Abstract
Hypertensive emergency, which occurs even in young adults, induces systemic organ damage and results in a poor prognosis. We herein report the case of a 27-year-old man who developed alveolar hemorrhaging with hypertensive emergency. He presented with bloody sputum, renal failure, and extremely high blood pressure (200/128 mmHg). Chest computed tomography revealed diffuse bilateral alveolar infiltrates suggestive of diffuse alveolar hemorrhaging. After intensive therapy with anti-hypertensive drugs, the alveolar hemorrhaging disappeared. Renal impairment was partially reversed. Therefore, we conclude that hypertensive emergency should be considered as a possible cause of hemoptysis, even in young adults.
Keywords: diffuse alveolar hemorrhaging, hypertensive emergency, heart failure, renal failure
Introduction
Hypertensive emergency induces acute and chronic targeted organ damage to the brain, heart, kidney, large blood vessels due to high blood pressure (1, 2). Generally, diffuse alveolar hemorrhaging (DAH) is uncommon in patients with hypertensive emergency (3, 4). Therefore, if a patient who presents with DAH develops renal failure, it might be difficult to rule out antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis or Goodpasture syndrome at presentation.
We herein report the case of a 27-year-old man who presented with hypertensive emergency, DAH, heart failure, and renal failure that was successfully treated with intensive and antihypertensive therapy including eplerenone, a mineralocorticoid receptor (MR) antagonist.
Case Report
A 27-year-old man was indicated to have hypertension at a medical checkup a few years ago, but he had never had a detailed examination nor taken any antihypertensive agents. His family history was not significant. He had a 5-year history of smoking 15 cigarettes per day (Brinkman Index=75), and he had continued to smoke until recently. He was obese with a weight of 80 kg and height of 169 cm, but he lost the weight and dropped from 80 kg to 65 kg due to a cough for 3 months. His cough was not resolved despite the continued administration of antitussive drugs. He visited our hospital, because he had bloody sputum and suffered from general fatigue.
His blood pressure was extremely high (200/128 mmHg) without a left-right difference. His percutaneous oxygen saturation (SpO2) level was 92% on room air. Coarse crackles were heard throughout the bilateral lung fields. A urine analysis showed proteinuria of 1 g/gCr. No urinary occult blood or urinary casts were observed. Renal dysfunction was noted, with a blood urea nitrogen level of 46.7 mg/dL and a serum creatinine level of 4.4 mg/dL. The levels of sodium, potassium, and chloride were 136 mEq/L, 2.8 mEq/L, and 95 mEq/L, respectively. The white blood cell count was 10,520 cells/μL, and the hemoglobin level was 10.0 g/dL. The platelet count was 15.3×104 cells/μL. Blood chemistry tests revealed elevated levels of lactate dehydrogenase (599 IU/L), alkaline phosphatase (181 IU/L), and C-reactive protein (14.8 mg/dL). The serum level of N-terminal-pro B-type natriuretic peptide (NT-proBNP) was 32,242 pg/mL. The plasma renin activity (PRA) was 28 ng/mL/h (normal: 0.3-2.9 ng/mL/hr) and plasma aldosterone concentration (PAC) was 447 pg/mL (normal: 29.9-159 pg/mL). The plasma levels of cortisol and catecholamines were within the normal range. The levels of thyroid-stimulating hormone and free thyroxine were also within the normal range.
