Abstract
BACKGROUND:
Although an estimated three million tuberculosis (TB) cases worldwide are missed by national TB programs annually, the level of under-reporting of diagnosed cases in high TB burden settings is largely unknown.
OBJECTIVE:
To quantify and describe under-reporting of sputum smear-positive TB cases in Kenya.
DESIGN:
A national-level retrospective TB inventory study was conducted. All sputum smear-positive TB cases diagnosed by public or private laboratories during 1 April–30 June 2013 were extracted from laboratory registers in 73 randomly sampled subcounties and matched to TB cases in the national TB surveillance system (TIBU). Bivariate and multivariate analyses were conducted.
RESULTS:
In the subcounties sampled, 715 of 3409 smear-positive TB cases in laboratory registers were not found in TIBU. The estimated level of under-reporting of smear-positive TB cases in Kenya was 20.7% (95%CI 18.4–23.0). Under-reporting was greatest in subcounties with a high TB burden. Unreported cases were more likely to be patients aged ≥55 years, have scanty smear results, and be diagnosed at large facilities, private facilities, and facilities in high TB burden regions.
CONCLUSION:
In Kenya, one fifth of smear-positive TB cases diagnosed during the study period went unreported, suggesting that the true TB burden is higher than reported. TB surveillance in Kenya should be strengthened to ensure all diagnosed TB cases are reported.
Keywords: surveillance, inventory study, under-reporting, TB
Abstract
CONTEXTE:
On estime que trois millions de cas de tuberculose (TB) sont manqués par les programme nationaux de lutte contre la TB dans le monde chaque année, mais le degré de sous-notification des cas diagnostiqués dans les contextes lourdement frappés est largement inconnu.
OBJECTIF:
Quantifier et décrire la sous-notification des cas de TB à frottis de crachats positif au Kenya.
SCHÉMA:
Une étude rétrospective de recensement de la TB au niveau national a été réalisée. Tous les cas de TB à frottis positif diagnostiqués par des laboratoires publics ou privés entre le 1er avril et le 30 juin 2013 ont été extraits des registres des laboratoires dans 73 sous-comtés choisis de façon aléatoire et appariés aux cas de TB dans le systéme national de surveillance de la TB (TIBU). Des analyses bivariée et multivariée ont été réalisées.
RÉSULTATS :
Dans les sous-comtés sélectionnés, 715 sur 3409 cas de TB à frottis positif dans les registres des laboratoires n’ont pas été retrouvés dans le TIBU. Le degré de sous-notification estimé des cas de TB à frottis positif au Kenya a été de 20,7% (IC95% 18,4-23,0).
Cette sous-notification a été la plus élevée dans les sous-comtés les plus touchés par la TB. Les cas non déclarés ont plus souvent été des patients âgés de ≥55 ans, avec un résultat de frottis de type « rare » et un diagnostic réalisé dans de grandes structures, dans des structures privées et des structures situeées dans les zones trés toucheées par la TB.
CONCLUSION:
Au Kenya, un cinquiéme des cas de TB à frottis positif diagnostiqués pendant la période de l’étude n’a pas été déclaré, ce qui suggére que le poids réel de la TB est plus important que la déclaration des cas. La surveillance de la TB au Kenya doit être renforcée afin de s’assurer que tous les cas de TB sont déclarés.
Abstract
MARCO DE REFERENCIA:
Se estima que en los Programas Nacionales contra la Tuberculosis se pasan por alto tres millones de casos de tuberculosis (TB) cada año en el mundo. Sin embargo, se desconoce en gran medida la tasa de infranotificación de los casos diagnosticados en los entornos con una alta carga de morbilidad por TB.
OBJETIVO:
Cuantificar y describir la infranotificacion de los casos de TB con baciloscopia positiva del esputo que se diagnostican en Kenya.
MÉTODO:
Se realizó un estudio retrospectivo de inventario de la TB de ámbito nacional. Se obtuvieron todos los casos de TB con baciloscopia positiva del esputo diagnosticados en los laboratorios del sector pÚblico y privado a partir de los registros de laboratorio de 73 subdistritos que se escogieron mediante un muestreo aleatorio y sus datos se cotejaron los datos de los casos de TB registrados en el sistema nacional de vigilancia (TIBU). Se practicaron análisis con modelos de regresión bivariantes y multivariantes.
RESULTADOS:
En los subdistritos muestreados, 715 de los 3409 casos de TB con baciloscopia positiva de los registros de laboratorio no se encontraban en el TIBU. Se calculÓ que en Kenya la tasa de infranotificaciÓn de casos de TB con baciloscopia positiva era 20,7% (IC95% 18,4–23,0). Esta infranotificaciÓn fue más altaen los subdistritos con una alta carga de morbilidad. Fue más probable que no se notificaran los casos de pacientes a partir de los 55 años,cuyo resultado de la baciloscopia comunicaba bacilos escasos, y de los pacientes diagnosticados en los grandes establecimientos, los centros privados y los centros de regiones con alta carga de morbilidad por TB.
