Abstract
BACKGROUND/OBJECTIVES
Although aged black garlic has various biological activities such as anti-allergy, anti-inflammation and neuroprotection, effect of aged black garlic on chemically contact dermatitis is unclarified.
MATERIALS/METHODS
To evaluate anti-dermatitic activity of aged black garlic extract, we investigated effects of a fraction of aged black garlic extract (BG10) on both in vivo and in vitro.
RESULTS
BG10 almost inhibited formation of nitric monoxide and interleukin-6 (IL-6; IC50, 7.07 µg/mL) at 25 µg/mL, and dose-dependently reduced production of tumor necrosis factor-α (TNF-α; IC50, 52.07 µg/mL) and prostaglandin E2 (IC50, 38.46 µg/mL) in lipopolysaccharide-stimulated RAW264.7 cells. In addition, BG10 significantly inhibited the expression of inducible nitric oxide synthase, cyclooxygenase-2 and nuclear NF-κB, and improved that of cytosolic levels of NF-κB and IκBα in the cells. Consistent with in vitro studies, BG10 (0.5 mg/mL) not only reduced ear edema but also suppressed the formation of IL-6 and TNF-α induced by 12-O-tetradecanoylphorbol-13-acetate in ear tissues of mice.
CONCLUSIONS
These findings suggest BG10 has anti-dermatitic activity through inhibiting activation of macrophages. Therefore, such effects of BG10 may provide information for the application of aged black garlic for prevention and therapy of contact dermatitis.
Keywords: Contact dermatitis, COX-2, cytokines, macrophage, NF-kappa B
INTRODUCTION
Contact dermatitis as one of acute inflammatory diseases in skin tissues includes allergic and irritant contact dermatitis [1]. Allergic contact dermatitis affiliated with hypersensitive reaction known as a type I allergy [2,3] is induced by various antigens such as foods, drugs and environmental factors [1]. The hypersensitive reaction is closely correlated with mast cells [3]. On the other hand, the initiation of irritant contact dermatitis by chemicals, such as 12-O-tetradecanoylphorbol-13-acetate (TPA), is associated with Langerhans cells and inflammatory dendritic epidermal cells [1]. The immune cells related with contact dermatitis produce inflammatory inducers such as inflammatory cytokines and eicosanoids, and then the inflammatory inducers initiate acute inflammation by recruiting various immune cells such as macrophages, monocytes and mast cells in surrounding normal tissues [1,4]. Especially, dermatitis bullosa belonging with contact dermatitis is involved in tissue necrosis by disruption of skin barrier [1]. If left untreated, the disease is able to progresses permanent tissue damage or septicemia. Therefore, the initial treatment or prevention of contact dermatitis is very important.
Garlic (Allium sativum) affiliated with a member of the lily family possesses beneficial effects on various biological events including anti-cancer, anti-hyperlipidemia and anti-inflammation [5,6,7]. Such effects are associated with different bioactive compounds such as phenolic compounds, flavonoids and organosulfur compounds [8,9]. However, the intake of garlic for a phytomedicine and a functional food is limited, because it has harmful effects correlated with rich organosulfides [10]. Moreover, raw garlic leads to severe contact dermatitis [11]. In contrast, aged black garlic (ABG), prepared from diverse processes [12,13,14], has an advantage in its beneficial effects with less side effects [12,15,16,17]. The reason is that ABG includes the elevated levels of S-allylcysteine [15] and polyphenols [15,18]. Recently, we found that ABG exerted anti-septic and anti-allergic actions [16,17], and its fraction (BG10) had more anti-allergic activity than the crude extract [16]. Nonetheless, effect of AGB and BG10 on contact dermatitis remains unknown.
Therefore, we were interested in effect of BG10 on contact dermatitis, after we found that ABG and BG10 possessed immunomodulatory actions. In this study, we hypothesized that BG10 could inhibit contact dermatitis through suppressing activation of immune cells such as macrophages, and then investigated anti-inflammatory actions of BG10 in both lipopolysaccharide (LPS)-activated RAW264.7 cells, a murine macrophage cell line [19], and TPA-induced contact dermatitis in mice. Herein, we showed that BG10 had anti-dermatitic actions in both in vivo and in vitro systems, and demonstrates how BG10 inhibits acute inflammatory reaction. Such effects of BG10 may provide further information for the development of a phytomedicine and a functional food for the treatment of contact dermatitis.
