Abstract
Regional standardized uptake value ratios (SUVRs) for tau positron emission tomography (PET) were compared among 19 cognitively normal human immunodeficiency virus (HIV)–negative control individuals, 20 HIV-negative patients with symptomatic Alzheimer disease, 15 cognitively normal HIV-positive individuals, and 17 cognitively impaired HIV-positive individuals. Among the HIV-positive participants, the correlation between tau PET SUVRs and both HIV loads and CD4+ T-cell counts (recent and nadir). Tau PET SUVRs were similar for HIV-positive individuals and HIV-negative control individuals. Individuals with symptomatic Alzheimer disease had elevated tau PET SUVRs. Tau PET SUVRs did not correlate with impairment or clinical markers in HIV-positive participants. Older HIV-positive individuals are not at increased risk of tau-mediated neurodegeneration.
Keywords: HIV dementia, positron emission tomography, Alzheimer’s disease
Older human immunodeficiency virus (HIV)–positive individuals do not have tau levels that are greater than those of HIV-negative individuals. Neither cognitive impairment nor disease variables (eg, CD4+ T-cell count and HIV load) in HIV-positive individuals are associated with tau standardized uptake value ratios.
Combination antiretroviral therapy (cART) has changed human immunodeficiency virus (HIV) infection from an acute, life-threatening illness to a long-term chronic condition. HIV-infected individuals are living longer, with an average life expectancy approaching that of HIV-uninfected individuals [1]. The majority of HIV-positive individuals in the United States are >50 years old and may experience premature aging and an increased risk of developing neurodegenerative diseases (eg, Alzheimer disease) [2].
Despite cART, cognitive impairment due to HIV persists, with less severe forms predominating [1]. A biomarker classification scheme proposed for Alzheimer disease may be applicable to other neurodegenerative diseases, including HIV infection [3]. Neuroimaging and cerebrospinal fluid (CSF) biomarkers could assist in differentiating effects due to HIV infection from those due to other neurodegenerative disorders [4].
Tau positron emission tomography (PET) with compounds such as flortaucipir (18F-AV-1451) allows for in vivo imaging of neurodegeneration. 18F-AV-1451 has a relatively high affinity for mature neurofibrillary paired helical filaments in neuritic tangles, correlates with CSF levels of total tau (t-tau) and phosphorylated tau (p-tau), and can generate topographies for and distinguish between multiple neurodegenerative diseases [5, 6]. However, a gap exists concerning whether tau deposition occurs in the brains of older HIV-positive individuals, especially those with cognitive impairment. Previous results suggest CSF tau levels may be elevated in HIV-positive individuals with cognitive impairment [7]. The ability to distinguish cognitive impairment due to HIV from that due to Alzheimer disease is clinically important for older HIV-positive individuals [2]. For the current study, tau PET performed on HIV-positive participants, cognitively normal HIV-negative controls, and patients with symptomatic Alzheimer disease. Within HIV-positive individuals, tau PET findings were assessed with regard to cognitive impairment and clinical markers of disease.
METHODS
Participants
Thirty-two older (age, >50 years) HIV-positive individuals who were chronically infected (duration, ≥12 months) and receiving stable cART were recruited. Most (81%) had an undetectable plasma viral load (defined as ≤20 copies/mL). Current plasma CD4+ T-cell count, plasma HIV RNA levels, and duration of HIV infection were also collected from medical records. The nadir CD4+ T-cell count was recorded as the lowest value from either self-report or medical record review. Nineteen cognitively normal HIV-negative community controls and 20 patients with symptomatic Alzheimer disease were included for comparison. The serostatus of all HIV-negative individuals was confirmed by testing. Patients with symptomatic Alzheimer disease were from studies performed at the Knight Alzheimer Disease Research Center [8]. Exclusion criteria for all participants included current or past history of confounding neurological disorders, severe depression, a positive result of a urine drug screen, head injury with loss of consciousness for >30 minutes, claustrophobia or seizures, and/or an education duration of <8 years. All participants provided informed written consent that was approved by Washington University in Saint Louis.
