Abstract
Background
Ebola vaccine development was accelerated in response to the 2014 Ebola virus infection outbreak. This phase 1 study (VAC52150EBL1004) assessed safety, tolerability, and immunogenicity of heterologous 2-dose Ad26.ZEBOV, MVA-BN-Filo vaccination regimens in the Lake Victoria Basin of Tanzania and Uganda in mid-level altitude, malaria-endemic settings.
Methods
Healthy volunteers aged 18–50 years from Tanzania (n = 25) and Uganda (n = 47) were randomized to receive placebo or active vaccination with Ad26.ZEBOV or MVA-BN-Filo (first vaccination), followed by MVA-BN-Filo or Ad26.ZEBOV (second vaccination) dose 2, respectively, with intervals of 28 or 56 days.
Results
Seventy-two adults were randomized to receive vaccine (n = 60) or placebo (n = 12). No vaccine-related serious adverse events were reported. The most frequent solicited local and systemic adverse events were injection site pain (frequency, 70%, 66%, and 42% per dose for MVA-BN-Filo, Ad26.ZEBOV, and placebo, respectively) and headache (57%, 56%, and 46%, respectively). Adverse event patterns were similar among regimens. Twenty-one days after dose 2, 100% of volunteers demonstrated binding antibody responses against Ebola virus glycoprotein, and 87%–100% demonstrated neutralizing antibody responses. Ad26.ZEBOV dose 1 vaccination induced more-robust initial binding antibody and cellular responses than MVA-BN-Filo dose 1 vaccination.
Conclusions
Heterologous 2-dose vaccination with Ad26.ZEBOV and MVA-BN-Filo against Ebola virus is well tolerated and immunogenic in healthy volunteers.
Clinical trials registration
Keywords: Ebola vaccine, heterologous 2-dose, Ad26.ZEBOV, MVA-BN-Filo, safety and immunogenicity
This phase 1 study demonstrated that heterologous prime-boost vaccination with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines at intervals of 28 or 56 days was well tolerated and immunogenic in healthy African adult volunteers.
(See the Major Article by Mutua et al on pages 57–67.)
Ebola virus disease is highly contagious and severe, with a high fatality rate [1, 2]. The 2014 Zaire Ebolavirus outbreak in West Africa received extensive global attention because of the large number of cases (>28 600) and deaths (>11 000) and the potential for further international spread [3].
Smaller outbreaks have occurred repeatedly in Central and East Africa since Ebola was first described in 1976 [4, 5]. In Uganda, 5 outbreaks of Ebola virus infection occurred between 2000 and 2012, with 606 suspected cases and 283 deaths (fatality rate, 47%) [6, 7]. More-recent cases have been reported in the Democratic Republic of the Congo (in 2017 and 2018) [8–10].
As a response to the 2013–2016 outbreak, efforts to develop Ebola vaccines were accelerated, with a variety of candidate vaccines under investigation that used platforms including DNA, recombinant or subunit proteins, virus-like particles, and recombinant viral vectors [11]. Heterologous 2-dose vaccination with Ad26.ZEBOV and MVA-BN-Filo is under development by Janssen Vaccines and Prevention [12, 13]. The heterologous 2-dose vaccination regimens comprise 1 dose of each of these vaccine candidates, with an intervening period of either 28 or 56 days; the second vaccine is administered to boost immune responses. In 2013, MVA-BN received market authorization in the European Union and Canada as a smallpox vaccine [14]. Protection against Ebola virus disease by using the Ad26.ZEBOV and MVA-BN-Filo vaccine regimen is being evaluated in an extensive clinical trial program that includes healthy adults, adolescents, children ≥1 year of age, and adults with human immunodeficiency virus infection from different sub-Saharan countries.
We conducted a phase 1 study in 2 urban/peri-urban, malaria-endemic areas of northwestern Tanzania and southwestern Uganda to assess the safety, tolerability, and immunogenicity of different Ad26.ZEBOV and MVA-BN-Filo heterologous 2-dose vaccination sequences, with a dosing interval of 28 or 56 days and a follow-up period of 1 year (clinical trials identifiers VAC52150EBL1004 and NCT02376400). This study was designed to complement the VAC52150EBL1003 study, which was located in a high-altitude, urban setting with low incidence of malaria (described by Mutua et al [15]).
METHODS
Study Population
Healthy adult volunteers (n = 72) aged 18–50 years were recruited from 2 malaria-endemic areas of East Africa: Mwanza (Tanzania) and Masaka (Uganda). Both sites are located near Lake Victoria at an altitude of approximately 1100 m. All study participants had to be considered healthy on the basis of physical examination, medical history, and the investigator’s clinical judgment (exclusion criteria are available in the Supplementary Materials).
Study Design
The study design of this trial was identical to that described for the VAC52150EBL1003 trial by Mutua et al [15]. Additional information is available in the Supplementary Materials.
Study Procedures
The protocol and procedures of this study followed exactly those of the VAC52150EBL1003 trial by Mutua et al [15], including screening, individual randomization, and placebo-controlled vaccine administration. The primary objective was to assess the safety and tolerability of different Ad26.ZEBOV and MVA-BN-Filo heterologous 2-dose vaccination regimens. Secondary outcomes included Ebolavirus glycoprotein–specific humoral and cellular immune responses induced by the vaccine regimens.
Randomization and Masking
Participants were randomized using a computer-generated block randomization schedule, and participants and study team members were blinded until 21 days after the second vaccination, as previously described [15].
Adverse Event (AE) Monitoring
The reporting of AEs was identical to that described for the VAC52150EBL1003 trial [15]. Briefly, solicited AEs were recorded in a diary by participants for 7 days following each vaccination, and unsolicited AEs were collected at all visits until 21 days after dose 2. Solicited AEs were previously defined [15] (Supplementary Materials). Clinical AEs were graded according to the scale of the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health [16], whereas laboratory toxicities were graded according to the Food and Drug Administration’s Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [17]. AEs of special interest were recorded because of particular concerns with historic early generation MVA-based vaccines [18].