MATERIALS AND METHODS
Reagents
Dulbecco's modified Eagle's minimum essential medium (DMEM), 1× DPBS and 1× PBS were obtained from WelGENE (Gyeongsan, Korea). Antibiotics and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Grand Island, NY, USA). Specific antibodies against inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), nuclear factor-κB (NF-κB), NF-κB inhibitor (IκB) and Lamin B were procured form Cell Signaling Technology (Beverly, MA, USA). A specific antibody against β-actin were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Enzyme-linked immunosorbent assay (ELISA) kit for interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were obtained from e-Bioscience, Inc. (Science Center Drive, San Diego, USA). Enzyme immunoassay (EIA) kit for prostaglandin E2 (PGE2) was purchased from Cayman Chemical (Ann Arbor, MI, USA). Ethylenediaminetetraacetic acid (EDTA), LPS (Escherichia coli O55:B5), methyl thiazolyl tetrazolium (MTT), thiobarbituric acid (TBA), trichoroacetic acid (TCA), 1,1-Diphenyl-2-picrylhydrazil (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), 2-deoxy-D-ribose, Ferric-reducing antioxidant power (FRAP), vitamin C, and all other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). All other chemicals were of analytical grade.
Plant material and fractionation
ABG and BG10 were prepared according to a process reported previously [16]. Homogenized ABG (2.6 kg) was extracted with 80% methanol (5 L) in a bath sonicator for 36 h, and then filtered by filter paper. The filtrate was evaporated, and then the residue of ABG was dissolved in deionized water. The ABG solution was mixed with three volumes of ethyl acetate, and then, the layer of ethyl acetate was separated and evaporated. The dried residue of ethyl acetate extract was dissolved in methanol, and then suspended in deionized water. The mixture was loaded onto an open column (C18, 40–63 µm), and then, the column was eluted by the methanol solution (500 mL of each) to yield 15 fractions. BG10 fraction was evaporated. The dried residue of BG10 was dissolved in ethanol for in vitro tests, and acetone for in vivo study.
Antioxidant activity assays
DPPH scavenging activity assay
DPPH radical scavenging activity was measured according to the method reported previously [20]. BG10 (0.1 mg) or vitamin C (0.05 mg) were dissolved in methanol (1 mL). The solution (0.2 mL) was mixed with 0.1 mM DPPH solution (4 mL), and then incubated for 30 min in the darkness. Its absorbance at 517 nm were determined using a micro-plate reader (DU650, Beckman Coulter, Inc., Brea, CA, USA).
FRAP assay
FRAP assay was determined following the method of the previous reported [20]. Briefly, diluted samples (0.1 mL) were mixed with 4.0 mL of FRAP reagent, and then incubated at 37℃ for 10 min in the dark. The absorbance of final solution at 593 nm was measured using a micro-plate reader.
ABTS radical scavenging activity assay
ABTS radical scavenging activity assay was determined carried out the protocol of the previous reported [20]. Briefly, samples (0.1 mL) were added to 2.9 mL of diluted ABTS solution, and then incubated for 20 min. The mixture's absorbance at 734 nm was measured using a micro-plate reader.
Hydroxyl radical scavenging activity assay
Hydroxyl radical scavenging activity was determined following the method reported previously [20]. Briefly, BG10 (0.1 mg) or vitamin C (0.05 mg) were dissolved in 1× PBS (1 mL). The sample solution (0.15 mL) was mixed with 0.2 mL of hydroxyl radical solution (10 mM FeSO4, 10 mM EDTA and 10 mM 2-deoxy-D-ribose), spiked with 10 mM hydrogen peroxide (0.2 mL), and then incubated for 4 h at 37℃. After incubation, the solution was mixed with 1 mL of 2.8% TCA and 1.0% TBA, and then heated in boiling water for 10 min. The final reaction mixture was cooled in ice water and then centrifuged (800 g, 10 min). The absorbance of supernatant at 532 nm was measured using a micro-plate reader.