CONCLUSIÓN:
Durante el presente estudio en Kenya, un quinto de los casos diagnosticados de TB con baciloscopia positiva del esputo no se notificÓ, lo cual significa que la carga de morbilidad por TB es más alta de lo que se ha comunicado hasta ahora. Es importante reforzar la vigilancia de la TB en Kenya, con el propÓsito de lograr la notificaciÓn de todos los casos que se diagnostican.
THE WORLD HEALTH ORGANIZATION (WHO) estimates that more than three million tuberculosis (TB) cases are missed by national TB surveillance systems annually.1 Although the WHO has declared that finding these missed TB cases is a top priority to reach global TB targets,1,2 the number and characteristics of missed cases are largely unknown in high TB burden countries. TB cases are missed when they are not reported to national TB programs (NTPs), which can occur if they are never diagnosed with TB, do not initiate treatment after diagnosis, are not recorded while on treatment, or are not reported to the national surveillance system.3,4 Missed cases can result in continued TB transmission in the community and hinder an NTP’s ability to effectively monitor TB care, target funding, and assess progress towards TB targets.3,5
Evaluating the extent of TB under-reporting in high TB burden settings is necessary to determine the true burden of diagnosed TB and to develop strategies to improve TB surveillance and control,3 yet few studies have assessed TB under-reporting in low-resource or high TB burden settings.5–22 Even fewer studies have quantified the extent of TB under-reporting at a national level,17–22 and none have been conducted in Eastern Africa. To address this gap, Kenya’s National TB, Leprosy, and Lung Disease (NTLD) Program, in collaboration with the Kenya Medical Research Institute (KEMRI; Kisumu, Kenya) and the US Centers for Disease Control and Prevention (CDC), sought to quantify and describe under-reporting of sputum smear-positive TB cases to the national TB surveillance system in Kenya to improve TB incidence estimates, strengthen TB surveillance, and inform TB control.
STUDY POPULATION AND METHODS
Setting
Kenya is a high TB burden country, with an estimated 110 000 incident TB cases per year.1 TB cases are predominantly diagnosed using sputum smear microscopy, which is available in approximately one third of health facilities in Kenya.23 If persons with presumptive TB visit a facility that lacks smear microscopy, they are referred for testing to the nearest diagnostic facility, but usually return to the facility closest to them to receive treatment. Kenya’s NTLD Program is responsible for active notification and follow-up of persons with smear-positive TB, but in practice, processes are known to vary widely between facilities.
In 2011, the NTLD Program reformed national TB surveillance with the roll-out of a national electronic case-based TB register called TIBU. Details on individual patient episodes of TB reported to the NTLD Program are stored in TIBU. Subcounty TB and leprosy coordinators (SCTLCs) transcribe demographic, clinical, and treatment outcome data electronically into TIBU on a quarterly basis using mobile computer tablets from the facility-based anti-tuberculosis treatment paper registers used in public and private sector facilities.24 The tablets transmit case-based data to a national server through Kenya’s mobile network.
Data from laboratory registers are not collected in TIBU. National guidelines stipulate that all sputum acid-fast bacilli (AFB) test results should be recorded in the paper laboratory TB registers within each diagnostic facility. There is no case-based register for sputum AFB TB laboratory test results at the national level.
Design
A national retrospective inventory study of sputum smear-positive TB cases was conducted following WHO guidelines.3 Sputum smear-positive TB cases were prioritized in this study because these cases are most infectious, experience high morbidity and mortality, and are a primary focus for TB control efforts.25,26
A national sample of subcounties was selected using stratified random sampling based on reported TB burden. Subcounties were classified as low, medium, or high burden if they reported <25, 25–49, or ≥50 sputum smear-positive TB cases, respectively, to the NTLD Program in the second quarter of 2013. Select subcounties near Kenya’s northern and eastern borders were excluded for security reasons. In the sampled subcounties, all facilities that provided diagnosis using sputum AFB smear were visited. Facilities were identified and defined (i.e., public, non-governmental organization, faith-based organization, private enterprise, or prison) based on the country’s Master Facility List (MFL), available from the Kenya Ministry of Health (MoH; Nairobi, Kenya), and through consultation with each SCTLC.
At each diagnostic facility, the paper TB laboratory register was reviewed. Laboratory records were included if they received baseline sputum examinations during 1 April–30 June 2013 (Quarter 2) and had at least one AFB sputum smear-positive result documented. Records of cases receiving follow-up sputum examinations were excluded. AFB smear was used in this study because facilities used this test universally for bacteriological diagnosis, whereas other diagnostics, such as culture and Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), were largely unavailable and, when used, were reserved primarily to detect drug resistance.
Data collection
Between September and December 2014, records were abstracted from paper-based laboratory registers and entered into computer tablets using electronic forms developed using KoBo Collect (Harvard Humanitarian Initiative, Cambridge, MA, USA). All fields in the laboratory register (i.e., name, age, sex, date of diagnosis, referring site, and sputum results) were abstracted for each eligible record. Information about laboratory facilities, including location, facility name, and facility ID (MFL) code, were collected. Anomalies in case reporting (e.g., 0 cases or missing registers) were also recorded. The data collection process had inbuilt quality assurance checks. All data were uploaded via mobile telecommunications networks to the Cloud and stored on a secure NTLD Unit server.