Imaging
Magnetic resonance imaging was performed using a 3 Tesla Siemens Biograph mMR scanner with a standard 12-channel head coil. A high-resolution structural scan was performed using a 3-dimensional sagittal T1-weighted magnetization-prepared rapid gradient echo (echo time, 16 msec; repetition time, 2400 msec; inversion time, 1000 msec; flip angle, 8°; acquisition matrix, 256 × 256 ; and voxel size, 1 mm × 1 mm × 1 mm). Tau PET was simultaneously performed using the same Biograph40 PET/CT scanner (Siemens Medical Solutions) after intravenous administration of 9–13 mCi of 18F-AV-1451. Data collected 80–100 minutes after injection were converted to regional standardized uptake value ratios (SUVRs). The cerebellar cortex served as the primary reference region. Values were also generated using unsegmented white matter as a reference region for comparison. SUVRs were corrected for partial-volume errors [8].
Neuropsychological Testing
HIV-positive participants and HIV-negative community controls completed a neuropsychological battery that assessed learning and memory, psychomotor/processing speed, and executive function. Raw test scores were converted to deficit scores, using established methods [9], and were averaged to create a global deficit score. Higher global deficit scores correspond to worse cognitive performance. HIV-positive participants were categorized as cognitively normal (HIVn) or impaired (HIVi; global deficit score, ≥0.5). All HIV-negative controls were cognitively normal. Patients with Alzheimer disease were evaluated by experienced clinicians, using a semistructured interview based on the clinical dementia rating scale, and were classified as having symptomatic Alzheimer disease (clinical dementia rating, >0) [10]. Notably, the impairment level for the majority (75%) of the symptomatic Alzheimer disease group was mild.
Analysis
Primary analysis compared tau PET SUVRs between HIV-positive individuals regardless of impairment and cognitively normal HIV-negative community controls and between HIV-negative community controls, HIV-positive individuals (HIVn and HIVi), and patients with symptomatic Alzheimer disease within regions of interest (ROIs) commonly affected by HIV (ie, the caudate, putamen, and frontal cortex) or Alzheimer disease (ie, the amygdala, entorhinal cortex, inferior temporal, and lateral occipital). Age was regressed out of SUVRs, and the unstandardized residuals were compared among groups, using an analysis of variance. Within HIV-positive individuals, regression analyses considered tau PET SUVR as a function of clinical markers (log nadir and recent CD4+ T-cell counts and infection duration). The Holm-Bonferroni method was used to correct for multiple comparisons. Tau PET SUVR was compared between HIV-positive individuals with undetectable (≤20 copies) versus detectable (>20 copies) virus loads, using the Mann-Whitney U test.
Identical analyses were conducted on tau PET SUVRs calculated using unsegmented white matter as a reference region. These results are presented in Supplementary Table 1.
RESULTS
Participant characteristics for the 4 groups are provided in Table 1.
Table 1.
