Abstract
Baleen whales (Mysticeti) are major ecosystem engineers, thanks to their enormous size and bulk filter feeding strategy. Their signature gigantism is thought to be a relatively recent phenomenon, resulting from a Plio-Pleistocene mode shift in their body size evolution. Here, we report the largest whale fossil ever described: an Early Pleistocene (1.5–1.25 Ma) blue whale from Italy with an estimated body length of up to 26 m. Macroevolutionary modelling taking into account this specimen, as well as additional material from the Miocene of Peru, reveals that the proposed mode shift occurred either somewhat earlier, or perhaps not at all. Large-sized mysticetes comparable to most extant species have existed since at least the Late Miocene, suggesting a long-term impact on global marine ecosystems.
Keywords: Mysticeti, body size, fossil, Miocene, macroevolution
1. Introduction
Baleen whales include the largest animals ever, and play a crucial role as major consumers and ecosystem engineers [1]. Their large body size is likely enabled by filter feeding, a strategy that dates back to at least the Late Oligocene [2,3]. Nevertheless, for much of their history, mysticetes stayed relatively small [4], albeit with some notable exceptions [5]. True gigantism is thought to have arisen only recently, in response to a Plio-Pleistocene (4.5–0.13 Ma) mode shift in mysticete body size evolution [4]. The latter was perhaps triggered by wind-driven upwelling, which in turn led to an increase in seasonally abundant, patchily distributed prey [4].
The mode shift model is highly plausible, yet also sensitive to changes in the temporal distribution of large-sized mysticete fossils. The latter are relatively scarce in current datasets on body size evolution [4,6], but it remains unclear whether this pattern reflects a genuine signal or a sampling bias (but see simulations in [4]). Especially alarming in this regard is the lack of information from the Pleistocene, and thus the very period during which the mode shift may have occurred [4]. Further, biases may have arisen from the practical difficulty of handling large specimens, especially in remote places with limited resources. For example, ongoing fieldwork has revealed a largely untapped Lagerstätte of Miocene mysticetes in the Ica Desert of Peru [7,8], several of which appear surprisingly large.
Here, we address these biases in two ways. First, we report a new blue whale specimen from the Early Pleistocene of Italy, the largest mysticete fossil ever described, and explore its implications for the timing of the proposed mode shift. Next, we extend our analysis further back in time by integrating additional material from the Middle–Late Miocene of Peru.
2. Material and methods
(a). Material
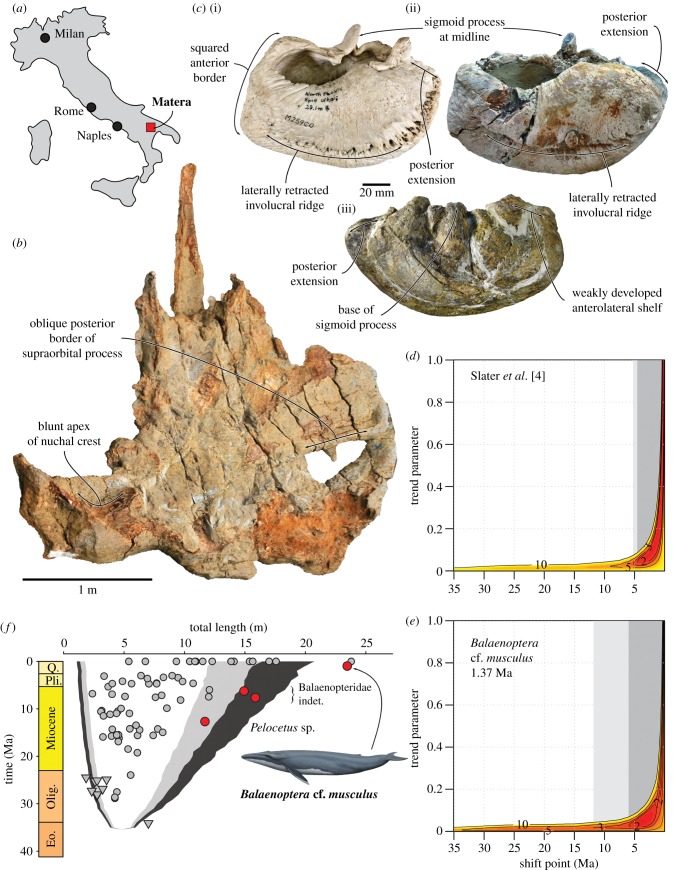
The most important of the new mysticete fossils is a partial blue whale skeleton from Lake San Giuliano, near Matera, southern Italy (figure 1), dated to 1.49–1.25 Ma (electronic supplementary material). In addition, we included three specimens from the Miocene strata of the Pisco Formation, exposed near the village of Ocucaje, Peru (electronic supplementary material, figure S1): Pelocetus sp. from the Middle Miocene locality of Mal Paso [9]; and two Late Miocene balaenopterids, one from the base (late Tortonian) and one from the top (Messinian) of the well-known site of Cerro Los Quesos [7] (electronic supplementary material).