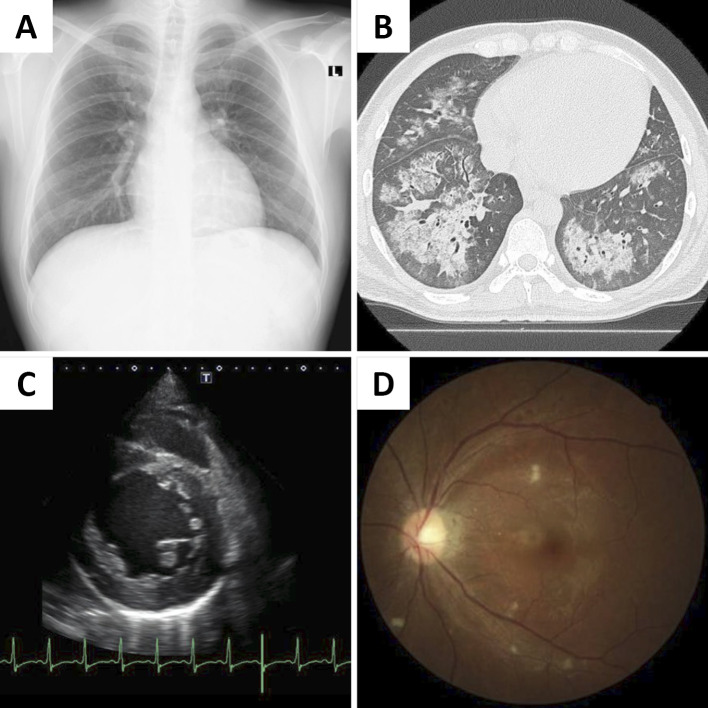
Chest radiography revealed frosted glass shadows and invasion shadows in the bilateral lung fields (Fig. 1A). An electrocardiogram showed sinus rhythm at a rate of 100 beats/min. An echocardiogram revealed a decrease in left ventricular function, an ejection fraction of 26%, and concentric hypertrophy of the left ventricle (thickness of the interventricular septum: 12 mm, thickness of the posterior wall: 12 mm; Fig. 1B). Chest computed tomography (CT) showed diffuse bilateral alveolar infiltrates suggestive of DAH (Fig. 1C). Renovascular ultrasonography showed no stenosis and a normal blood flow pattern in both renal arteries (peak systolic velocity was 71 cm/sec and 81 cm/sec; systolic renal-to-aortic ratio was 1.18 and 1.34, right and left renal artery, respectively). Therefore, we considered hypertensive emergency, Goodpasture syndrome, and ANCA-associated vasculitis as conceivable diseases at that time.
Figure 1.
Chest X-ray and computed tomography, echocardiography, and fundus images obtained on admission. (A) Chest X-ray showing frosted glass shadows and invasion shadows in the bilateral lung fields. (B) Chest computed tomography showing bilateral alveolar hemorrhaging. (C) Echocardiography showing left ventricular dilation and concentric hypertrophy. (D) Fundus image showing hypertensive retinopathy (Scheie classification H3S3, Keith-Wagner classification III).
He was admitted to the intensive-care unit (ICU). A bronchoscopy was not performed, because the patient did not consent to the procedure. We started plasma exchange with hemodialysis and intravenous pulse therapy because it was difficult to rule out Goodpasture syndrome and ANCA-associated vasculitis. No intracranial lesions or masses in the adrenal glands were observed on CT. An abdominal ultrasound examination showed bilateral renal atrophy without renal vascular stenosis. On the second day after hospital admission, his symptoms improved. The plasma exchange was stopped after only one procedure because the anti-neutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody were not detectable. After the diffuse bilateral alveolar infiltrates improved, the steroid was promptly reduced and discontinued. A fundus examination confirmed hypertensive retinopathy (Scheie classification H3S3, Keith-Wagener classification III) (Fig. 1D). Thus, the patient was diagnosed with hypertensive emergency with heart and renal failure and DAH.