Immunogenicity Measurements
Measurements of immunogenicity were identical to those previously described [15]. Immune responses were measured using serum samples taken before first and second vaccinations, 7 days after first and second vaccinations, and 21 days after the second vaccinations. Participants who received vaccines with a 56-day interval had an additional blood specimen collected 28 days after the first vaccination. Long-term follow-up samples were collected in all groups at days 180, 240, and 360. Immunoglobulin G (IgG) binding and neutralizing antibody responses were analyzed using an enzyme-linked immunosorbent assay (ELISA) [13] and pseudovirion neutralization assay [15], respectively. Exploratory objectives included evaluation of CD4+ and CD8+ T-cell responses, using intracellular cytokine staining combined with flow cytometry [12, 19].
Data Analysis and Statistics
The primary analysis sets for safety and immunogenicity were as described previously [15]; the primary analysis set for safety (full analysis set) comprised all randomized participants who received at least 1 dose of study vaccine. The primary analysis set for immunogenicity included all vaccinated participants with immunogenicity data at baseline and data from at least 1 measurement after vaccination. All data were analyzed using descriptive statistics without formal hypothesis testing. Immunogenicity data are presented using methods similar to those of other phase 1 studies of this 2-dose regimen [12, 15, 20]. A participant was defined as a responder for ELISA, virus-neutralizing antibody (VNA), or intracellular cytokine staining at each time point if the test result was negative at baseline and positive after baseline or if a test result that was positive at baseline was followed by a result that increased by at least 3-fold, as described previously [15]. Given the small sample sizes in each vaccination group and minimal evidence available regarding statistical hypothesis testing, no formal statistical testing of safety data or immune responses was planned or performed.
RESULTS
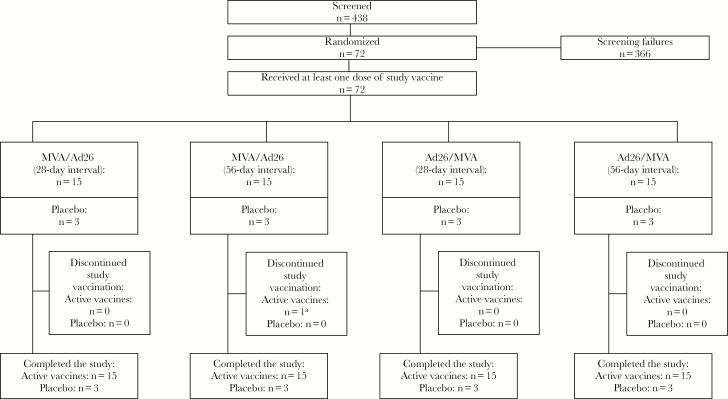
The study was conducted between 18 February 2015 and 16 September 2016. Seventy-two healthy adult African volunteers (25 from Tanzania and 47 from Uganda) were randomized into 4 groups of 18 volunteers; 15 were randomized to receive active vaccine, and 3 were assigned to receive placebo (Figure 1). Active-vaccine recipients were vaccinated with Ad26.ZEBOV or MVA-BN-Filo as the first vaccination and MVA-BN-Filo or Ad26.ZEBOV, respectively, as the second vaccination (n = 30 each). For all participants, the interdose interval was 28 or 56 days. Baseline characteristics of placebo recipients, MVA-BN-Filo first dose recipients, and Ad26.ZEBOV first dose recipients are shown in Table 1.
Figure 1.
Subject disposition aOne subject did not receive Ad26.ZEBOV second dose vaccination, because he met a protocol-specific criterion for contraindication to dose 2. This subject remained in the study.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Dose 2 at Day 28 | Dose 2 at Day 56 | ||||
|---|---|---|---|---|---|---|
| MVA/Ad26 (n = 15) | Ad26/MVA (n = 15) | Placebo (n = 6) | MVA/Ad26 (n = 15) | Ad26/MVA (n = 15) | Placebo (n = 6) | |
| Sex | ||||||
| Female | 1 (6.7) | 3 (20.0) | 2 (33.3) | 3 (20.0) | 5 (33.3) | 1 (16.7) |
| Male | 14 (93.3) | 12 (80.0) | 4 (66.7) | 12 (80.0) | 10 (66.7) | 5 (83.3) |
| Age, y | 25 (20–41) | 24 (20–37) | 27 (24–49) | 27 (18–38) | 25 (19–42) | 23 (20–43) |
| Body mass indexa | 21.1 (18.2–23.6) | 23.2 (16.1–35.4) | 20.4 (18.4–28.5) | 22.2 (15.9–30.7) | 21.8 (18.6–33.8) | 21.0 (17.5–26.9) |
Abbreviations: Ad26, Ad26.ZEBOV; MVA, MVA-BN-Filo.
Data are no. (%) of participants or median value (range).
aCalculated as the weight in kilograms divided by the height in meters squared.
Safety and Tolerability
Solicited local and systemic AEs were mostly mild to moderate in severity and transient following MVA-BN-Filo (n = 60) and Ad26.ZEBOV (n = 59) administration. The most frequently reported solicited local AE for both vaccines and placebo was injection site pain (Table 2). One grade 3 solicited local AE was documented following Ad26.ZEBOV first dose vaccination (injection site swelling). No grade 3 solicited local AEs were observed following administration of MVA-BN-Filo or placebo. The most frequently reported solicited systemic AEs for both vaccines and placebo were headache and fatigue (Table 3). Grade 3 solicited systemic AEs (headache in all cases) occurred in 1 MVA-BN-Filo second dose recipient (considered by the investigator as doubtfully related to study vaccine because the participant had concurrent clinical malaria), 1 Ad26.ZEBOV second dose recipient (considered by the investigator as doubtfully related to study vaccine and thought to be attributable to recurrent toothache), and 1 placebo recipient. The median duration of frequently reported solicited AEs ranged from 1 to 3 days following MVA-BN-Filo vaccination and from 1 to 2 days following Ad26.ZEBOV vaccination.