Figure 1.
New whale fossils from Italy and Peru imply an early origin of modern mysticete gigantism. (a) Map of Italy showing the fossil locality of Balaenoptera cf. musculus. (b) Cranium of Balaenoptera cf. musculus, in dorsal view. (c) Right tympanic bulla of B. musculus (National Museum of Nature and Science specimen M25900), in dorsal view (i), and B. cf. musculus in dorsal (ii) and ventrolateral (iii) view. (d) Support surface for the mode shift model from Slater et al. [4]; dark and light grey bars denote the range of the 2- and 3-unit support regions, respectively. (e) Support surface for the mode shift model with B. musculus truncated at 1.37 Ma, but with the Peruvian fossils excluded. (f) Mysticete body length plotted against time, and compared with the 80 (white), 90 (grey) and 95% (black) quantiles of 1000 BM simulations on the baleen whale phylogeny of [4]; grey circles are chaeomysticetes, triangles toothed mysticetes, and red circles the new fossils from Italy and Peru. Note that the BM simulations were carried out on a phylogeny that did not include the specimens described here; their placement relative to the quantiles is thus merely indicative. (d–f) Modified from Slater et al. [4]. Photo in (b) by Akhet s.r.l. (www.akhet.it). Drawing of B. musculus by Carl Buell. (Online version in colour.)
(b). Body size
We estimated the total length (TL) of all specimens based on their bizygomatic width (BZW), using the ‘stem mysticete’ equation of [10]:
In addition, we confirmed the length of the blue whale fossil based on the ‘general mysticete’ equation of [6, suppl. fig. 9], and a range of new equations focusing on extant rorquals only (data from [6], electronic supplementary material, table S1).
(c). Macroevolutionary model fitting
We integrated our new fossils into the recent study of Slater et al. [4], which analysed mysticete body size evolution by fitting several macroevolutionary models to a comprehensive phylogeny and associated body size dataset. Specifically, we considered the following models: (i) simple Brownian motion (BM); (ii) single peak Ornstein–Uhlenbeck (OU); (iii) a biased random walk (trend); (iv) time-dependent rates (accelerating/decelerating: AC/DC); (v) temperature-dependent rates (based on the global δ18O curve [11]); (vi) a shift between two rate regimes, with the timing of the shift (tshift) treated as a free parameter; and (vii) a mode shift from BM to a biased random walk, with the shift point (tshift) again treated as a free parameter.