Record linkage
As there were no unique patient-level identifiers (e.g., national ID) in the laboratory register or TIBU, abstracted laboratory records were matched to corresponding cases reported in TIBU from 1 January to 31 December 2013 using all possible variables in the laboratory register, including name, age, sex, location of laboratory, sputum results, and date of diagnosis. All reported cases in 2013 were searched, regardless of recorded smear status (i.e., positive, negative, not done), to maximize the number of matches found between the laboratory and the treatment records. This allowed matches to be identified even if there were large delays between diagnosis and treatment initiation (i.e., up to 9 months) and despite inevitable recording differences between the laboratory register and TIBU (e.g., differences in name, age, or baseline sputum results).
Potential matches were identified through a multiple-step deterministic matching algorithm using ‘sounds-like’ operators (e.g., Soundex) and compare functions in SAS 9.3 (Statistical Analysis System, Cary, NC, USA) and Microsoft Access 2013 (Redmond, WA, USA), and subsequent manual review.3,27,28 Each potential match was reviewed for similarities for name and age. If additional information was needed to determine match status, similarities between sex, smear status, location of care, and date of diagnosis vs. date of treatment initiation were reviewed and scored. Each potential match was then classified as a ‘match’ or ‘non-match’ based on similarities between names and age, followed by this supplementary score. If more than one potential match was identified per laboratory record, the match with the highest score was used. A single TIBU record could not be paired to more than one laboratory record. Duplicate laboratory cases were identified and removed. Following record linkage, a sensitivity analysis was conducted using expanded matching criteria to evaluate the impact of accepting matches with large incongruence between names and age and lack of similarity between supplementary variables.
Data analysis
A laboratory-diagnosed smear-positive case was defined as reported to the NTLD Program if a match was identified in TIBU. All other laboratory-diagnosed cases were defined as unreported; under-reporting could thus be due to pre-treatment loss to follow-up or reporting errors. A national estimate for under-reporting, adjusted for study design, was calculated using R software, 3.1.0 (R Computing, Vienna, Austria).3 To identify possible risk factors for non-reporting, unreported cases were compared to reported cases using bivariate analyses conducted using SAS 9.3. Adjusted odds ratios (aORs) and 95% confidence intervals (95%CIs) for risk factors were calculated using multivariate logistic regression.
Ethics
The study was determined to be non-research by the CDC and the Kenya NTLD Program, as it involved routine record review for programmatic purposes. Data were collected and submitted to the NTLD Program following the NTLD Program’s standard procedures for surveillance data.
RESULTS
Of the 224 subcounties in Kenya, 15 (7%) were excluded due to insecurity. Of the remaining 209 subcounties, 73 (35%) were sampled (21 low-, 27 medium-, and 25 high-burden) (Figure 1). All 541 diagnostic facilities in the subcounties sampled were visited, 418 (77%) of which had records of sputum smear-positive TB cases during the study period. In the subcounties sampled, a total of 3455 smear- positive TB cases were abstracted from laboratory registers, yielding 3409 unique cases; 46 cases were identified as duplicates (Figure 2). Of the 3409 smear-positive TB cases, 715 (21.0%) were not found in TIBU in 2013; in the sensitivity analysis, which applied the broadest matching criteria, the number of unmatched cases was reduced to 525 (15.4%). Adjusting for sampling design, the estimated level of under-reporting of smear-positive TB cases in Kenya was 20.7% (95%CI 18.4–23.0).
Figure 1.
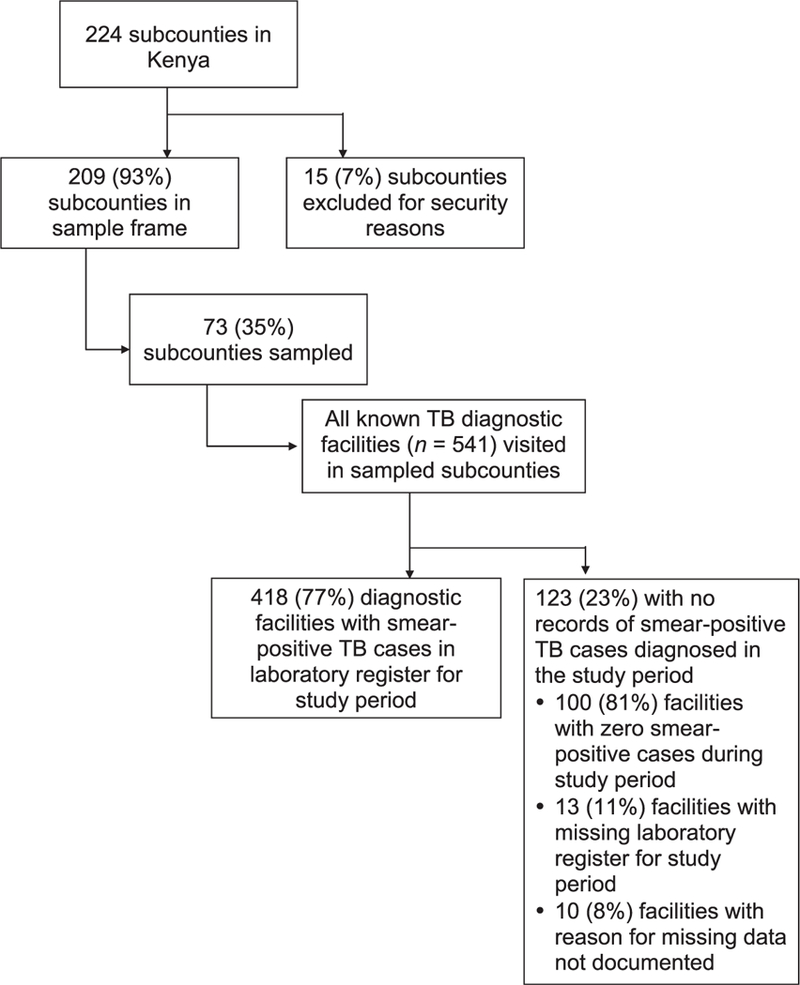
Flow diagram of sampling strategy and data collection for the Kenya national tuberculosis inventory study. TB = tuberculosis.