Animals
ICR mice, known as Swiss CD-1 mice [21], (male, 6 weeks and 25–30 g) were procured from Nara Biotech Co. (Pyeongteak, Korea), and housed in cages (5 mice per cage) under specific pathogen-free condition (21–24℃ and 40–60% relative humidity) with a 12 h light/dark cycle, and free access to standard rodent food (Orientbio Inc., Seongnam, Korea) and water. All animal experiments were approved by the Committee of Animal Care and Experiment of Chungnam National University, Korea (CNU-00137), and performed according to the guidelines of the Animal Care and Use Committee at Chungnam National University.
12-O-tetradecanoylphorbol-13-acetate-induced contact dermatitis
TPA-induced contact dermatitis was evaluated following the previous method [22]. After adaptation, mice were applied with BG10 (0.5 mg/mL) to the back of ears once a day. After 7 days, they were spread with TPA dissolved in acetone (0.1 mg/mL) on the ears after BG10 application. Next day, the ears were isolated from sacrificed mice after measurement of ear thickness. The ear tissues were frozen in liquid nitrogen, and then quickly pulverized. The powder of ear tissue was lysed with a lysis buffer, and then centrifuged (15,000 g, 15 min) at 4℃. The lysates were stored at −80℃ until use.
Cell culture
RAW264.7 cells were procured form the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in DMEM medium containing 10% (v/v) FBS, 100 units/mL penicillin and 100 µg/mL streptomycin at 37℃ in a humidified atmosphere of 5% CO2. All in vitro tests contain a vehicle control group (0.1% ethanol).
Cell viability assay
Cell viability was determined following a modification of the method reported previously [17]. Briefly, RAW264.7 cells were seeded on a 96-well plate (1 × 104 cells/well) in DMEM with 10% FBS, and then incubated for 24 h. The cells were preincubated with BG10 (0–100 µg/mL) for 1 h, stimulated by LPS (1 µg/mL) for 22 h, and then further incubated with 200 µL culture media containing 500 µg/mL MTT reagent for 2 h. 100 µL of DMSO was added to the plate after supernatnat was removed, and then incubated for 15 min. Cell viability was determined at 570 nm using a micro-plate reader.
Nitrite assay
The amount of nitrite, an indicator of nitric monoxide (NO), was determined as described previously [23]. RAW264.7 cells were seeded on a 96-well plate and cultured. After 24 h, the cells were preincubated with BG10 for 1 h, and then stimulated with LPS for 23 h. The amount of nitrite were measured using Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid). 50 µL of cell culture medium was mixed with 50 µL of Griess reagent. Then, the mixture was incubated for 15 min, and then the absorbance at 540 nm was measured using a micro-plate reader.
Enzyme-linked immunosorbent assay of IL-6 and TNF-α
To measure the amounts of IL-6 and TNF-α in cultured media, all the cultured media were centrifuged, and then stored at −80℃ until use. IL-6 and TNF-α were detected by ELISA kits according to the manufacturer's instructions.
Enzyme immunoassay analysis of PGE2
To determine the level of PGE2 in cultured media, all cultured media were centrifuged, and stored at −80℃ until use. PGE2 was measured by EIA kits according to the manufacturer's instructions.
Extraction of Nuclear and Cytosolic protein
Nuclear and cytosolic proteins were performed following a process reported previously [23] with a Nuclear Extraction Kit from (Cayman Chemical Company, Inc., Ann Arbor, MI, USA). Nuclear and cytosolic proteins were fractionated according to the instructions of the manufacturer.
Immunoblotting analysis
Immunoblotting analysis was evaluated following the method reported previously [23]. PVDF membranes containing blotted proteins were visualized by WEST One™ western blot detection system (iNtRON Biotechnology, Inc., Seongnam, Korea). The level of target proteins was compared to that of a loading control (β-actin or Lamin B), and the results were expressed as a ratio of density of each protein identified by a protein standard size marker (BIOFACT Co., Ltd., Daejeon, Korea). The density of each inverted band was measured using ImageJ software (version 1.50i for Windows; NIH, USA).
Statistical analyses
The experimental results were listed as means ± SD or SEM. One-way analysis of variance (ANOVA) was used for multiple comparisons (GraphPad Prism version 5.03 for Windows, San Diego, CA, USA). If there was a significant variation between treated groups, the Dunnett test was applied. Differences at the *P < 0.05 and **P < 0.01 levels were considered statistically significant.