Table 2.
Solicited Local Adverse Events (AEs) Following First and Second Dose Vaccinations With Standard Doses of MVA-BN-Filo (MVA) and Ad26.ZEBOV (Ad26)
| AE, Severity | MVA (n = 60) | Ad26 (n = 59) | Placebo (n = 24) |
|---|---|---|---|
| Any | |||
| Grade 1 | 30 (50) | 24 (41) | 12 (50) |
| Grade 2 | 14 (23) | 15 (25) | 0 (0) |
| Grade 3 | 0 (0) | 1 (2) | 0 (0) |
| Total | 44 (73) | 40 (68) | 12 (50) |
| Injection site paina | |||
| Grade 1 | 31 (52) | 26 (44) | 10 (42) |
| Grade 2 | 11 (18) | 13 (22) | 0 (0) |
| Total | 42 (70) | 39 (66) | 10 (42) |
| Injection site warmtha | |||
| Grade 1 | 15 (25) | 13 (22) | 6 (25) |
| Grade 2 | 6 (10) | 2 (3) | 0 (0) |
| Total | 21 (35) | 15 (25) | 6 (25) |
| Injection site pruritusa | |||
| Grade 1 | 12 (20) | 8 (14) | 6 (25) |
| Grade 2 | 2 (3) | 1 (2) | 0 (0) |
| Total | 14 (23) | 9 (15) | 6 (25) |
| Injection site swellingb | |||
| Grade 3 | 0 (0) | 1 (2) | 0 (0) |
| Total | 0 (0) | 1 (2) | 0 (0) |
Data are no. (%) of doses and reflect pooled first and second dose data from all 4 vaccination regimens.
aNo grade 3 AEs were reported.
bNo grade 1 or 2 AEs were reported.
Table 3.
Solicited Systemic Adverse Events (AEs) Following First and Second Dose Vaccinations With Standard Doses of MVA-BN-Filo (MVA) and Ad26.ZEBOV (Ad26)
| AE, Severity | MVA (n = 60) |
Ad26 (n = 59) |
Placebo (n = 24) |
|---|---|---|---|
| Any | |||
| Grade 1 | 27 (45) | 18 (31) | 10 (42) |
| Grade 2 | 18 (30) | 25 (42) | 4 (17) |
| Grade 3 | 1 (2) | 1 (2) | 1 (4) |
| Total | 46 (77) | 44 (75) | 15 (63) |
| Headache | |||
| Grade 1 | 23 (38) | 19 (32) | 8 (33) |
| Grade 2 | 10 (17) | 13 (22) | 2 (8) |
| Grade 3 | 1 (2) | 1 (2) | 1 (4) |
| Total | 34 (57) | 33 (56) | 11 (46) |
| Fatiguea | |||
| Grade 1 | 21 (35) | 18 (31) | 5 (21) |
| Grade 2 | 9 (15) | 15 (25) | 2 (8) |
| Total | 30 (50) | 33 (56) | 7 (29) |
| Myalgiaa | |||
| Grade 1 | 18 (30) | 12 (20) | 4 (17) |
| Grade 2 | 10 (17) | 8 (14) | 1 (4) |
| Total | 28 (47) | 20 (34) | 5 (21) |
| Arthralgiaa | |||
| Grade 1 | 14 (23) | 9 (15) | 3 (13) |
| Grade 2 | 4 (7) | 6 (10) | 0 (0) |
| Total | 18 (30) | 15 (25) | 3 (13) |
| Nauseaa | |||
| Grade 1 | 12 (20) | 12 (20) | 1 (4) |
| Grade 2 | 3 (5) | 5 (9) | 1 (4) |
| Total | 15 (25) | 17 (29) | 2 (8) |
| Chillsa | |||
| Grade 1 | 8 (13) | 11 (19) | 1 (4) |
| Grade 2 | 3 (5) | 1 (2) | 0 (0) |
| Total | 11 (18) | 12 (20) | 1 (4) |
| Pruritus (generalized)a | |||
| Grade 1 | 5 (8) | 2 (3) | 2 (8) |
| Grade 2 | 2 (3) | 3 (5) | 1 (4) |
| Total | 7 (12) | 5 (8) | 3 (13) |
| Rasha | |||
| Grade 1 | 5 (8) | 3 (5) | 1 (4) |
| Grade 2 | 1 (2) | 0 (0) | 0 (0) |
| Total | 6 (10) | 3 (5) | 1 (4) |
| Pyrexiaa | |||
| Grade 1 | 1 (2) | 2 (3) | 0 (0) |
| Grade 2 | 0 (0) | 2 (3) | 0 (0) |
| Total | 1 (2) | 4 (7) | 0 (0) |
| Vomitingb | |||
| Grade 1 | 0 (0) | 3 (5) | 0 (0) |
| Total | 0 (0) | 3 (5) | 0 (0) |
Data are no. (%) of doses and reflect pooled first and second dose data from all 4 vaccination regimens.
aNo grade 3 AEs were reported.
bNo grade 2 or 3 AEs were reported.
The percentages of doses associated with an unsolicited AE were 66.7%, 57.6%, and 79.2% following vaccination with MVA-BN-Filo, Ad26.ZEBOV, and placebo, respectively. The most-frequent unsolicited AEs were proteinuria (after 14 doses, with 9 cases occurring after MVA-BN-Filo receipt [15% of MVA-BN-Filo doses], 2 after Ad26.ZEBOV receipt [3.4% of Ad26.ZEBOV doses], and 3 after placebo receipt [12.5% of placebo doses]), headache (after 11, with 4 after MVA-BN-Filo receipt [6.7%], 3 after Ad26.ZEBOV receipt [5.1%], and 4 after placebo receipt [16.7%]), decreased neutrophil count (after 10, with 4 after MVA-BN-Filo receipt [6.7%], 3 after Ad26.ZEBOV receipt [5.1%], and 3 after placebo receipt [12.5%]), and malaria (after 4, with 1 after MVA-BN-Filo receipt [1.7%] and 3 after placebo receipt [12.5%]). These unsolicited AEs occurred more frequently in placebo recipients than in the active vaccine groups, with the exception of proteinuria, which was based on protein dipstick results from a mid-stream urine sample.