Figure 2.
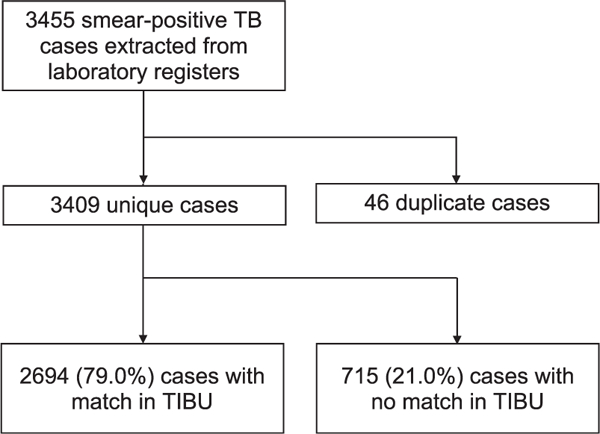
Matching smear-positive TB cases from laboratory registers to those reported to the national TB surveillance system (TIBU), process and outcomes. TB = tuberculosis.
Under-reporting was greatest in high TB burden subcounties (23%) and lowest in low TB burden subcounties (13%) (Table 1). Under-reporting in subcounties ranged from 0% to 36%. In six (8%) subcounties, there was complete reporting of smear-positive TB cases (data not shown). The highest level of under-reporting occurred in the Nairobi region (33%), while the lowest occurred in the North Eastern region (12%) (Figure 3).
Table 1.
Reporting of diagnosed smear-positive TB cases to the national TB surveillance system (TIBU) by TB burden
| Subcounties | Smear-positive cases in laboratory registers (diagnosed cases) n |
Smear-positive cases in TIBU (reported cases) n |
Unreported smear-positive cases n |
Under-reporting % |
|---|---|---|---|---|
| High TB burden | 2221 | 1721 | 500 | 22.5 |
| Medium TB burden | 918 | 738 | 180 | 19.6 |
| Low TB burden | 270 | 235 | 35 | 13.0 |
| Total | 3409 | 2694 | 715 | 21.0 |
TB = tuberculosis.
Figure 3.
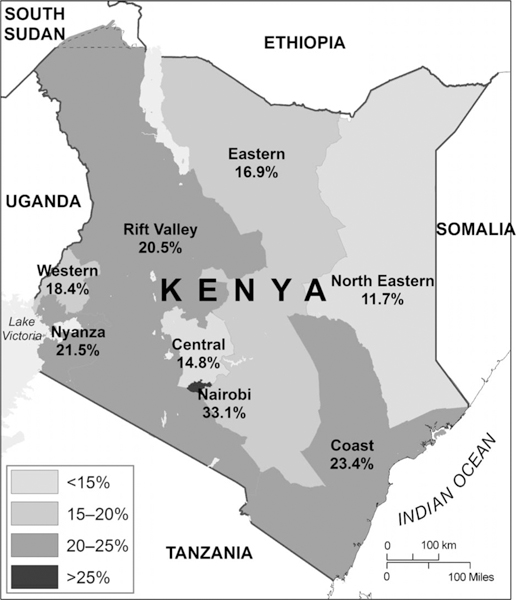
Percentage of diagnosed smear-positive TB cases not reported to the National TB, Leprosy and Lung Disease Program by region, Kenya, 2013. TB = tuberculosis.
The 3409 smear-positive TB cases were predominantly male (62%) and aged 25–54 years (63%), and many had at least 3+ grade sputum smears (38%) (Table 2). The majority of cases were diagnosed in hospitals (67%) and the public sector (85%). In bivariate analysis, age, smear grade, facility ownership, facility type, facility size, and region were all significantly associated with under-reporting (for each, P < 0.0001). In multivariable analysis, unreported cases were significantly more likely to be aged ≥55 years (aOR 1.6, 95%CI 1.1–2.3) or lack age data (aOR 3.3, 95%CI 2.3–4.6), diagnosed at a private facility (aOR 2.6, 95%CI 1.8–3.9), diagnosed at a large (i.e., 50–249 beds) (aOR 1.8, 95%CI 1.2–2.7) or extremely large (i.e., ≥250 beds) (aOR 1.9, 95%CI 1.2–2.9) facility, and diagnosed in high-burden regions, including the Coast (aOR 1.8, 95%CI 1.3–2.7), Nairobi (aOR 2.4, 95%CI 1.6–3.5), Nyanza (aOR 1.5; 95%CI 1.1–2.2), or Rift Valley (aOR 1.5, 95%CI 1.0–2.1). Unreported cases were significantly less likely to have a high (i.e., 3 or 4+) smear grade (aOR 0.71, 95%CI 0.6–0.9). There was no significant association between facility type (i.e., clinic, dispensary, health center, or hospital) and whether or not a case was reported.