Participant Demographic Characteristics, by Study Group
| HIV Negative, Cognitively Normal (n = 19) | HIV Positive, Cognitively Normal (n = 15) | HIV Positive, Cognitively Impaired (n = 17) | HIV-Negative, Symptomatic Alzheimer Disease (n = 20) | P a | |
|---|---|---|---|---|---|
| Age, y, mean ± SD | 61.4 ± 6.1 | 62.1 ± 7.4 | 58.5 ± 6.3 | 73.1 ± 7.2b | <.001 |
| Sex | .4 | ||||
| Male | 11 (58) | 9 (60) | 13 (76) | 10 (50) | |
| Female | 8 (42) | 6 (40) | 4 (24) | 10 (50) | |
| Education duration, y, mean ± SD | 14.1 ± 2.3 | 13.1 ± 3.0 | 12.5 ± 2.9 | 16.9 ± 2.6b | .001 |
| CD4+ T-cell count, median (IQR) | |||||
| Nadir | NA | 50 (10–113) | 110 (11–454) | NA | .2 |
| Recent | NA | 527 (372–680) | 568 (423–866) | NA | .5 |
| Duration of HIV infection, mo, median (IQR) | NA | 312 (172–338) | 229 (90–273) | NA | .06 |
| HIV load | .7 | ||||
| Undetectable | NA | 13 | 13 | NA | |
| Detectable | NA | 2 | 4 | NA | |
| Self-reported marijuana use in past 6 mo, participants, % | 11 | 40 | 35 | … | .13 |
| Self-reported alcohol use in past 6 mo, participants, % | 74 | 53 | 59 | … | .30 |
| Current smoker, participants, % | 16 | 33 | 41 | … | .27 |
| Tau PET SUVR, by ROI | |||||
| Amygdala | 1.0 (0.2) | 1.1 (0.3) | 1.0 (0.3) | 1.6 (0.8)b | .04 |
| Entorhinal | 0.9 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 1.4 (0.7)b | .017 |
| Inferior temporal | 1.2 (0.1) | 1.3 (0.2) | 1.2 (0.2) | 2.0 (1.0)b | .005 |
| Lateral occipital | 1.2 (0.3) | 1.4 (0.3) | 1.1 (0.2) | 1.6 (0.8) | .19 |
| Caudate | 1.1 (0.3) | 1.1 (0.4) | 1.0 (0.4) | 1.5 (0.2)b | .07 |
| Putamen | 1.4 (0.3) | 1.4 (0.3) | 1.2 (0.3) | 1.7 (0.3) | .51 |
| Frontal | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 1.3 (0.6)b | .05 |
Data are no. (%) of participants, unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; PET, positron emission tomography; ROI, region of interest; SUVR, standardized uptake value ratio.
aBy analysis of variance, the t test, or χ2 analysis.
bSignificantly different from values in the other 3 groups. No other significant group differences were observed.
Effects of HIV and Cognitive Impairment on Tau PET
Tau PET SUVRs were first analyzed between HIV-negative controls and HIV-positive individuals. No significant differences were identified between HIV-negative controls and HIV-positive individuals in any of the analyzed ROIs (P > .05).
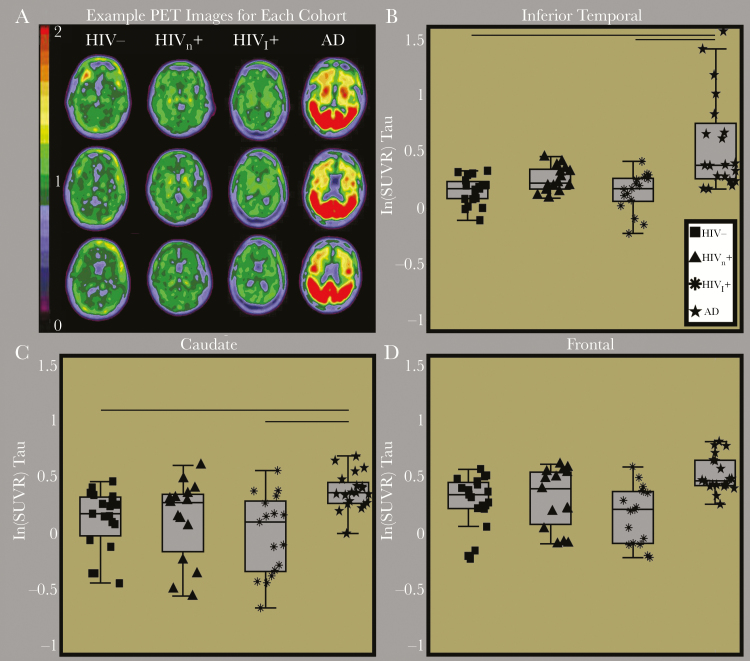
Representative tau PET images are shown in Figure 1 for a cognitively normal HIV-negative community control, 1 participant each from the HIVn and HIVi groups, and a patient with symptomatic Alzheimer disease. Subcortical tau PET binding was elevated for individuals across all groups and may reflect nonspecific binding. Results indicated significant group differences for SUVRs within the inferior temporal (P = .005), entorhinal cortex (P = .017), and amygdala (P = .04) and a trend-level difference in the frontal cortex (P = .05) and caudate (P = .07). Pair-wise comparisons revealed that, among HIV-negative community controls and individuals in the HIVn and HIVi groups, the tau PET SUVRs did not significantly differ for any region. However, the Alzheimer disease group exhibited significantly elevated SUVRs, compared with the other 3 groups, in each of the above regions (P < .05; Supplementary Table 1).