RESULTS
Inhibitory effect of BG10 on TPA-induced contact dermatitis
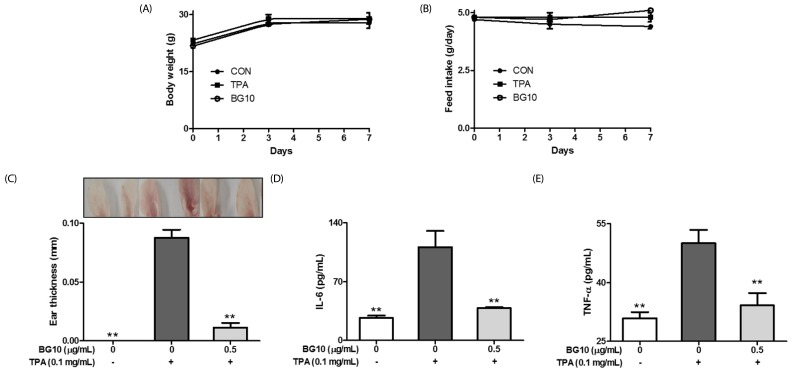
First, we investigated whether BG10 attenuated TPA-induced contact dermatitis in mice, after we found that anti-allergic [16] and anti-septic properties [17] of ABG previously. TPA-induced dermatitis model is commonly used as the contact dermatitis model [22]. When mice were applied with TPA to ears, TPA induced an increment in ear edema in mice, whereas BG10 (0.5 mg/mL) significantly reduced the ear edema (Fig. 1C) without toxicity (Fig. 1A and 1B) in TPA-treated mice. In addition, BG10 significantly inhibited the formation of IL-6 and TNF-α in TPA-treated ear tissues (Fig. 1D and 1E). These results indicate that BG10 possesses anti-dermatitic activity through inhibiting the production of inflammatory cytokines such as IL-6 and TNF-α. Therefore, BG10 may be used for treatment or prevention of contact dermatitis.
Fig. 1. Inhibitory effects of BG10 on TPA-induced dermatitis in mice.
ICR mice were spread with BG10, a fraction of aged black garlic extract (0.5 mg/mL), on the back of ears prior to 12-O-tetradecanoylphorbol-13-acetate (TPA; 0.1 mg/mL) challenge. Ear thickness, and the levels of IL-6 and TNF-α in ear tissues were determined as described in the Materials and Methods section. The data are expressed as the mean ± SEM values of septuple determinations. **P < 0.01 versus the TPA-treated group. A, body weight; B, food intake; C, ear thickness; D, IL-6; E, TNF-α.
Inhibitory effects of BG10 on the formation of inflammatory mediators in LPS-activated macrophages
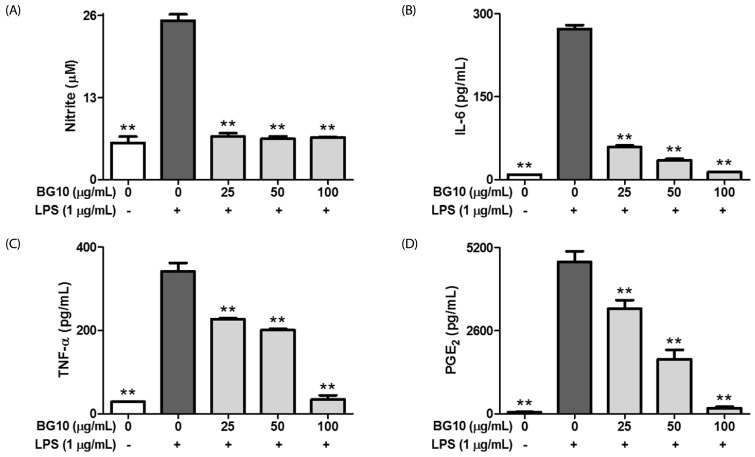
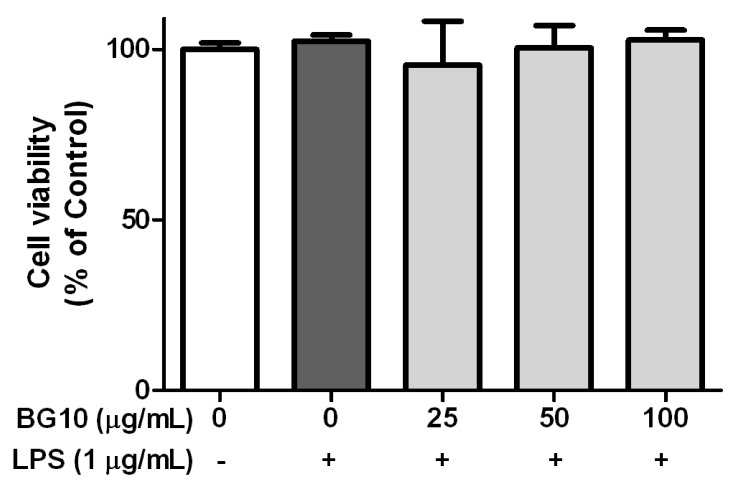
Since we found inhibitory effects of BG10 on the formation of inflammatory mediators in the TPA-induced dermatitis model, we further investigated effect of BG10 on LPS-activated macrophages. As shown in Fig. 2, BG10 at ≥ 25 µg/mL not only dramatically suppressed the formation of NO and IL-6 with IC50 value of 7.07 µg/mL, but also dose-dependently inhibited that of TNF-α with IC50 value of 52.07 µg/mL and PGE2 with IC50 value of 38.46 µg/mL. Moreover, such effects of BG10 did not show any significant cytotoxicity (Fig. 3). Taken together, these findings suggest that BG10 regulates LPS-mediated inflammatory reaction through inhibiting the formation of inflammatory mediators in macrophages at non-cytotoxic concentrations. Therefore, the anti-dermatitic action of BG10 may be closely associated with the inhibition of LPS-activated macrophages.