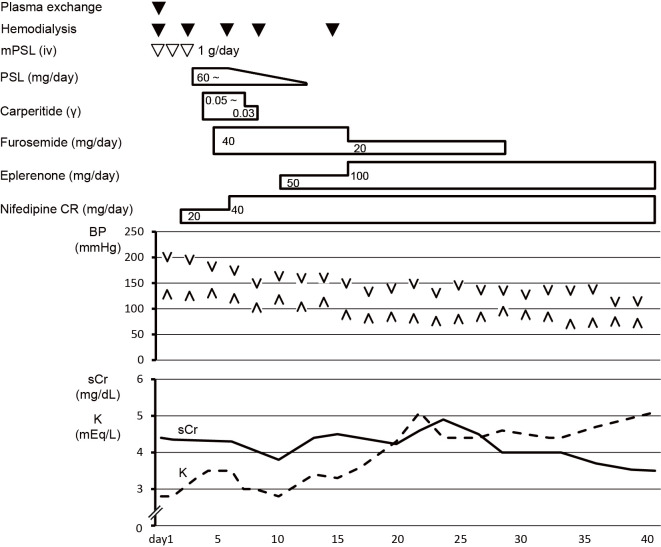
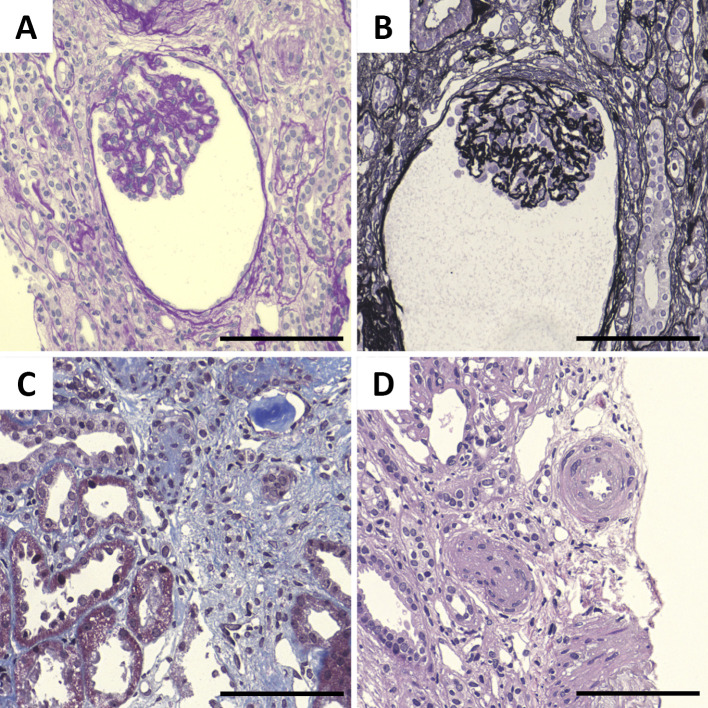
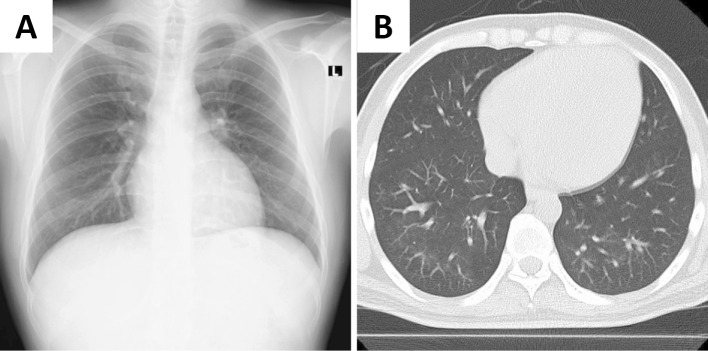
To control the blood pressure and excessive body fluid volume, oral nifedipine CR, intravenous furosemide, and carperitide treatments were started immediately (Fig. 2). After the treatment, the urine output was maintained at 1,000-2,000 mL/day. His renal function improved, and hemodialysis was discontinued after the fifth treatment. The intravenous furosemide and carperitide treatments were tapered off, and oral eplerenone was started. Hypertension was subsequently controlled at 130-140/80-90 mmHg; therefore, a renal biopsy was performed on day 28 (Fig. 2). Renal pathology results showed glomerular prostration, basement membrane wrinkling, tubular atrophy, interstitial fibrosis, and arteriole intimal hyperplasia (Fig. 3); therefore, the patient was diagnosed with hypertensive nephropathy. After hypertension control, the left ventricular dysfunction was improved with an ejection fraction of 39%. DAH completely disappeared (Fig. 4). His blood pressure was finally maintained at around 130/80 mmHg with a combination therapy with 40 mg/day of oral nifedipine and 100 mg/day of eplerenone, and he was discharged on day 43 (Fig. 2).
Figure 2.
Clinical course after admission. Oral nifedipine CR 20 mg/day, intravenous furosemide 40 mg/day, and intravenous carperitide 0.05 γ were initiated. The dose of oral nifedipine CR was increased to 40 mg/day, and oral eplerenone 50 mg/day was started on day 10. His renal function improved, and hemodialysis was discontinued after the fifth treatment. The patient’s blood pressure was finally maintained at approximately 130/80 mmHg by combination therapy with 40 mg/day of oral nifedipine and 100 mg/day of eplerenone, and he was discharged on day 43.