Table 2.
Associations between diagnosed sputum smear-positive TB cases and incomplete reporting (n = 3409)
| Total cases n (%) |
Unreported cases n (%) |
Reported cases n (%) |
Bivariate |
Multivariate |
|||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | aOR (95%CI) | P value | ||||
| Patient characteristics | |||||||
| Sex | |||||||
| Male | 2114 (62.0) | 425 (59.4) | 1689 (62.7) | Reference | 0.1187 | Reference | 0.26 |
| Female | 1295 (38.0) | 290 (40.6) | 1005 (37.3) | 1.2 (1.0–1.4) | 1.1 (0.9–1.3) | ||
| Age, years | |||||||
| <25 | 822 (24.1) | 149 (20.8) | 673 (25.0) | Reference | <0.0001 | Reference | <0.001 |
| 25–54 | 2132 (62.5) | 415 (58.0) | 1717 (63.7) | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) | ||
| ≥55* | 254 (7.5) | 61 (8.5) | 193 (7.2) | 1.4 (1.0–2.0) | 1.6 (1.1–2.3) | ||
| Missing* | 201 (5.9) | 90 (12.6) | 111 (4.1) | 3.7 (2.6–5.1) | 3.3 (2.3–4.6) | ||
| Grade of smear result † | |||||||
| Scanty | 319 (9.4) | 89 (12.5) | 230 (8.5) | Reference | <0.0001 | Reference | 0.0041 |
| 1+ | 863 (25.3) | 210 (29.4) | 653 (24.3) | 1.2 (0.9–1.6) | 1.1 (0.8–1.5) | ||
| 2+ | 929 (27.3) | 187 (26.2) | 742 (27.6) | 0.8 (0.6–1.0) | 0.8 (0.7–1.0) | ||
| 3 or 4+* | 1296 (38.0) | 229 (32.0) | 1067 (39.6) | 0.7 (0.5–0.8) | 0.7 (0.6–0.9) | ||
| Facility characteristics | |||||||
| Facility ownership | |||||||
| Public | 2881 (84.5) | 578 (80.8) | 2303 (85.5) | Reference | <0.0001 | Reference | 0.0002 |
| NGO | 54 (1.6) | 15 (2.1) | 39 (1.5) | 1.5 (0.8–2.8) | 0.9 (0.5–1.8) | ||
| FBO | 301 (8.8) | 64 (9.0) | 237 (8.8) | 1.1 (0.8–1.4) | 1.0 (0.7–1.4) | ||
| Private* | 129 (3.8) | 53 (7.4) | 76 (2.8) | 2.8 (1.9–4.0) | 2.6 (1.8–3.9) | ||
| Prisons | 44 (1.3) | 5 (0.7) | 39 (1.5) | 0.5 (0.2–1.3) | 0.7 (0.3–2.0) | ||
| Facility type | |||||||
| Clinic | 57(1.7) | 16(2.2) | 41 (1.5) | Reference | <0.0001 | Reference | 0.51 |
| Dispensary | 402 (11.8) | 60 (8.4) | 342 (12.7) | 0.5 (0.2–0.9) | 1.0 (0.5–2.0) | ||
| Health center | 663 (19.5) | 89 (12.5) | 574 (21.3) | 0.4 (0.2–0.7) | 0.9 (0.5–2.0) | ||
| Hospital | 2287 (67.1) | 550 (76.9) | 1737 (64.5) | 0.8 (0.5–1.5) | 1.2 (0.6–2.6) | ||
| Facility size (number of beds and cots)† | |||||||
| 0 | 537 (15.9) | 92 (12.9) | 445 (16.7) | Reference | <0.0001 | Reference | 0.0009 |
| 1–10 | 401 (11.9) | 52 (7.3) | 349 (13.1) | 1.4 (1.0–2.0) | 1.2 (0.8–1.9) | ||
| 11–49 | 827 (24.5) | 130 (18.3) | 697 (26.1) | 1.3 (0.9–1.8) | 1.0 (0.7–1.5) | ||
| 50–249* | 913 (27.0) | 244 (34.3) | 669 (25.1) | 2.5 (1.8–3.4) | 1.8 (1.2–2.7) | ||
| ≥250* | 702 (20.8) | 193 (27.1) | 509 (19.1) | 2.6 (1.8–3.6) | 1.9 (1.2–2.9) | ||
| Region | |||||||
| Central | 364 (10.7) | 54 (7.6) | 310 (11.5) | Reference | <0.0001 | Reference | <0.0001 |
| Coast* | 499 (14.6) | 117 (16.4) | 382 (14.2) | 1.8 (1.2–2.5) | 1.8 (1.3–2.7) | ||
| Eastern | 444 (13.0) | 75 (10.5) | 369 (13.7) | 1.2 (0.8–1.7) | 1.6 (1.0–2.3) | ||
| Nairobi* | 411 (12.1) | 136 (19.0) | 275 (10.2) | 2.8 (2.0–4.0) | 2.4 (1.6–3.5) | ||
| North Eastern | 128 (3.8) | 15 (2.1) | 113 (4.2) | 0.8 (0.4–1.4) | 0.7 (0.4–1.3) | ||
| Nyanza* | 478 (14.0) | 103 (14.4) | 375 (13.9) | 1.6 (1.1–2.3) | 1.5 (1.1–2.2) | ||
| Rift Valley* | 716 (21.0) | 147 (20.6) | 569 (21.2) | 1.5 (1.1–2.1) | 1.5 (1.0–2.1) | ||
| Western | 369 (10.8) | 68 (9.5) | 301 (11.2) | 1.3 (0.9–1.9) | 1.4 (0.9–2.0) | ||
Significance on multivariate analysis.