Fig. 2. Inhibitory effects of BG10 on the formation of NO, IL-6, TNF-α and PGE2 in LPS-activated RAW 264.7 cells.
RAW 264.7 cells were seeded on 96-well plate (5 × 104 cells/well) in DMEM with 10% FBS at 37℃ overnight. The cells were simultaneously exposed to BG10 (0–100 µg/mL) with or without lipopolysaccharide (LPS; 1 µg/mL) for 24 h. The levels of NO, IL-6, TNF-α and PGE2 were determined as described in the Materials and Methods section. The data are expressed as the mean ± SD values of triple determinations. **P < 0.01 versus the LPS-treated group. A, NO; B, IL-6; C, TNF-α; D, PGE2.
Fig. 3. Effect of BG10 on cell viability in LPS-activated RAW 264.7 cells.
RAW 264.7 cells were seeded on 96-well plate in DMEM with 10% FBS at 37℃ overnight. The cells were simultaneously exposed to BG10 with or without LPS for 22 h, and then further incubated with methyl thiazolyl tetrazolium (MTT) reagent (500 µg/mL) for 2 h. Cell viability was determined as described in Materials and Methods section. The data are expressed as the mean ± SD values of quadruple determinations.
Inhibitory effects of BG10 on expression of iNOS and COX-2
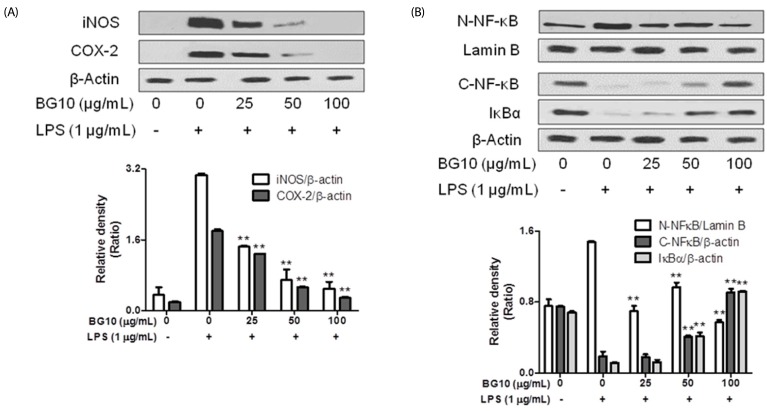
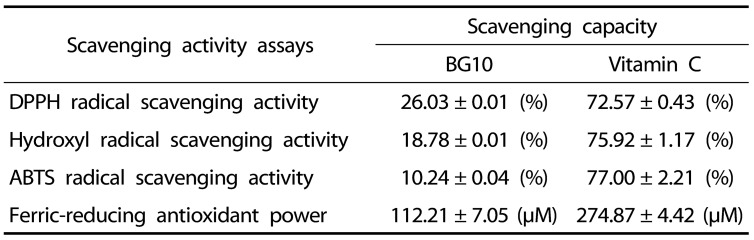
Next, we examined effects of BG10 on expression of iNOS, a NO synthase, and COX-2, a rate-limiting enzyme for prostaglandin biosynthesis, in LPS-stimulated macrophages and antioxidant activity of BG10, after we found the inhibition of BG10 on the formation of NO and PGE2. LPS-activated RAW264.7 cells elevated the expression of iNOS and COX-2, whereas BG10 significantly suppressed that of iNOS and COX-2 in the cells (Fig. 4A). Interestingly, BG10 at 100 µg/mL completely blocked the expression levels of iNOS and COX-2 as the levels of control group. Table. 1 summarizes the antioxidant activities of BG10. It suggests that BG10 possessing strong antioxidant activity directly inhibit the expression of iNOS and COX-2 in LPS-activated macrophages. Such effects of BG10 may be an important factor of its anti-dermatitic actions.
