Shared Resources used in this study.
Abbreviations:
- PCa
prostate cancer
- AR
androgen receptor
- B2G2
procyanidin B2 3,3″-di-O-gallate
- GSE
grape seed extract
- CSCs
cancer stem cells
- FACS
Fluorescent-activated cell sorting
- AI
androgen independent
- PSA
prostate specific antigen
- PCA3
prostate cancer antigen3/DD3
Footnotes
Conflict of Interest Disclosures: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a Cancer Journal for Clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ting H, Deep G, Agarwal C, Agarwal R. The strategies to control prostate cancer by chemoprevention approaches. Mutation Research 2014;760:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasper S Stem cells: The root of prostate cancer? Journal of Cellular Physiology 2008;216:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. Journal of Clinical Oncology 2008;26:2862–2870. [DOI] [PubMed] [Google Scholar]
- 5.Tang DG, Patrawala L, Calhoun T et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Molecular Carcinogenesis 2007;46:1–14. [DOI] [PubMed] [Google Scholar]
- 6.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Research 2007;67:6796–6805. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 2002;23:1869–1876. [DOI] [PubMed] [Google Scholar]
- 8.Dhanalakshmi S, Agarwal R, Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. International Journal of Oncology 2003;23:721–727. [PubMed] [Google Scholar]
- 9.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. The Journal of Nutrition 2009;139:1806S–1812S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Molecular Cancer Therapeutics 2006;5:1265–1274. [DOI] [PubMed] [Google Scholar]
- 11.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Research 2007;67:5976–5982. [DOI] [PubMed] [Google Scholar]
- 12.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. International Journal of Cancer 2004;108:733–740. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene 2003;22:1302–1316. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2–3,3’-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2007;28:1478–1484. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Deep G, Wempe MF et al. Procyanidin B2 3,3”-di-O-gallate induces oxidative stress-mediated cell death in prostate cancer cells via inhibiting MAP kinase phosphatase activity and activating ERK1/2 and AMPK. Molecular Carcinogenesis 2018;57:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi A, Raina K, Shrestha SP et al. Procyanidin B2 3,3(“)-di-O-gallate, a biologically active constituent of grape seed extract, induces apoptosis in human prostate cancer cells via targeting NF-kappaB, Stat3, and AP1 transcription factors. Nutrition and Cancer 2014;66:736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bokhoven A, Varella-Garcia M, Korch C et al. Molecular characterization of human prostate carcinoma cell lines. The Prostate 2003;57:205–225. [DOI] [PubMed] [Google Scholar]
- 18.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 2006;27:1445–1453. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Raina K, Agarwal C, Agarwal R. Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget 2014;5:4972–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietras A Cancer stem cells in tumor heterogeneity. Advances in Cancer Research 2011;112:255–281. [DOI] [PubMed] [Google Scholar]
- 21.Patrawala L, Calhoun T, Schneider-Broussard R et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006;25:1696–1708. [DOI] [PubMed] [Google Scholar]
- 22.Bin Hafeez B, Adhami VM, Asim M et al. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix metalloproteinase-9 and urokinase plasminogen activator. Clinical Cancer Research 2009;15:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannuti A, Foreman K, Rizzo P et al. Targeting Notch to target cancer stem cells. Clinical cancer research 2010;16:3141–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Research 2001;61:7291–7297. [PubMed] [Google Scholar]
- 25.Credence Research I. Grape Seed Extracts Market By Form Type (Powder, Liquid, and Gel), By Application (Food, Pharmaceutical, Personal care and others) - Growth, Share, Opportunities & Competitive Analysis, 2016 – 2023. Report Code: 58056–08-16. http://wwwcredenceresearchcom/report/grape-seed-extracts-market Aug 2016
- 26.Derry MM, Raina K, Agarwal C, Agarwal R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Frontiers in Oncology 2013;3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinent M, Blade C, Salvado MJ et al. Procyanidin effects on adipocyte-related pathologies. Critical Reviews in Food Science and Nutrition 2006;46:543–550. [DOI] [PubMed] [Google Scholar]
- 28.Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. Journal of the American Dietetic Association 2011;111:1173–1181. [DOI] [PubMed] [Google Scholar]
- 29.Preuss HG, Wallerstedt D, Talpur N et al. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: a pilot study. Journal of Medicine 2000;31:227–246. [PubMed] [Google Scholar]
- 30.Razavi SM, Gholamin S, Eskandari A et al. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. Journal of Medicinal Food 2013;16:255–258. [DOI] [PubMed] [Google Scholar]
- 31.Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabetic Medicine : a Journal of the British Diabetic Association 2009;26:526–531. [DOI] [PubMed] [Google Scholar]
- 32.Fine AM. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Alternative Medicine Review : a Journal of Clinical Therapeutic 2000;5:144–151. [PubMed] [Google Scholar]
- 33.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Research Communications in Molecular Pathology and Pharmacology 1997;95:179–189. [PubMed] [Google Scholar]
- 34.FDA Response letter GRAS Notice no. GRN000124. http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm153940.htm, accessed 1 October 2018.
- 35.Brasky TM, Kristal AR, Navarro SL et al. Specialty supplements and prostate cancer risk in the VITamins and Lifestyle (VITAL) cohort. Nutrition and Cancer 2011;63:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter RB, Brasky TM, Milano F, White E. Vitamin, mineral, and specialty supplements and risk of hematologic malignancies in the prospective VITamins And Lifestyle (VITAL) study. Cancer Epidemiology, Biomarkers & Prevention 2011;20:2298–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scopelliti A, Cammareri P, Catalano V, Saladino V, Todaro M, Stassi G. Therapeutic implications of Cancer Initiating Cells. Expert Opinion on Biological Therapy 2009;9:1005–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GSE procyanidins (A) inhibit growth, and (B) induce death in LNCaP, C4–2B, 22Rv1, PC3, and DU145 PCa cells, in both dose- and time dependent manner. Cells (1.05 × 105) were plated in 60 mm dishes, treated with DMSO (control) or different concentrations of GSE procyanidins, and after 12, 24, 48 or 72 h, cells were harvested and counted as detailed in ‘Materials and Methods’ section. C) GSE procyanidins inhibit the clonogenic potential of human PCa cells. LNCaP, C4–2B, 22Rv1, PC3, and DU145 cells (∼1 × 103) were plated in 6-well plates, after 24 h cells were treated with DMSO or different doses of procyanidins. Fresh media with DMSO or respective GSE dose was added every 48 h till 10 days after which the colonies were stained with crystal violet and true colonies (≥ 50 cells) were counted. The data shown are mean of 3 wells (in different plates-treated similarly). In all bar graphs statistical differences are shown w.r.t. respective DMSO controls. $, P<0.05; #, P<0.01; *, P<0.001. C, control; GSE, grape seed extract.