The majority of unsolicited AEs were grade 1 and 2 in severity, with grade 3 unsolicited AEs reported in 12 participants (20%) after MVA-BN-Filo receipt, in 9 (15.3%) after Ad26.ZEBOV receipt, and in 4 (16.7%) after placebo receipt. One grade 3 bradycardia event (heart rate, <50 beats/minute) was reported as an AE of special interest following MVA-BN-Filo first dose vaccination because it was considered to be a cardiac sign or symptom. The bradycardia was considered to be probably related to study vaccine. The symptoms resolved within 1 hour, without treatment. This participant did not receive a second vaccination. All other grade 3 unsolicited AEs were related to abnormal laboratory findings and, therefore, were reported as AEs regardless of clinical significance, as per protocol. These reported AEs were decreased neutrophil count (5 occurred after MVA-BN-Filo receipt, 3 after Ad26.ZEBOV receipt, and 3 after placebo receipt), decreased hemoglobin level (1 after MVA-BN-Filo receipt and 2 after Ad26.ZEBOV receipt), prolonged prothrombin time (1 after MVA-BN-Filo receipt, 2 after Ad26.ZEBOV receipt, and 1 after placebo receipt), proteinuria (3 after MVA-BN-Filo receipt), hyperkalemia (1 after MVA-BN-Filo receipt and 1 after Ad26.ZEBOV receipt), and increased blood potassium level (1 after MVA-BN-Filo receipt). However, in all subjects reporting prolonged prothrombin time, the international normalized ratio was a grade 0 or grade 1 AE.
No differences were seen in AE patterns when comparing the periods after first vaccination to the periods after second vaccination or between different vaccine sequences or intervals. One serious AE (grade 2 typhoid fever) was reported 36 days following Ad26.ZEBOV first vaccination and was not considered by the investigator as related to study vaccine. In addition to the grade 3 bradycardia, there was 1 other AE of special interest: 1 Ad26.ZEBOV first dose individual had grade 1 hypertension on day 1 that was considered to be probably related to study vaccine. However, as the event was reported after Ad26.ZEBOV vaccination and prior to receiving MVA-BN-Filo, it was no longer considered an AE of special interest after unblinding of the study.
Immunogenicity
Binding-Antibody Responses
High levels of binding antibodies to EBOV glycoprotein were generated in response to all 4 vaccination regimens. In general, responses to placebo were low or not quantifiable.
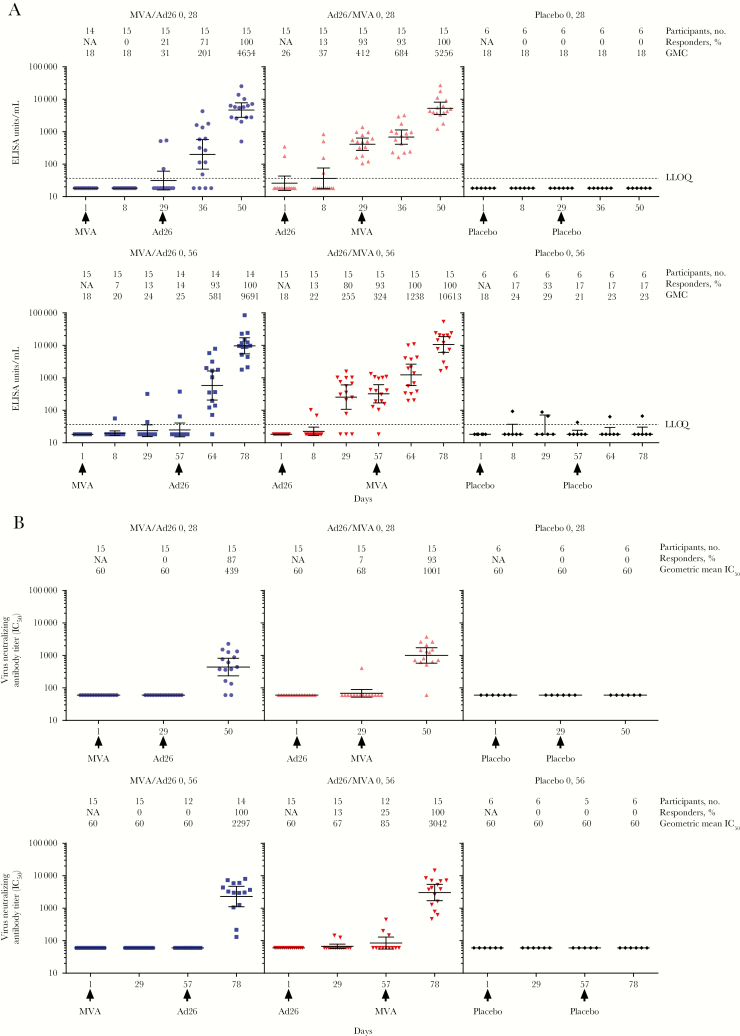
For both dosing intervals with Ad26.ZEBOV dose 1 vaccination, binding antibody responder rates (ie, the proportion of participants showing a true response) increased to 93% at the time of MVA-BN-Filo dose 2 (Figure 2A). At 21 days after dose 2 (day 50 or 78), 100% of participants in both Ad26.ZEBOV dose 1 groups demonstrated an antibody response, with geometric mean concentrations (GMCs) rising to 5256 and 10 613 ELISA units [EU]/mL in the 28-day and 56-day interval groups, respectively (Supplementary Table 1).
Figure 2.