Fig. 4. Effects of of BG10 on the expression of iNOS, COX-2, nuclear NF-κB, or cytosolic NF-κB and IκB.
RAW 264.7 cells were seeded on 6-well plate (1 × 106 cells/well) in DMEM with 10% FBS at 37℃ overnight. The cells were simultaneously exposed to BG10 with or without LPS for 15 min or 6 h. They were washed, and then lysed in a cell lysis buffer. The expression of iNOS, COX-2 and nuclear NF-κB as well as cytosolic NF-κB and IκBα was determined as described in Materials and Methods section. Similar results were obtained in three independent experiments. **P < 0.01 versus LPS-treated group. A, iNOS and COX-2; B, nuclear NF-κB, cytosolic NF-κB and cytosolic IκBα.
Table 1. Antioxidant activities of BG10.
Data are the mean ± SD values of triple determinations.
Regulatory effects of BG10 on nuclear translocation of NF-κB
Finally, we investigated that effect of BG10 on NF-κB translocation into nuclei in LPS-activated macrophages. The nuclear translocation of NF-κB is associated with the formation of NO, inflammatory cytokines and eicosanoids in LPS-activated macrophages [24]. As shown in Fig. 4B, when RAW264.7 cells were stimulated by LPS, they increased the nuclear level of NF-κB, and decreased the cytosolic levels of NF-κB and IκBα. Contrastively, BG10 reduced the nuclear level of NF-κB, and recovered the cytosolic levels of NF-κB and IκBα dose-dependently. Overall, it indicated that BG10 could directly inhibit the nuclear translocation of NF-κB in LPS-activated macrophages. The effect of BG10 may be another important factor for its anti-dermatitic actions. Therefore, it suggests that BG10 possesses anti-dermatitic properties through directly down-regulating the activation of NF-κB pathway in LPS-activated macrophages.
DISCUSSION
Garlic has been used as a spice and a component of traditional medicine in Northeast Asia for a long time, and known to possess various biological actions including anti-arteriosclerosis, antibiotic, anti-cancer and anti-inflammation [5,6,7]. Although garlic has the various beneficial effects, the intake and application of garlic for a phytomedicine and a functional food have been always limited by its severe side effects including contact dermatitis [10,11]. In contrast, aged garlic has more beneficial effects than raw garlic, and its side effects is lower than that [12,15]. Recently, we reported that ABG, one of aged garlics, exerted both anti-allergic and anti-septic activities [16,17]. In addition, BG10 is stronger anti-allergic action than the crude extract of ABG [16]. Such effect of BG10 is associated with rich phenolic compounds and flavonoids [16]. Although phenolic compounds and flavonoids are well known to have antioxidant and anti-inflammatory properties [25], effect of BG10 on contact dermatitis remains unknown.
Herein, we demonstrated that BG10 has strong antioxidant activity, and anti-dermatitic actions through inhibiting activated macrophages. Such effects of BG10 are closely related with inhibiting the formation of inflammatory mediators such as NO, TNF-α, IL-6 and PGE2 in the cells. The inhibitory effects of BG10 on inflammatory mediator production are closely associated with the directly inhibition of iNOS, COX-2 and NF-κB. Moreover, the anti-inflammatory actions of BG10 are more potent than the crude extract of ABG [17]. It suggests that BG10 may be useful for treatment and prevention of contact dermatitis.