To place the blue whale fossil from Matera, we used its midpoint age (1.37 Ma) to truncate the branch representing Balaenoptera musculus, following Slater et al. [4]. Limited resources prevented the collection of the three Peruvian fossils, and thus their formal inclusion in the phylogeny. We therefore approximated their position by grouping them with three species of similar age and morphology, namely, Pelocetus calvertensis from the early Middle Miocene of the eastern United States [12]; Balaenoptera siberi from the Tortonian of the Pisco Formation [13,14]; and Parabalaenoptera baulinensis from the Tortonian–Messinian of California [15]. We assigned branch lengths of 1 Ma to the two balaenopterids, and a branch length of 2 Ma to Pelocetus sp. to reflect its somewhat younger age relative to P. calvertensis.
Finally, we fitted all models in R 3.4.4 [16], and assessed their relative support based on the second-order Akaike information criterion (AICc) and Akaike weights (wi). Where appropriate, we furthermore calculated the support surface for the mode shift model [4, fig. 4] to gauge the effect of our new specimens on its timing.
3. Results and discussion
The blue whale fossil from Matera is poorly preserved but nonetheless shares several traits with extant Balaenoptera musculus, including: a somewhat anteriorly oriented posterior border of the supraorbital process of the frontal; a relatively blunt apex of the nuchal crest; and a large (145 mm long) tympanic bulla with a squared anterior border, a posteriorly extended involucrum, a sigmoid process located approximately at the transverse midline, a laterally retracted involucral ridge and a weakly developed anterolateral shelf [17] (figure 1b,c). Crucially, the Matera specimen also matches blue whales in overall size: at a BZW of 294 cm, we estimate a total body length of 23.4–26.1 m, depending on the exact equation used. In the light of these similarities, we preliminarily refer the new fossil to Balaenoptera cf. musculus.
The specimen from Matera provides a rare insight into the poorly known Pleistocene marine record, which was largely rendered inaccessible by rising sea levels following the end of widespread glaciation [18]. Its size is truly titanic and confirms previous suggestions of a complex Quaternary history of mysticetes in the Mediterranean [19], which was once home to larger whales than are found there today [20]. Truncating B. musculus at 1.37 Ma still recovers a mode shift as the best-fitting model, albeit with less support than in the original analysis (wi = 0.88 versus 0.99; table 1). The timing of the mode shift is pushed back from 0.31 to 3.62 Ma, and its two-unit support region now extends into the latest Miocene, as opposed to the Early Pliocene [4] (figure 1d,e). Truncating B. musculus at 1.25 or 1.49 Ma yields similar results (electronic supplementary material, table S2).
Table 1.
Parameter estimates and support for each model. σ2, evolutionary rate; Θ, root state; tshift, timing of the rate/mode shift; lnL, log-likelihood; k, number of free parameters; AICc, sample-size corrected Akaike information criterion; ΔAICc, difference in the AICc; wi, Akaike weight; n.a., not applicable.