Anti–Ebola virus glycoprotein immunoglobulin G binding antibody responses (detected by enzyme-linked immunosorbent assay [ELISA]) binding antibody responses (A) and virus neutralizing antibody (VNA) responses (B) following dose 1 with MVA-BN-Filo (MVA) or Ad26.ZEBOV (Ad26) and heterologous dose 2 with Ad26 or MVA on day 29 or day 57, up to 21 days post dose 2 (day 50 or 78). Data are geometric mean concentration (GMC), for ELISA, and geometric mean 50% inhibitory concentration (IC50), for VNA analysis. Error bars represent 95% confidence intervals. NA, not applicable.
With MVA-BN-Filo dose 1 vaccination, binding-antibody responder rates were low at the time of Ad26.ZEBOV dose 2 (21% and 14% with 28-day and 56-day intervals, respectively). At 21 days after dose 2, responder rates rose to 100% for both dosing intervals (Figure 2A), with GMCs rising to 4654 and 9691 ELISA units/mL (EU/mL) in the 28-day and 56-day interval groups, respectively (Supplementary Table 1).
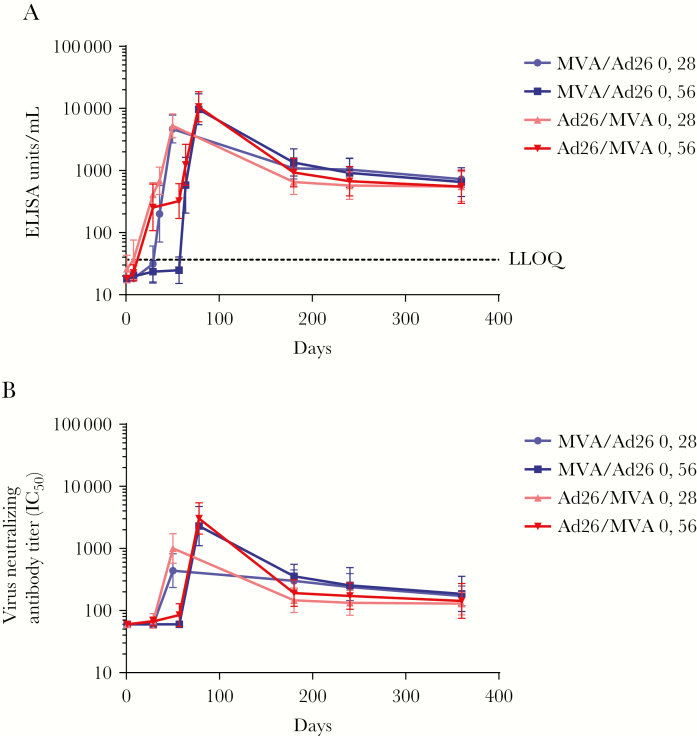
The magnitude of the binding antibody responses decreased toward day 180 after dose 1 but stabilized thereafter. Across all regimens, responses persisted in 100% of participants until day 360 after dose 1, with GMCs ranging from 550 to 730 EU/mL (Figure 3A).
Figure 3.
Durability of anti–Ebola virus glycoprotein immunoglobulin G binding (A) and neutralizing (B) antibody responses following dose 1 with MVA-BN-Filo (MVA) or Ad26.ZEBOV (Ad26) and heterologous dose 2 with Ad26 or MVA on day 29 or day 57. Data are geometric mean values; error bars represent 95% confidence intervals. ELISA, enzyme-linked immunosorbent assay; IC50, 50% inhibitory concentration.
Virus-Neutralizing Antibody (VNA) Response
Neutralizing antibody titers were low following Ad26.ZEBOV first dose vaccination but increased by 21 days after second dose vaccination so that 93% and 100% of participants on the 28- and 56-day interval regimens, respectively, showed neutralizing antibody responses (Figure 2B). Geometric mean 50% inhibitory concentration (IC50) values were elevated 21 days after dose 2, at 1001 and 3042 in the 28-day and 56-day interval groups, respectively (Figure 2B and Supplementary Table 1).
Neutralizing antibody titers were low following MVA-BN-Filo first dose vaccination but increased by 21 days after second dose vaccination so that 87% and 100% of participants on the 28- and 56-day interval regimens, respectively, showed neutralizing antibody responses (Figure 2B). In the MVA-BN-Filo dose 1 groups, geometric mean IC50 values 21 days after dose 2 were 439 and 2297 for the 28- and 56-day intervals, respectively (Supplementary Table 1).
In all Ad26.ZEBOV and MVA-BN-Filo dose 1 groups, geometric mean titers declined to a stable level observed by day 180, which was sustained until day 360 (Figure 3B).