Figure 3.
Histopathology of the kidney. (A) Periodic acid-Schiff (PAS) staining showing glomerular prostration. (B) Periodic acid-methenamine-silver (PAM) staining showing basement membrane wrinkling. (C) Masson’s trichrome (MT) staining showing tubular atrophy. (D) Hematoxylin and Eosin staining showing arteriole intimal hyperplasia. Scale bars: 100 μm
Figure 4.
Alveolar hemorrhaging had completely disappeared on chest computed tomography images.
Discussion
We herein report a 27-year-old man who developed DAH with hypertensive emergency, which is a rare but important cause of hemoptysis. DAH with hypertensive emergency can successfully be treated with steroid pulse therapy and multiple antihypertensive drugs, including eplerenone.
DAH represents a medical emergency, and clinicians must adopt a rapid approach to its identification (5). There are many causes of DAH, including Goodpasture syndrome, ANCA-associated vasculitis, and idiopathic conditions (6, 7). In our case, it was difficult to rule out Goodpasture syndrome and ANCA-associated vasculitis. We therefore selected plasma exchange with hemodialysis and intravenous pulse therapy, as corticosteroids are used for patients with DAH (5). Of note, plasma exchange was recently reported to also be effective for severe vasculitis with DAH (6). Secondary hypertension, such as renovascular hypertension, Cushing's syndrome, pheochromocytoma, and primary aldosteronism, was not suggested by endocrinological studies or the findings of renovascular ultrasonography and abdominal computed tomography.
Table shows the previously published cases of DAH associated with hypertensive emergency (8-12), including a case where the patient required artificial respiration to treat his deteriorating respiratory function (9). All of these patients were men. Concomitant complications of other organ damage, such as renal failure, heart failure and retinopathy, were observed. Half of them were smokers, suggesting that nicotine-induced endothelial injury may be associated with the induction of DAH. In case 5 in Table, a trans-bronchial lung biopsy was performed and showed macrophage infiltration into alveolar spaces and goblet cell metaplasia in bronchial mucosa, findings that are generally seen in heavy smokers (12). Although the mechanism by which hypertensive emergency can cause DAH remains unclear, several mechanisms can be considered. First, humoral factors, such as renin, aldosterone, vasopressin, catecholamines, and endothelin might be involved in the alveolar capillary injury (9). Second, alveolar edema as a result of left ventricular dysfunction may also cause DAH, as previously reported (10). Third, the bronchopulmonary arteries might have suffered directly from severe systemic hypertension in some patients, as the bronchopulmonary arteries derive their blood supply from the systemic and pulmonary circulations or alveolar septal vessels (13).
Table.
Previous Published Cases of Diffuse Alveolar Hemorrhage with Hypertension Emergency.
| Case | Ref | Age | Sex | Time after diagnosis | Background | Complication | Blood pressure (mmHg) | Serum creatinine (mg/dL) | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | [8] | 34 | M | Unknown | Alveolar hemorrhage | 220/135 | 4.9 | Calcium channel blocker ACEI Hemodialysis Prednisolone Cyclophosphamide |
|
| 2 | [9] | 26 | M | 3 years | Adjacent travel by airplane | Alveolar hemorrhage | 210/150 | 2.2 | Intubation+Artificial respirator Calcium channel blocker β-blocker (Type unknown) ARB (Type unknown) |
| 3 | [10] | 38 | M | 3 months | Smoker Cocaine use |
Alveolar hemorrhage Retinopathy Nasal bleeding Left intension infarction |
220/120 | 3.16 | β-blocker (Atenolol) Calcium channel blocker (Amlodipine) ACEI (Enalapril) ARB (Candesartan) α-blocker (Doxazosin) |
| 4 | [11] | 32 | M | 5 years | Alveolar hemorrhage Retinopathy Heart failure |
290/150 | 8.24 | Calcium channel blocker (Amlodipine) β-blocker (Bisoprolol) |
|
| 5 | [12] | 27 | M | Unknown | Smoker | Alveolar hemorrhage Retinopathy |
180/100 | 5.15 | Pulse steroid therapy Antihypertensive drugs (Type unknown) |
| 6 | Our case | 27 | M | 2 years | Smoker | Alveolar hemorrhage Retinopathy Heart failure |
200/128 | 4.35 | Hemodialysis and plasma exchange Steroid pulse therapy Human atrial natriuretic peptide (Carperitide) Furosemide Calcium channel blocker (Nifedipine CR) MR antagonist (Eplerenone) |