| σ2 | Θ | parameter | tshift (Ma) | lnL | k | AICc | ΔAICc | wi | |
|---|---|---|---|---|---|---|---|---|---|
| Balaenoptera cf. musculus 1.37 Ma, Peruvian specimens excluded | |||||||||
| BM | 0.00294 | 2.68 | n.a. | n.a. | 43.99 | 2 | −83.81 | 6.28 | 0.04 |
| AC/DC | 0.00190 | 2.67 | 0.018 | n.a. | 44.28 | 3 | −82.23 | 7.87 | 0.02 |
| trend | 0.00280 | 2.65 | 0.006 | n.a. | 44.67 | 3 | −83.00 | 7.09 | 0.03 |
| OU | 0.00294 | 2.68 | 0.000 | n.a. | 43.99 | 3 | −81.65 | 8.44 | 0.01 |
| temp.-dep. rates | 0.00076 | 2.68 | 0.002 | n.a. | 44.27 | 3 | −82.20 | 7.89 | 0.02 |
| rate shift | 0.00232 | 2.67 | 0.003 | 19.92 | 44.18 | 4 | −79.79 | 10.31 | 0.01 |
| mode shift | 0.00232 | 2.67 | 0.050 | 3.62 | 49.33 | 4 | −90.09 | 0.00 | 0.88 |
| Balaenoptera cf. musculus 1.37 Ma, Peruvian specimens included | |||||||||
| BM | 0.00338 | 2.69 | n.a. | n.a. | 40.37 | 2 | −76.59 | 3.25 | 0.13 |
| AC/DC | 0.00218 | 2.67 | 0.019 | n.a. | 40.68 | 3 | −75.04 | 4.80 | 0.06 |
| trend | 0.00330 | 2.66 | 0.005 | n.a. | 40.73 | 3 | −75.14 | 4.70 | 0.06 |
| OU | 0.00338 | 2.69 | 0.000 | n.a. | 40.37 | 3 | −74.43 | 5.41 | 0.04 |
| temp.-dep. rates | 0.00177 | 2.69 | 0.002 | n.a. | 40.49 | 3 | −74.66 | 5.18 | 0.05 |
| rate shift | 0.00187 | 2.66 | 0.004 | 17.93 | 41.18 | 4 | −73.82 | 6.02 | 0.03 |
| mode shift | 0.00287 | 2.68 | 0.054 | 3.00 | 44.19 | 4 | −79.84 | 0.00 | 0.64 |
Among the Peruvian specimens, Pelocetus is relatively common in the vicinity of Mal Paso and identified by its narrowly triangular supraoccipital shield, an exoccipital that projects posteriorly beyond the occipital condyle, a narrow nasal, a wide squamosal fossa, a proportionately small tympanic bulla, and a well-developed subcondylar furrow on the posterior face of the mandibular ramus. Based on its BZW of 140 cm, we estimate a TL of 11.8 m. The balaenopterids from the Pisco Formation are less well studied, but clearly identifiable by their recurved mandibular neck [21]. We estimate 15.8 m for the Tortonian rorqual from the base of Cerro Los Quesos (BZW = 192 cm), and 14.9 m for its Messinian counterpart (BZW = 180 cm). Inclusion of the Peruvian specimens reveals a more gradual emergence of mysticete gigantism (figure 1f), and causes support for the mode shift model to reduce substantially (wi = 0.64). BM (wi = 0.13) is the best-supported alternative (table 1).
Crucially, these results are robust to varying assumptions, including truncating B. musculus at either 1.25 or 1.49 Ma (electronic supplementary material, table S2), and smaller body size estimates (electronic supplementary material). They are also conservative. To maintain comparability, we followed Slater et al. [4] in using the ‘stem mysticete’ equation of [10], which—for balaenopteroids—yields lower estimates than alternative approaches, such as the ‘general mysticete’ equation of [6, suppl. fig. 9] and the ‘stem balaenopteroid’ equation of [10] (table 2). If applied here, either of these equations would further weaken support for the mode shift model.
Table 2.
Total length (m) of the Peruvian fossil mysticetes analysed here, based on the ‘stem mysticete’ and ‘stem balaenopteroid’ equations of [10] and the ‘general mysticete’ equation of [6, suppl. fig. 9].