Concerning the mechanism for anti-inflammatory actions of BG10, one possible mechanism may be related to a direct suppression of activation toll-like receptor 4 (TLR4) signaling cascade in macrophages. Various immune cells including macrophages are found in the inflammatory tissue lesion in contact dermatitis [1]. Especially, macrophages are well known as be associated with chronic inflammatory diseases because they express TLR4 located on the plasma membrane [24,26]. When TLR4 is combined with LPS-endotoxin binding protein complex (LPS-binding protein, the cluster differentiation antigen14 and the myeloid differentiation protein-2), TLR4 activates myeloid differentiation factor 88 (MyD88) [26]. Then, the activated MyD88 leads to produce various inflammatory mediators such as IL-6, TNF-α and PGE2 through NF-κB translocation into nucleus by the activation of mitogen-activated protein kinases (MAPKs) [26]. Moreover, the released inflammatory mediators cause severe or chronic inflammatory responses through recruiting other immune cells [24]. Therefore, NF-κB activation in LPS-activated macrophages is an important intracellular signaling mediator in early signaling pathway of TLR4 signaling cascades. In support of this, BG10 not only reduced nuclear NF-κB level, but also increased the cytosolic levels of NF-κB and IκB in LPS-activated RAW264.7 cells. In addition, BG10 also inhibited the expression of iNOS and COX-2, which belong to down-stream proteins of TLR4 signaling cascade. Subsequently, BG10 suppressed the formation of NO, IL-6 and TNF-α in LPS-activated RAW264.7 cells and TPA-mediated dermatitis in mice. Taken together, it suggests that anti-dermatitic activity of BG10 is correlated with the direct inhibition of IκB kinase (IKK) and/or NF-κB translocation in activated macrophages.
Another possible mechanism may be correlated with antioxidant activity of BG10. LPS-activated macrophages can produce NO and ROS [23,24]. In addition, inflammatory cytokines, chemicals and ultraviolet radiation are able to induce the generation of NO and ROS in skin tissues [27,28]. Moreover, the NO and ROS are capable of inducing inflammation and apoptosis in skin tissues [28]. Therefore, the regulation of NO and ROS production in immune cells and skin tissues is another important event for anti-dermatitic action of BG10. Actually, the crude extract of ABG exerts antioxidant activity through elevating the expression of antioxidant enzymes such as heme oxygenase-1 in LPS-activated macrophages [17,29]. In our data, BG10 itself showed strong antioxidant activity. The antioxidant activity of BG10 may be associated with its components such as phenolic compounds and flavonoids [16]. Although garlic and ABG include rich sulfur compounds such as diallyl sulfide, diallyl disulfide and diallyl trisulfide, the sulfur compounds are not able to inhibit allergic response in IgE/antigen-activated mast cells [16]. Separately, in previous report, when mice were orally administrated with BG10 form 33.3 to 66.7 mg/kg, BG10 showed anti-allergic action in mice [16]. The concentrations are equal to 2.7 to 5.4 mg/kg (162.0 to 324.0 mg/60 kg) in human based on body surface area [30]. Overall, it suggests that anti-dermatitic action of BG10 attenuates dermatitis through down-regulating oxidative stress in immune cells and skin tissues.
In conclusion, this study demonstrates that BG10 exerting anti-allergic activity possesses both antioxidant activity and anti-inflammatory actions in in vitro and in vivo. These findings reveal a novel feature of BG10 in LPS-mediated inflammation and TPA-induced dermatitis. The mechanisms for its anti-inflammatory actions may include multiple targets such as IKK, iNOS, COX-2 and NF-κB. Such effects may be due to rich phenolic compounds and flavonoids in BG10, and may provide further information for the application of BG10 as a phytomedicine and a functional food for therapies of inflammatory diseases including contact dermatitis.
Footnotes
This work was supported by research fund of Chungnam National University.