Figure 1.
A, Representative tau positron emission tomography (PET) images from a cognitively normal human immunodeficiency virus (HIV)–negative community control (HIV-), a cognitively normal HIV-infected participant (HIVn), a cognitively impaired HIV-positive participant (HIVi), and an HIV-negative participant with symptomatic Alzheimer disease (AD). On visual inspection, tau PET binding potentials were similar for the cognitively normal community control and the HIV-positive participants. Only the participant with symptomatic Alzheimer disease had significantly elevated tau PET binding. Box plots of the natural log (ln) of tau PET standardized uptake value ratios (SUVRs) for representative regions of interest, including the inferior temporal (B), caudate (C), and frontal cortex (D). Tau PET SUVRs were only elevated in participants with symptomatic Alzheimer disease, compared with the 3 other groups.
Relationship Between Tau PET SUVRs and Clinical Variables in HIV-Positive Individuals
Tau PET SUVRs within ROIs were similar between 6 HIV-positive participants with detectable virus loads and 26 HIV-positive participants with undetectable virus loads. There were no significant relationships between tau PET SUVRs and nadir CD4+ T-cell count, recent CD4+ T-cell count, or duration of infection, after adjustment for multiple comparisons (P > .5).
DISCUSSION
The population of older (age, >50 years) HIV-positive individuals continues to grow and could be at increased risk of developing Alzheimer disease. This study focused on the role of tau deposition in older HIV-positive participants, compared with that in cognitively normal HIV-negative community controls and patients with symptomatic Alzheimer disease. We demonstrated that HIV infection was not associated with an increased tau PET SUVR. Tau PET binding was not elevated in HIV-positive patients with or those without cognitive impairment, compared with changes typically seen in symptomatic Alzheimer disease [11]. Tau PET SUVRs did not correlate with clinical markers. These results suggest that older HIV-positive individuals may not be at increased risk of the tau-mediated neurodegeneration seen with Alzheimer disease.
Mixed results exist concerning the role of tau, a marker of neurodegeneration, in cognitive impairment due to HIV [7]. CSF studies have demonstrated increases in t-tau and p-tau [12], increases in only t-tau [13], or no changes in t-tau and p-tau in HIV-positive individuals [14]. Differences among these various studies may reflect relatively small samples, the combination of HIV-positive individuals receiving or not receiving cART, the selection of appropriate age-matched controls, and differences in tau quantification methods. The tau entity measured by CSF tau analysis and tau PET may differ. Findings of CSF tau analysis may reflect current tau levels within an individual, while findings of tau PET may reflect the cumulative combination of both previous and current tau. Furthermore, CSF analysis and PET may measure different soluble forms of tau.
This study focused on older (age, >50 years) HIV-positive participants because multiple neurodegenerative conditions are seen in this population. Older HIV-positive individuals may be at increased risk for earlier onset of adverse health conditions (so-called accelerated aging) [1, 2]. A potential mechanism could involve atrophy from neurodegeneration, coupled with cognitive impairment due to HIV [4]. Our current results suggest that HIV-positive individuals, regardless of the degree of cognitive impairment or the presence of virus, are not at increased risk for tau-mediated neurodegeneration due to neuritic plaques. We have previously shown that amyloid PET did not detect elevated amyloid levels in HIV-positive individuals [15]. Our results suggest that molecular markers of Alzheimer disease are not increased in older HIV-positive patients. PET (including tau- and/or amyloid-specific imaging) may assist in differentiating Alzheimer disease from cognitive impairment due to HIV in this population. For clinicians, it may be difficult to differentiate these disorders on the basis of symptoms alone in older HIV-positive individuals.