CD8+ T-Cell Responses
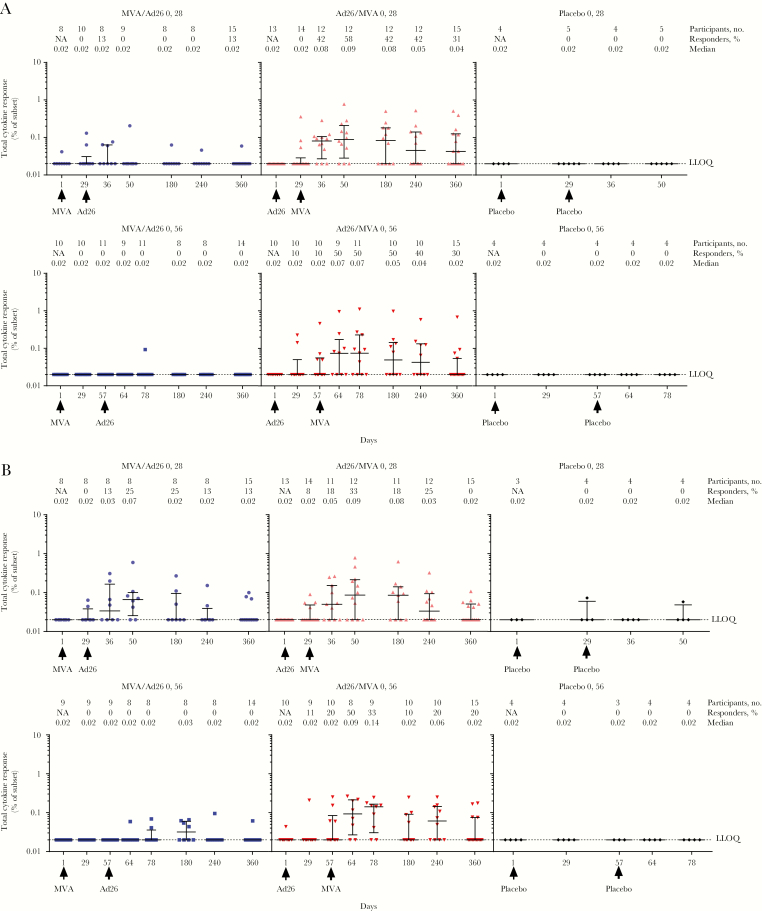
In the Ad26.ZEBOV dose 1 groups, T-cell responses were only observed following MVA-BN-Filo dose 2 administration. Responder rates peaked 21 days after dose 2, at 58%, for the 28-day interval group and remained elevated at day 360, at 31% (Figure 4A). For the 56-day interval group, a peak responder rate of 50% was observed 7 days after dose 2 and was sustained until day 180, after which it declined to 30% at day 360. Median CD8+ T-cell response rates were highest 7 and 21 days after dose 2 for the 56-day interval group (0.07%) and 28-day interval group (0.09%), respectively (Figure 4A).
Figure 4.
Median CD8+ T-cell responses (A) and CD4+ T-cell responses (B) following first dose with MVA-BN-Filo (MVA) or Ad26.ZEBOV (Ad26) and heterologous second dose with Ad26 or MVA on day 29 or day 57. NA, not applicable.
In the MVA-BN-Filo dose 1 groups, a peak responder rate of 13% was observed 7 days after dose 2 for the 28-day interval regimen. There were no responders at any time point in the 56-day interval group (Figure 4A).
CD4+ T-Cell Responses
Responder rates peaked at 33% 21 days after dose 2 in the Ad26.ZEBOV dose one 28-day interval group. In the 56-day interval group, the proportion of responders peaked 7 days after dose 2, at 50% (Figures 4B). In the Ad26.ZEBOV dose one 28-day interval regimen, the median response rate among CD4+ T cells was 0.09% 21 days after dose 2. For the 56-day interval group, the highest median CD4+ T-cell response rates were reached 21 days after dose 2, at 0.14%. By day 360, CD4+ T-cell responses in all Ad26.ZEBOV dose 1 groups were detected in 0%–20% of individuals.
While no responders were detected at any time point in the MVA-BN-Filo dose 1 group with a 56-day interval, a peak responder rate of 25% was observed 21 days after dose 2 in the 28-day interval regimen. The responder rate was sustained until day 180 (Figure 4B). The highest median CD4+ T-cell response rate was also recorded 21 days after dose 2, at 0.07% of cells.
DISCUSSION
This is the first clinical trial of the novel Ad26.ZEBOV and MVA-BN-Filo vaccination strategy that recruited participants from a malaria-endemic region of sub-Saharan Africa. The results of this study are similar to the findings from other phase 1 studies of heterologous 2-dose regimens with Ad26.ZEBOV and MVA-BN-Filo, respectively, and with MVA-BN-Filo and Ad26.ZEBOV, respectively, performed in Nairobi, Kenya [15], and the United Kingdom [12, 20], showing that these 2-dose vaccination regimens consistently demonstrate a favorable safety profile, eliciting few grade 3 AEs and no serious AEs, in different regions. For both vaccines, AE patterns were similar regardless of their use as first or second vaccines and irrespective of whether a 28- or 56-day dosing interval was used. Strong humoral immune responses were observed, reaching binding and neutralizing antibody responder rates of 100% and 87%–100%, respectively, 21 days after dose 2, regardless of vaccine interval and sequence. Although the magnitude of responses declined over time, the responder rate remained high 1 year after dose 1 (100% of volunteers showed binding antibodies, and 53%–87% showed neutralizing antibodies). After dose 1, T-cell responses were low or not quantifiable, and after dose 2, the highest responses were observed for participants receiving the Ad26.ZEBOV first dose regimen with either the 28-day or 56-day first or second dose interval. This finding is consistent with data reported previously [12]. Extending the dosing interval from 28 days to 56 days led to an increase in humoral responses. Efficacy data for heterologous 2-dose vaccination with Ad26.ZEBOV and MVA-BN-Filo, respectively, or with MVA-BN-Filo and Ad26.ZEBOV, respectively, are not yet available in humans; however, nonhuman primate data have shown a strong correlation between binding antibody responses and survival after challenge with Ebola virus [21].
Both this study and that of Mutua et al [15] are part of the clinical development program for Ad26.ZEBOV and MVA-BN-Filo 2-dose vaccination, designed to ascertain the potential of this vaccination strategy to play a role in the prevention and/or containment of future Zaire Ebolavirus outbreaks. The need for effective control measures was highlighted by the recent outbreaks in the Democratic Republic of the Congo [8–10]. The reemergence of Ebola virus is unpredictable, suggesting a need either for population-wide protection, healthcare worker protection, or alternative effective control measures that can be implemented rapidly.
With our findings and those of Mutua et al [15], there are now data from multiple studies demonstrating the safety and tolerability of these vaccines in different African populations from geographical areas representative of the region. In addition, data have also been captured from the United Kingdom in a population geographically and ethnically distinct from those experiencing the Ebola virus outbreak [12, 20]. In our study, similar to the findings from the United Kingdom, injection site pain was the most frequently reported solicited local AE, and the most common solicited systemic AEs were headache, fatigue, and myalgia, all of which occurred with mild-to-moderate severity and were of short duration [12]. In Uganda and Tanzania, fever, a common symptom of Ebola [22], was not a frequent solicited systemic AE, and no vaccine-related serious AEs were reported.