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Dhingra N, Gulati N, Guttman-Yassky E. Mechanisms of contact sensitization offer insights into the role of barrier defects vs. intrinsic immune abnormalities as drivers of atopic dermatitis. J Invest Dermatol. 2013;133:2311–2314. doi: 10.1038/jid.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Pimiento AJ, Santaolalla M, de Paz S, Fernández-Parra B, Domínguez-Lázaro AR, Moneo I. Anaphylactic reaction to young garlic. Allergy. 1999;54:626–629. doi: 10.1034/j.1398-9995.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 3.Itoh T, Ohguchi K, Iinuma M, Nozawa Y, Akao Y. Inhibitory effect of xanthones isolated from the pericarp of Garcinia mangostana L. on rat basophilic leukemia RBL-2H3 cell degranulation. Bioorg Med Chem. 2008;16:4500–4508. doi: 10.1016/j.bmc.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 5.Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature's protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 6.Liu CT, Sheen LY, Lii CK. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 2007;51:1353–1364. doi: 10.1002/mnfr.200700082. [DOI] [PubMed] [Google Scholar]
- 7.Thomson M, Ali M. Garlic [Allium sativum]: a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- 8.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 9.Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Borrelli F, Capasso R, Izzo AA. Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res. 2007;51:1386–1397. doi: 10.1002/mnfr.200700072. [DOI] [PubMed] [Google Scholar]
- 11.Burden AD, Wilkinson SM, Beck MH, Chalmers RJ. Garlic-induced systemic contact dermatitis. Contact Dermatitis. 1994;30:299–300. doi: 10.1111/j.1600-0536.1994.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 12.Moriguchi T, Saito H, Nishiyama N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin Exp Pharmacol Physiol. 1997;24:235–242. doi: 10.1111/j.1440-1681.1997.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 14.Bae SH, Lee SW, Kim MR, Kim JM, Suh HJ. Influence of steeping solution and storage temperature on the color change of garlic. J Food Sci. 2010;75:C108–C112. doi: 10.1111/j.1750-3841.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 15.Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60:417–420. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- 16.Yoo JM, Sok DE, Kim MR. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J Med Food. 2014;17:92–102. doi: 10.1089/jmf.2013.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MJ, Yoo YC, Kim HJ, Shin SK, Sohn EJ, Min AY, Sung NY, Kim MR. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in LPS-stimulated raw 264.7 macrophages and LPS-induced septicemia mice. J Med Food. 2014;17:1057–1063. doi: 10.1089/jmf.2013.3043. [DOI] [PubMed] [Google Scholar]
- 18.Nencini C, Menchiari A, Franchi GG, Micheli L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum Nutr. 2011;66:11–16. doi: 10.1007/s11130-010-0204-2. [DOI] [PubMed] [Google Scholar]
- 19.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 20.Birasuren B, Kim NY, Jeon HL, Kim MR. Evaluation of the antioxidant capacity and phenolic content of agriophyllum pungens seed extracts from Mongolia. Prev Nutr Food Sci. 2013;18:188–195. doi: 10.3746/pnf.2013.18.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz JN, Daniels CA, Shivers JC, Klintworth GK. Experimental cytomegalovirus ophthalmitis. Am J Pathol. 1974;77:477–492. [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeon HL, Yoo JM, Lee BD, Lee SJ, Sohn EJ, Kim MR. Anti-inflammatory and antioxidant actions of N-arachidonoyl serotonin in RAW264.7 cells. Pharmacology. 2016;97:195–206. doi: 10.1159/000443739. [DOI] [PubMed] [Google Scholar]
- 24.Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol. 2017;79:567–592. doi: 10.1146/annurev-physiol-022516-034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015;2015:504253. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002;23:296–300. doi: 10.1016/s1471-4906(02)02222-6. [DOI] [PubMed] [Google Scholar]
- 27.Seo SH, Jeong GS. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int Immunopharmacol. 2015;29:246–253. doi: 10.1016/j.intimp.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Bito T, Nishigori C. Impact of reactive oxygen species on keratinocyte signaling pathways. J Dermatol Sci. 2012;68:3–8. doi: 10.1016/j.jdermsci.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Park HJ, Jeon BT, Kim HC, Roh GS, Shin JH, Sung NJ, Han J, Kang D. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta Physiol (Oxf) 2012;205:61–70. doi: 10.1111/j.1748-1716.2012.02425.x. [DOI] [PubMed] [Google Scholar]
- 30.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]