Some limitations must be noted. The AV-1451 tau PET ligand is likely to bind in subcortical regions in individuals, even with no known pathology (ie, via nonspecific binding). However, a large recent study has shown that the ligand is useful in discriminating Alzheimer disease from other dementias without tau pathology [6]. Our study complements this research by demonstrating similar results when comparing patients with Alzheimer disease to HIV-positive individuals. Additionally, the use of the cerebellum as a reference region may not be ideal owing to its involvement in HIV. However, we conducted additional analyses using alternate reference regions, and the results did not change.
While imaging methods demonstrate that neurodegeneration coincides with cognitive impairment due to HIV, these results suggest that the mechanism is not through pathological changes seen in Alzheimer disease. Other potential etiologies include continued neuroinflammation or brain HIV reservoirs. Future studies using MRI and PET to measure neuroinflammation are needed, especially in older HIV-positive individuals with well-controlled HIV loads, to assess potential reservoirs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. S. A. C. contributed to analysis, interpretation of data, and writing the manuscript. J. F. S. contributed to analysis and writing the manuscript. H. B. contributed to analysis, interpretation of data, and writing the manuscript. A. H. B. contributed to analysis and writing the manuscript. J. D. contributed to analysis and writing the manuscript. J. C. M. contributed to study conception and design and writing the manuscript. T. L. B. contributed to study conception and design, interpretation of data, and writing the manuscript. B. M. A. contributed to study conception and design, analysis, interpretation of data, and writing the manuscript.
Acknowledgments. We thank the study participants, who helped make this work possible.
Financial support. This work was supported by the National Institutes of Health - National Institute of Nursing Research (grants R01NR012907, R01NR012657, and R01NR014449 to B.M.A), National Institutes of Health - National Institute on Aging (grants P01AG00391, P01AG026276, and P01AG005681 to J.C.M.); Barnes-Jewish Hospital; the National Institutes of Health - National Center for Advancing Translational Sciences (grant UL1 TR000448); the Hope Center for Neurological Disorders; the Paula and Rodger O. Riney Fund; the Daniel J Brennan MD Fund; Fred Simmons and Olga Mohan; and Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly; provision of the precursor for AV-1451, chemistry support for the production of this compound, and doses of the compound).
Potential conflicts of interest. J. C. M. is currently participating in clinical trials of antidementia drugs developed by Eli Lilly, Biogen, and Janssen; serves as a consultant for Lilly USA; and receives support from Eli Lilly/Avid Radiopharmaceuticals. T. L. B. is involved in a clinical trial that uses AV-1451, sponsored by Avid. B. M. A. is involved in a clinical trial that uses AV-1451, sponsored by Avid. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 2009; 4:163–74. [DOI] [PubMed] [Google Scholar]
- 2. Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer’s disease: an emerging issue in geriatric neuroHIV. Curr HIV/AIDS Rep 2017; 14:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016; 87:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol 2014; 34:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015; 78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 2018; 320:1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown LA, Scarola J, Smith AJ, Sanberg PR, Tan J, Giunta B. The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener 2014; 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mishra S, Gordon BA, Su Y, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 2017; 161:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris JC. The Clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–4. [DOI] [PubMed] [Google Scholar]
- 11. Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016; 89:971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 2005; 65:1490–2. [DOI] [PubMed] [Google Scholar]
- 13. Steinbrink F, Evers S, Buerke B, et al. ; German Competence Network HIV/AIDS Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol 2013; 20:420–8. [DOI] [PubMed] [Google Scholar]
- 14. Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009; 73:1982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ances BM, Christensen JJ, Teshome M, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology 2010; 75:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.