In our study, conducted in malaria-endemic communities on Lake Victoria [23, 24], 4 of 72 participants (5.5%) developed clinical malaria, of whom 3 were in the placebo arm. The total proportion of participants reporting malaria was the same as in the VAC52150EBL1003 study [15], conducted in Nairobi, where malaria is not endemic and where participants were therefore considered to be at low risk of the infection unless they traveled out of the city [25]. Our study showed a pattern similar to that of a previous study from West Africa involving the ChAd3-EBO-Z Ebola vaccine, as both demonstrated an unexpected finding of a lower incidence of malaria in participants receiving the active vaccine as compared to participants receiving placebo [26]. However, the small sample size in our study makes it difficult to assess any association between the vaccines and a possible lower risk of malaria.
Several other candidate prophylactic Ebola vaccines have also been tested in clinical trials in Africa. Over 4000 individuals received rVSV-ZEBOV vaccine in a phase 3 ring vaccination study; vaccine efficacy was estimated to be 100% (95% CI, 68.9%–100%), and only 3 serious AEs occurred that were judged to be at least possibly related to vaccination (1 febrile reaction, 1 case of anaphylaxis, and 1 influenza-like illness; all resolved without further sequelae) [27]. Consequently, this vaccine was used in the 2018 outbreaks in the Democratic Republic of the Congo [28, 29]. Comparisons of immune responses in different studies need to be made with caution, as they may be confounded by differences in population characteristics, dosing regimens, or the use of different assays to measure antibody responses. However, when using the same ELISA protocol, the Ad26.ZEBOV and MVA-BN-Filo heterologous regimens in this study and that of Mutua et al [15], especially the 56-day-interval regimens, induced higher EBOV-specific glycoprotein IgG concentrations after dose 2 than single-dose rVSV-ZEBOV [30]. Another candidate vaccine, ChAd3-EBO-Z, is an adenovirus-based vaccine expressing Zaire Ebolavirus glycoprotein and can be boosted by MVA-BN-Filo [31]. The persistence of binding antibody responses observed in the current study up to 1 year after Ad26.ZEBOV and MVA-BN-Filo vaccination has also been observed for both rVSV-ZEBOV and ChAd3-EBO-Z in study participants in Liberia [26].
Our study provides support to the further development of the Ad26.ZEBOV and MVA-BN-Filo heterologous 2-dose vaccination strategy. Further clinical studies are currently underway to evaluate Ad26.ZEBOV dose 1 and MVA-BN-Filo dose 2 vaccination with dosing intervals of 28, 56, and 84 days (clinical trials registration NCT02564523 and NCT02509494). Different populations are being included in these studies (eg, children and individuals with human immunodeficiency virus infection). Clinical trials of the Ad26.ZEBOV and MVA-BN-Filo strategy are also investigating short-duration regimens, with intervals between first and second dose vaccinations of 7 or 14 days (clinical trials registration NCT02325050 and NCT02598388). Two-dose regimens with longer dosing intervals, such as 56 days, tend to elicit higher antibody responses, as demonstrated in this study, and therefore may be more suitable for long-term protection strategies. Conversely, short intervals between first and second dose vaccinations may enable reactive use and early onset of immunity in the context of an outbreak.
In 2 placebo recipients (1 from Uganda and 1 from Tanzania) who received the 56-day interval second dose vaccination depending on time point, binding antibodies were detected. A potential explanation may be previous asymptomatic infection after exposure to the virus, particularly in Uganda, where Ebola virus outbreaks have previously occurred, or exposure to unknown but closely related virus strains circulating in these settings. There have been approximately 25 outbreaks of Ebola virus infection in Africa alone since 1976, in addition to transmission of the virus to countries of nonendemicity [5], and this demonstrates the need for continued development of vaccines against Ebola, even after the end of the last epidemic.
Although the relatively small number of participants might be considered to be a limitation of our study, the similarity between the findings of our study and those of Mutua et al [15], conducted independently in different countries of East Africa, strengthen the conclusions that can be made. Strengths of the study include the exploration of multiple vaccination regimens and the 12-month follow-up period, which enabled the durability of immune responses to be assessed.
In conclusion, this study has demonstrated that heterologous Ad26.ZEBOV and MVA-BN-Filo 2-dose vaccination against Ebola is well tolerated and immunogenic in healthy African adult volunteers, regardless of whether the dosing interval is 28 or 56 days. Ad26.ZEBOV dose 1 vaccination induced more-robust initial antibody and T-cell responses than MVA-BN-Filo dose 1 vaccination, and immune responses were shown to persist for at least 360 days. The results of later-phase trials of Ad26.ZEBOV and MVA-BN-Filo 2-dose vaccination will be reported elsewhere.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank our partners in the EBOVAC1 Study Program (which is part of the Innovative Medicines Initiative Ebola+ Program), the London School of Hygiene and Tropical Medicine, the French National Institute for Health and Medical Research (INSERM), and the University of Oxford, for their important contributions to the clinical development of these vaccines; the ethical bodies and regulatory authorities of the participating countries, for their review and approval of the study; all of the volunteers who took part in the study; the community advisory boards of the participating sites and the research staff who worked on the trial; all members of the clinical and laboratory teams of the MRC/UVRI and LSHTM Uganda Research Unit in Entebbe, the Mwanza Intervention Trials Unit, and the National Institute for Medical Research in Mwanza, for their work on the study; and all members of the Clinical Operations Group at Janssen who contributed to the successful completion of the trial. Medical writing support was provided by Kaedy Bryson and Morgan McKenzie of Zoetic Science, an Ashfield Company. All authors had full access to the data. The corresponding author had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Innovative Medicines Initiative 2 Joint Undertaking (grants 115854 [to the EBOVAC 1 trial], 115861 [to the EBOVAC 2 trial], 115850 [to the EBOMAN Project via the Janssen Ebola Vaccine Program], and 115847 [to the EBODAC Project via the Janssen Ebola Vaccine Program]), Janssen Vaccines and Prevention, the European Union’s Horizon 2020 Research and Innovation Programme (to the Innovative Medicines Initiative 2 Joint Undertaking), the European Federation of Pharmaceutical Industries and Association (to the Innovative Medicines Initiative 2 Joint Undertaking), and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract HHSN272200800056C to the Janssen Filovirus Project).