| ‘stem mysticete’ | ‘stem balaenopteroid’ | ‘general mysticete’ | |
|---|---|---|---|
| Balaenoptera cf. musculus | 23.4 | 27.1 | 24.8 |
| Pelocetus sp. | 11.8 | 13.7 | 12.2 |
| Tortonian balaenopterid | 15.8 | 18.3 | 16.4 |
| Messinian balaenopterid | 14.9 | 17.2 | 15.4 |
Overall, our results thus suggest that the origin of modern mysticete gigantism dates back to at least 3.6 Ma, and likely even further. A mode shift around this time remains possible, but seems questionable in light of our preliminary observations from Peru. This does not mean, however, that large-bodied baleen whales have always been dominant. On the contrary, relatively small species were the norm throughout much of mysticete history [4,6] and appear to have remained so until their disappearance around 3 Ma [3]. Nevertheless, maximum body size increased steadily, and reached essentially modern levels by the Middle–Late Miocene. Considering the importance of living whales as major predators and nutrient distributors [1], it seems likely that these early giants had a profound, though currently underappreciated, impact on the evolution of global marine ecosystems.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank V. Ventricelli for reporting the discovery of the Matera fossil and hosting us during the excavation; Akhet s.r.l. (www.akhet.it) for collecting the skull under the auspices of the Soprintendenza Archeologica, Belle Arti e Paesaggio della Basilicata (scientific direction A. De Siena, technical direction F. Copeta); A. D'Andrea, Akhet s.r.l. and E. Ghezzo for information and providing photographs; A. M. Patrone (Museo Archeologico Nazionale Domenico Ridola) and F. Canestrini (Soprintendenza Archeologica, Belle Arti e Paesaggio della Basilicata) for general support; R. Sartini for his efforts in bringing the Matera whale to our attention; M. Urbina for his help in locating fossils in Peru; G. Slater for advice and providing R scripts; C. Buell for his illustration; and G. Slater and two anonymous reviewers for their constructive comments.
Ethics
This study does not include live animals and therefore was not subject to an ethics assessment. Balaenoptera cf. musculus has been deposited at the Museo Archeologico Nazionale Domenico Ridola (Matera, Italy; specimen number 2016-MT-GIU).
Data accessibility
Additional data are available as electronic supplementary material.
Authors' contributions
G.B., W.L. and A.V. took part in the excavation of the Matera fossil whale. G.B., A.C., F.G.M. and W.L. took part in the fieldwork in Peru. G.B., F.G.M., A.D.S. and C.M conducted the analyses. G.B., F.G.M. and A.C. organized the collaborative project. All authors contributed to and approve the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
F.G.M. was funded by an FNRS postdoctoral fellowship (32795797) and an EU Marie Skłodowska-Curie Global postdoctoral fellowship (656010/MYSTICETI).
References
- 1.Roman J, et al. 2014. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. ( 10.1890/130220) [DOI] [Google Scholar]
- 2.Tsai C-H, Fordyce RE. 2015. The earliest gulp-feeding mysticete (Cetacea: Mysticeti) from the Oligocene of New Zealand. J. Mamm. Evol. 22, 535–560. ( 10.1007/s10914-015-9290-0) [DOI] [Google Scholar]
- 3.Marx FG, Fordyce RE. 2015. Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. R. Soc. open sci. 2, 140434 ( 10.1098/rsos.140434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slater GJ, Goldbogen JA, Pyenson ND. 2017. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proc. R. Soc. B 284, 20170546 ( 10.1098/rspb.2017.0546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fordyce RE, Marx FG. 2018. Gigantism precedes filter feeding in baleen whale evolution. Curr. Biol. 28, 1670–1676.e2. ( 10.1016/j.cub.2018.04.027) [DOI] [PubMed] [Google Scholar]
- 6.Lambert O, Bianucci G, Post K, de Muizon C, Salas-Gismondi R, Urbina M, Reumer J. 2010. The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru. Nature 466, 105–108. ( 10.1038/nature09067) [DOI] [PubMed] [Google Scholar]