Numbers do not sum to total due to missing cases.
TB = tuberculosis; OR = odds ratio; CI = confidence interval; aOR = adjusted OR; NGO = non-governmental organization; FBO = faith-based organization.
DISCUSSION
In this national-level study of TB under-reporting in Kenya, approximately one of every five smear-positive TB cases was unknown to the NTLD Program, indicating that the TB burden was higher than reported. This is an important public health concern, as unreported patients may not be started on treatment, thus continuing to transmit TB in the community, or worse yet, die.3,4 Complete reporting of TB disease is essential to ensure proper patient care, timely public health response, and accurate evaluation of trends. Without reliable TB surveillance systems, NTPs are hindered in their ability to target resources, develop national plans, and monitor and report progress towards targets for TB control.3 TB surveillance should be strengthened in Kenya to ensure that all diagnosed TB cases—not just TB cases started on treatment—are reported to the NTLD Program.
Although the level of TB under-reporting in Kenya is relatively high, it is consistent with levels observed in other high-burden or low-resource settings. National studies recently conducted in the Middle East have found levels of under-reporting ranging between 27% and 31%,17–20 while national studies in South Africa and Malawi identified respectively 34% and 14% under-reporting.21,22 Similarly, small subnational studies conducted in Ghana, Malawi, and South Africa have found under-reporting rates ranging between 15% and 38%.6,13,14 Although the ultimate goal of TB surveillance data is to provide a direct measure of TB incidence,1,3 this study and others demonstrate that the surveillance systems in many high TB burden countries are not robust enough to do so. Inventory studies can be used to improve current estimates of TB incidence in settings where suboptimal surveillance systems exist.3
Under-reporting was not uniform across Kenya: it was greatest in high TB burden areas, with up to one in three smear-positive cases unknown to the NTLD Program in some regions. Significantly higher levels of under-reporting were found in predominantly urban areas (e.g., Nairobi) and larger facilities, similar to findings in other studies.9,22 High patient load at these facilities may impact quality of recording and timeliness of health services, leading to lower reporting levels. A TB surveillance evaluation in Kenya highlighted that health centers and hospitals had challenges maintaining complete TB records for patients.24 Studies elsewhere report that patients diagnosed with TB may not initiate treatment because of dissatisfaction with and barriers to TB services, due to factors that could be exasperated by heavy caseloads (e.g., clinic crowdedness).5,8–11,13 Levels of under-reporting were also significantly higher among patients with scanty smears, possibly because clinics may focus on contacting the most infectious patients, while those with scanty smears are left to seek treatment on their own. Nonetheless, hundreds of highly infectious patients were diagnosed but unreported in TIBU, thus raising concerns about continued disease transmission; of the 715 patients who were not reported, 229 (32%) had a smear grade of at least 3+ and 416 (58%) had a grade of at least 2+. Similarly, although the majority of unreported cases were aged <55 years, lack of reporting was significantly greater among patients of older age, perhaps because older patients are at greater risk for death in general, a primary reason for pre-treatment loss to follow-up identified in other countries.5,8,12,22 Older patients may also have been more likely to be missing age data due to uncertainty about age, which could have contributed to the significant lack of reporting observed among patients with ages missing in laboratory registers. Additional research is needed to understand why these groups are more likely to be excluded from the TB surveillance system.
In Kenya, extra efforts are needed to ensure that persons diagnosed in the growing private sector are reported and initiated on treatment. The public-private mix initiative for TB in Kenya has demonstrated success,29 but increased efforts to coordinate with private facilities, including those outside the initiative, are needed. Financial incentives and non-financial enablers, such as access to free training and continuing education, are examples of strategies to further engage and promote reporting by the private sector to the NTLD Program.30–32
While this study was not designed to differentiate between pre-treatment loss to follow-up and cases started on treatment but not reported, the data suggest that under-reporting may be in part due to the former. The differences in patient characteristics and failure to report, as observed in the inventory study, suggest that under-reporting may be due to pre-treatment loss to follow-up, assuming failure to record information should happen randomly and not more frequently in certain groups (i.e., older age, scanty smears). Furthermore, a small evaluation of TIBU found that 99% of cases from facility TB treatment registers were recorded in TIBU, and that facility TB treatment registers were more complete than patient files.24 This suggests that under-reporting in Kenya is unlikely to be due solely to recording and reporting challenges.