ACEI: Angiotensin converting enzyme inhibitor, ARB: Angiotensin II receptor blocker
Even young adults develop hypertensive emergency with progressive organ damage. Therefore, the possibility of hypertensive emergency should be kept in mind when young adults present with nonspecific symptoms such as general fatigue and chronic cough with DAH. When severe hypertension is observed occasionally in young adults, a further detailed examination should be conducted. In patients with DAH and renal involvement, a kidney biopsy should be considered to identify the underlying cause and help direct therapy. Indeed, it was through the kidney biopsy that we confirmed hypertensive nephropathy in the present case. Endothelial cell injury is thought to be related to kidney injury, via an increase in apoptosis and the detachment of non-viable podocytes, as angiotensin II production has been reported to increase in adjunct mesangial cells (14). Although such kidney injury occasionally requires dialysis, as in our case, the reduction in the kidney function may be reversed by long-term blood pressure control. Furthermore, eplerenone may be administered as an antihypertensive drug in combination therapy for patients with malignant-phase hypertension and activation of the renin-angiotensin-aldosterone system, as we reported previously (4).
In conclusion, we should consider hypertensive emergency as a possible cause of DAH, even in young adults. Further studies are required to elucidate how we should best manage and treat such patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Shantsila A, Lip GYH. Malignant hypertension revisited-does this still exist? Am J Hypertens 30: 543-549, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Polgreen LA, Suneja M, Tang F, Carter BL, Polgreen PM. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension 65: 1002-1007, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Mitaka H, Yamada Y, Hamada O, Kosaka S, Fujiwara N, Miyakawa Y. Malignant hypertension with thrombotic microangiopathy. Intern Med 55: 2277-2280, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi F, Goto M, Wada Y, Hasebe N. Successful treatment with an antihypertensive drug regimen including eplerenone in a patient with malignant phase hypertension with renal failure. Intern Med 54: 2467-2470, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Ioachimescu OC, Stoller JK. Diffuse alveolar hemorrhage: diagnosing it and finding the cause. Cleve Clin J Med 75: 258, 260,, 264-255 passim, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Goto K, Nakai K, Fujii H, Nishi S. The effects of plasma exchange on severe vasculitis with diffuse alveolar hemorrhage. Intern Med 56: 55-59, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakaguchi Y, Uehata T, Kawabata H, et al. An autopsy-proven case of myeloperoxidase-antineutrophil cytoplasmic antibody-positive polyarteritis nodosa with acute renal failure and alveolar hemorrhage. Clin Exp Nephrol 15: 281-284, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Hida K, Wada J, Odawara M, et al. Malignant hypertension with a rare complication of pulmonary alveolar hemorrhage. Am J Nephrol 20: 64-67, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Hara S, Yamada K, Fujimoto S. A rare case of alveolar haemorrhage due to malignant hypertension. Nephrol Dial Transplant 20: 2289-2290, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Aithal S, Marley N, Venkat-Raman G. An unusual non-immunological cause of renal pulmonary syndrome. Clin Nephrol 72: 322-325, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Nanba K, Yahata K, Kikuchi Y, Okamoto C, Seta K, Sugawara A. A rare case of malignant-phase hypertension with pulmonary alveolar hemorrhage. Clin Exp Nephrol 15: 303-307, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Park HS, Hong YA, Chung BH, et al. Malignant hypertension with an unusual presentation mimicking the immune mediated pulmonary renal syndrome. Yonsei Med J 53: 1224-1227, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita Y, Takase K, Ichikawa H, et al. Bronchial artery anatomy: preoperative 3D simulation with multidetector CT. Radiology 255: 934-943, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Abu Hamad R, Berman S, Hachmo Y, et al. Response of renal podocytes to excessive hydrostatic pressure: a pathophysiologic cascade in a malignant hypertension model. Kidney Blood Press Res 42: 1104-1118, 2017. [DOI] [PubMed] [Google Scholar]