- 7.Bianucci G, et al. 2016. Fossil marine vertebrates of Cerro Los Quesos: distribution of cetaceans, seals, crocodiles, seabirds, sharks, and bony fish in a late Miocene locality of the Pisco Basin, Peru. J. Maps 12, 1037–1046. ( 10.1080/17445647.2015.1115785) [DOI] [Google Scholar]
- 8.Marx FG, Lambert O, de Muizon C. 2017. A new Miocene baleen whale from Peru deciphers the dawn of cetotheriids. R. Soc. open sci. 4, 170560 ( 10.1098/rsos.170560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Celma C, et al. 2017. Sequence stratigraphy and paleontology of the Upper Miocene Pisco Formation along the western side of the lower Ica Valley (Ica Desert, Peru). Riv. Ital. Paleontol. Stratigr. 123, 255–274. ( 10.13130/2039-4942/8373) [DOI] [Google Scholar]
- 10.Pyenson ND, Sponberg SN. 2011. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. J. Mamm. Evol. 18, 269–288. ( 10.1007/s10914-011-9170-1) [DOI] [Google Scholar]
- 11.Zachos JC, Dickens GR, Zeebe RE. 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283. ( 10.1038/nature06588) [DOI] [PubMed] [Google Scholar]
- 12.Kellogg R. 1965. Fossil marine mammals from the Miocene Calvert Formation of Maryland and Virginia, part 1: a new whalebone whale from the Miocene Calvert Formation. US Nat. Mus. Bull. 247, 1–45. [Google Scholar]
- 13.Pilleri G. 1989. Balaenoptera siberi, ein neuer spätmiozäner Bartenwal aus der Pisco-Formation Perus [Balaenoptera siberi, a new Late Miocene baleen whale from the Pisco Formation of Peru]. In Beiträge zur Paläontologie der Cetaceen Perus [Contributions to the palaeontology of the cetaceans of Peru] (ed. Pilleri G.), pp. 63–84 (in German). Bern, Switzerland: Hirnanatomisches Institut Ostermundingen. [Google Scholar]
- 14.Pilleri G. 1990. Paratypus von Balaenoptera siberi (Cetacea: Mysticeti) aus der Pisco Formation Perus [Paratype of Balaenoptera siberi (Cetacea: Mysticeti) from the Pisco Formation of Peru]. In Beiträge zur Paläontologie der Cetaceen und Pinnipedier der Pisco Formation Perus II [Contributions to the palaeontology of the cetaceans and pinnipeds of the Pisco Formation of Peru] (ed. Pilleri G.), pp. 205–215 (in German). Bern, Switzerland: Hirnanatomisches Institut Ostermundingen. [Google Scholar]
- 15.Zeigler CV, Chan GL, Barnes LG. 1997. A new Late Miocene balaenopterid whale (Cetacea: Mysticeti), Parabalaenoptera baulinensis, (new genus and species) from the Santa Cruz Mudstone, Point Reyes Peninsula, California. Proc. Calif. Acad. Sci. 50, 115–138. [Google Scholar]
- 16.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.r-project.org/. [Google Scholar]
- 17.Ekdale EG, Berta A, Demere TA. 2011. The comparative osteology of the petrotympanic complex (ear region) of extant baleen whales (Cetacea: Mysticeti). PLoS ONE 6, e21311 ( 10.1371/journal.pone.0021311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyenson ND, Lindberg DR. 2011. What happened to gray whales during the Pleistocene? The ecological impact of sea-level change on benthic feeding areas in the North Pacific Ocean. PLoS ONE 6, e21295 ( 10.1371/journal.pone.0021295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collareta A, Regattieri E, Zanchetta G, Lambert O, Catanzariti R, Bosselaers M, Covelo P, Varola A, Bianucci G. 2018. New insights on ancient cetacean movement patterns from oxygen-isotope analyses of a Mediterranean Pleistocene whale barnacle. Neues Jb. Geol. Palaeontol. Abh. 288, 143–159. ( 10.1127/njgpa/2018/0729) [DOI] [Google Scholar]
- 20.Notarbartolo-Di-Sciara G, Zanardelli M, Jahoda M, Panigada S, Airoldi S. 2003. The fin whale Balaenoptera physalus (L. 1758) in the Mediterranean Sea. Mammal Rev. 33, 105–150. ( 10.1046/j.1365-2907.2003.00005.x). [DOI] [Google Scholar]
- 21.Deméré TA, Berta A, McGowen MR. 2005. The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes. J. Mamm. Evol. 12, 99–143. ( 10.1007/s10914-005-6944-3) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are available as electronic supplementary material.