In Kenya, a system that traces persons from the point of TB diagnosis until treatment initiation could reduce the level of pre-treatment loss to follow-up and ensure all diagnosed cases are reported, regardless of treatment initiation. Examples of such strategies include establishing standard operating procedures to collect contact information on presumptive TB cases in laboratory or TB unit registers, training staff in the field to reconcile laboratory and treatment registers and to proactively trace patients who have not started treatment, and implementing a unique patient identification number across all TB registers.4–6,12 These small changes can ensure that NTLD Program reports are truly reflective of program performance and that all diagnosed TB cases receive care.4,5
The study has several limitations. First, transcription errors during routine reporting or data collection may have hindered record linkage, but this was likely minimized as the matching algorithm was designed to accommodate multiple discrepancies by examining all available variables. Second, select subcounties were excluded from the sampling frame for security reasons. While these subcounties largely had low TB burden, it is unclear how reporting in those sites differed from those that were included. Third, although Kenya is a country with a high burden of human immunodeficiency virus (HIV) infection, information on HIV status was not available in laboratory registers, and it was not possible to assess whether under-reporting differed by HIV status; similarly, using smear microscopy to identify TB cases in this study may have excluded many HIV- positive TB cases from analysis. Finally, following WHO guidelines, data for this study reflect the level of under-reporting over a 3-month period in 2013.3 Variations in practices related to reporting, diagnosis, or treatment could have affected under-reporting in subsequent quarters.
CONCLUSION
In 2013, approximately 39100 smear-positive TB cases were reported in Kenya; however, results from this study suggest that the real number of diagnosed smear-positive TB cases was closer to 47 000. The results of this study can help improve estimates of diagnosed TB disease in Kenya while also informing new procedures to ensure all diagnosed cases are reported to the national TB surveillance system and included in routine cohort reviews. With more than three million TB cases missed globally per year, there is a need to conduct similar national-level studies in other high TB burden countries to further elucidate under-reporting of diagnosed TB cases and pave the way to find and treat missing cases.
Acknowledgements
The authors thank J Tobias (US Centers for Disease Control and Prevention [CDC]) for producing the map for this article.
This project was supported by the United States Agency for International Development through the CDC, and the Global Fund for HIV, TB, and Malaria through the Kenya National Tuberculosis, Leprosy, and Lung Disease Program.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2014 WHO/HTM/TB/2014.08. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 2.World Health Organization. The End TB Strategy: Global strategy and targets for tuberculosis, prevention, care and control after 2015. Geneva, Switzerland: WHO, 2014. http://www.who.int/tb/post2015_TBstrategy.pdf. Accessed June 2016. [Google Scholar]
- 3.World Health Organization. Assessing tuberculosis under-reporting through inventory studies WHO/HTM/TB/2012.12. Geneva, Switzerland: WHO, 2012. http://www.who.int/tb/publications/inventory_studies/en/. Accessed June 2016. [Google Scholar]
- 4.Harries AD, Rusen ID, Chiang CY, Hinderaker SG, Enarson DA. Registering initial defaulters and reporting on their treatment outcomes. Int J Tuberc Lung Dis 2009; 13: 801–803. [PubMed] [Google Scholar]
- 5.MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ 2014; 92: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afutu FK, Zachariah R, Hinderaker SG, et al. High initial default in patients with smear-positive pulmonary tuberculosis at a regional hospital in Accra, Ghana. Trans R Soc Trop Med Hyg 2012; 106: 511–513. [DOI] [PubMed] [Google Scholar]
- 7.Botha E, den Boon S, Lawrence KA, et al. From suspect to patient: tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis 2008; 12: 936–941. [PubMed] [Google Scholar]
- 8.Botha E, Den Boon S, Verver S, et al. Initial default from tuberculosis treatment: how often does it happen and what are the reasons? Int J Tuberc Lung Dis 2008; 12: 820–823. [PubMed] [Google Scholar]
- 9.Buu TN, Lonnroth K, Quy HT. Initial defaulting in the National Tuberculosis Programme in Ho Chi Minh City, Viet Nam: a survey of extent, reasons and alternative actions taken following default. Int J Tuberc Lung Dis 2003; 7: 735–741. [PubMed] [Google Scholar]