Potential conflicts of interest. The study sponsor was involved in the design and conduct of the trial, and in the collection and analysis of data. V. B., G. S., D. A., K. L., C. R., and M. D. are all full-time employees of Janssen Pharmaceuticals or its affiliates. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ngatu NR, Kayembe NJ, Phillips EK, et al. Epidemiology of ebolavirus disease (EVD) and occupational EVD in health care workers in Sub-Saharan Africa: Need for strengthened public health preparedness. J Epidemiol 2017; 27:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nyakarahuka L, Kankya C, Krontveit R, et al. How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence. BMC Infect Dis 2016; 16:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC). 2014 Ebola outbreak in West Africa - case counts 2016. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed April 2018.
- 4. Holmes EC, Dudas G, Rambaut A, Andersen KG. The evolution of Ebola virus: Insights from the 2013-2016 epidemic. Nature 2016; 538:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC). Outbreaks chronology: Ebola virus disease 2015. https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html. Accessed April 2018.
- 6. Centers for Disease Control and Prevention (CDC). Ebola outbreaks 2000–2017 2017. https://www.cdc.gov/vhf/ebola/outbreaks/history/summaries.html Accessed April 2018.
- 7. Oyok T, Odonga C, Mulwani E, et al. Outbreak of Ebola hemorrhagic fever: Uganda, August 2000 – January 2001. MMWR Weekly 2001; 50:73–7. [PubMed] [Google Scholar]
- 8. World Health Organization (WHO). Ebola virus disease – Democratic Republic of the Congo 2017 http://www.who.int/csr/don/13-may-2017-ebola-drc/en/. Accessed April 2018.
- 9. World Health Organization (WHO). Ebola virus disease Democratic Republic of Congo: external situation report 1 2018. http://apps.who.int/iris/bitstream/handle/10665/272509/SITREP-EVD-DRC-20180511.pdf?ua=1. Accessed July 2018.
- 10. World Health Organization (WHO). Ebola virus disease - Ebola situation reports: Democratic Republic of the Congo 2018. http://www.who.int/ebola/situation-reports/drc-2018/en/. Accessed August 2018.
- 11. Lambe T, Bowyer G, Ewer KJ. A review of Phase 1 trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Philos Trans R Soc Lond B Biol Sci 2017; 372:20160295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Milligan ID, Gibani MM, Sewell R, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA 2016; 315:1610–23. [DOI] [PubMed] [Google Scholar]
- 13. Shukarev G, Callendret B, Luhn K, Douoguih M; EBOVAC1 consortium A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 2017; 13:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO Intelligence Blueprint. MVA platform partnership http://www.who.int/blueprint/what/research-development/MVA-presentation.pdf. Accessed March 2019.
- 15. Mutua G, Anzala O, Luhn K, et al. Randomized clinical trial examining safety and immunogenicity of heterologous prime-boost Ebola vaccines, Ad26.ZEBOV and MVA-BN-Filo: 12-month results in Kenyan healthy volunteers. J Infect Dis 2018. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Division of Microbiology and Infectious Diseases (DMID). Adult toxicity table (draft) 2007. https://www.niaid.nih.gov/sites/default/files/dmidadulttox.pdf. Accessed April 2018.
- 17. Guidance for Industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf. Accessed March 2019. [DOI] [PubMed]
- 18. Neff J, Modlin J, Birkhead GS, et al. ; Advisory Committee on Immunization Practices; Armed Forces Epidemiological Board Monitoring the safety of a smallpox vaccination program in the United States: report of the joint Smallpox Vaccine Safety Working Group of the advisory committee on immunization practices and the Armed Forces Epidemiological Board. Clin Infect Dis 2008; 46 Suppl 3:S258–70. [DOI] [PubMed] [Google Scholar]
- 19. Frahm N, DeCamp AC, Friedrich DP, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest 2012; 122:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winslow RL, Milligan ID, Voysey M, et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus ankara-vectored Ebola vaccines at 1 year. JAMA 2017; 317:1075–7. [DOI] [PubMed] [Google Scholar]
- 21. Wong G, Richardson JS, Pillet S, et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci Transl Med 2012; 4:158ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ 1983; 61:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization (WHO). World Malaria report. United Republic of Tanzania 2010. http://www.who.int/malaria/publications/country-profiles/profile_tza_en.pdf?ua=1 Accessed April 2018.
- 24. World Health Organization (WHO). Malaria publications. Country profiles http://www.who.int/malaria/publications/country-profiles. Accessed April 2018.
- 25. Mudhune SA, Okiro EA, Noor AM, et al. The clinical burden of malaria in Nairobi: a historical review and contemporary audit. Malar J 2011; 10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kennedy SB, Bolay F, Kieh M, et al. ; PREVAIL I Study Group Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization (WHO). Ebola virus disease – Democratic Republic of the Congo: update on ring vaccination 2018. http://www.who.int/csr/don/21-may-2018-ebola-drc/en/. Accessed July 2018.
- 29. World Health Organization (WHO). Report.Ebola vaccination begins in North Kivu https://reliefweb.int/report/democratic-republic-congo/ebola-vaccination-begins-north-kivu Accessed August 2018.
- 30. Regules JA, Beigel JH, Paolino KM, et al. ; rVSVΔG-ZEBOV-GP Study Group A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tapia MD, Sow SO, Lyke KE, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2016; 16:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.