- 10.Mehra D, Kaushik RM, Kaushik R, Rawat J, Kakkar R. Initial default among sputum-positive pulmonary TB patients at a referral hospital in Uttarakhand, India. Trans R Soc Trop Med Hyg 2013; 107: 558–565. [DOI] [PubMed] [Google Scholar]
- 11.Rao NA, Anwer T, Saleem M. Magnitude of initial default in pulmonary tuberculosis. J Pak Med Assoc 2009; 59: 223–225. [PubMed] [Google Scholar]
- 12.Sai Babu B, Satyanarayana AV, Venkateshwaralu G, et al. Initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Andhra Pradesh, India. Int J Tuberc Lung Dis 2008; 12: 1055–1058. [PubMed] [Google Scholar]
- 13.Squire SB, Belaye AK, Kashoti A, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis 2005; 9: 25–31. [PubMed] [Google Scholar]
- 14.Dunbar R, van Hest R, Lawrence K, et al. Capture-recapture to estimate completeness of tuberculosis surveillance in two communities in South Africa. Int J Tuberc Lung Dis 2011; 15: 1038–1043. [DOI] [PubMed] [Google Scholar]
- 15.Dunbar R, Lawrence K, Verver S, et al. Accuracy and completeness of recording of confirmed tuberculosis in two South African communities. Int J Tuberc Lung Dis 2011; 15: 337–343. [PubMed] [Google Scholar]
- 16.Guernier V, Guegan JF, Deparis X. An evaluation of the actual incidence of tuberculosis in French Guiana using a capture-recapture model. Microbes Infect 2006; 8: 721–727. [DOI] [PubMed] [Google Scholar]
- 17.Fatima R, Harris RJ, Enarson DA, et al. Estimating tuberculosis burden and case detection in Pakistan. Int J Tuberc Lung Dis 2014; 18: 55–60. [DOI] [PubMed] [Google Scholar]
- 18.Bassili A, Al-Hammadi A, Al-Absi A, et al. Estimating the tuberculosis burden in resource-limited countries: a capture-recapture study in Yemen. Int J Tuberc Lung Dis 2013; 17: 456–461. [DOI] [PubMed] [Google Scholar]
- 19.Bassili A, Grant AD, El-Mohgazy E, et al. Estimating tuberculosis case detection rate in resource-limited countries: a capture-recapture study in Egypt. Int J Tuberc Lung Dis 2010; 14: 727–732. [PubMed] [Google Scholar]
- 20.Huseynova S, Hashim DS, Tbena MR, et al. Estimating tuberculosis burden and reporting in resource-limited countries: a capture-recapture study in Iraq. Int J Tuberc Lung Dis 2013; 17: 462–467. [DOI] [PubMed] [Google Scholar]
- 21.Podewils LJ, Bantubani N, Bristow C, et al. Completeness and Reliability of the Republic of South Africa National Tuberculosis (TB) Surveillance System. BMC Public Health 2015; 15: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyirenda T, Harries AD, Banerjee A, Salaniponi FM. Registration and treatment of patients with smear-positive pulmonary tuberculosis. Int J Tuberc Lung Dis 1998; 2: 944–945. [PubMed] [Google Scholar]
- 23.National Coordinating Agency for Population and Development, Ministry of Medical Services, Ministry of Public Health and Sanitation, Kenya National Bureau of Statistics, ICF Macro Kenya Service Provision Assessment Survey 2010. Nairobi, Kenya: NCAPD, MOMS, MOPHS, Kenya National Bureau of Statistics & ICF Macro, 2011. http://esaro.unfpa.org/publications/kenya-service-provision-assessment-survey-2010-kspa Accessed June 2016. [Google Scholar]
- 24.Sharma A, Ndisha M, Ngari F, et al. A review of data quality of an electronic tuberculosis surveillance system for case-based reporting in Kenya. Eur J Public Health 2015; 25: 1095–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Tuberculosis Controllers Association; Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2005; 54(RR-15): 1–47. [PubMed] [Google Scholar]
- 26.Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000; 161(4 Pt 1): 1376–1395. [DOI] [PubMed] [Google Scholar]
- 27.Dusetzina SB, Tyree S, Meyer AM, Meyer A, Green L, Carpenter WR. Linking data for health services research: a framework and instructional guide. Rockville, MD, USA: The University of North Carolina at Chapel Hill, 2014. [PubMed] [Google Scholar]
- 28.Roesch A Matching data using sounds-like operators and SAS® compare functions Paper 122–2012. SAS Global Forum 2012. Cary, NC, USA: Statistical Analysis System, 2012. http://support.sas.com/resources/papers/proceedings12/122-2012.pdf. Accessed June 2016. [Google Scholar]
- 29.Sitienei J, Kipruto H, Mansour O, et al. Correlates of default from anti-tuberculosis treatment: a case study using Kenya’s electronic data system. Int J Tuberc Lung Dis 2015; 19: 1051–1056. [DOI] [PubMed] [Google Scholar]
- 30.Stop TB, World Health Organization. Engaging all health care providers in TB control: guidance on imlementing public-private mix approaches WHO/HTM/TB/2006.360. Geneva, Switzerland: WHO, 2006. [Google Scholar]
- 31.Khan AJ, Khowaja S, Khan FS, et al. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. Lancet Infect Dis 2012; 12: 608–616. [DOI] [PubMed] [Google Scholar]
- 32.Lal SS, Sahu S, Wares F, Lonnroth K, Chauhan LS, Uplekar M . Intensified scale-up of public-private mix: a systems approach to tuberculosis care and control in India. Int J Tuberc Lung Dis 2011; 15: 97–104. [PubMed] [Google Scholar]


