Abstract
Although numerous recent studies have reported the development of sarcoidosis in patients treated with tumor necrosis factor alpha (TNF-α) inhibitors, it is unclear whether the pathogenesis of drug-induced sarcoidosis is identical to that of spontaneous sarcoidosis. We herein present the case of a patient who developed sarcoidosis 6 months after the introduction of etanercept as treatment for rheumatoid arthritis. Typical clinical symptoms with noncaseating epithelioid granulomas detected in a mediastinal lymph node specimen were consistent with the diagnosis of sarcoidosis. Immunohistochemistry revealed Propionibacterium acnes in the noncaseating granulomas. The present findings suggest that Propionibacterium acnes is a cause of sarcoidosis, even when the disease is induced by TNF-α inhibitors.
Keywords: sarcoidosis, etanercept, TNF-α, Propionibacterium acnes
Introduction
Sarcoidosis is a systemic inflammatory disorder characterized by noncaseating granuloma and commonly manifests in the lungs, eyes, skin, and heart (1). Previous reports indicate that tumor necrosis factor alpha (TNF-α) is critical in the formation of granulomas and is therefore a potential therapeutic target for the treatment of granulomatous diseases. However, some patients with chronic inflammatory disorders such as rheumatoid arthritis (RA) unexpectedly develop sarcoidosis after treatment with TNF-α inhibitors (2, 3). Numerous recent studies have reported cases of sarcoidosis associated with drugs such as TNF-α inhibitors and immune checkpoint inhibitors (4, 5). These cases have been referred to by some researchers as drug-induced sarcoidosis-like reactions (DISRs) (6). Chopra et al. reported that the clinical manifestations of sarcoidosis and DISRs are similar; however, unlike sarcoidosis, DISRs often resolve when the offending drug is discontinued and recur with re-challenge (6). Although the clinical characteristics of these conditions differ to some extent, it is unclear whether the pathogeneses of DISRs and sarcoidosis are distinct.
Propionibacterium acnes (P. acnes) has been extensively studied as a causative microorganism in sarcoidosis (7-11). Interestingly, Negi et al. reported that immunohistochemistry using a specific monoclonal antibody of P. acnes can discriminate sarcoid and non-sarcoid granulomas, including the sarcoid reaction of malignant tumors (11).
We herein report a case in which sarcoidosis developed during etanercept treatment. Immunohistochemistry revealed P. acnes in the patient's noncaseating granulomas.
Case Report
A 54-year-old woman was admitted to another hospital in August X for pain in multiple joints. RA was diagnosed and etanercept treatment was started. Congestion of the bulbar conjunctiva developed 6 months after the start of etanercept treatment, and iritis was diagnosed by an ophthalmologist. Bilateral hilar lymphadenopathy was noted in a health exam in December X+1, after which she was referred to our institution for further examination.
A physical examination revealed mild congestion in both eyes but no fever, skin lesions, or abnormal sounds on chest auscultation. A purified protein derivative skin test yielded a negative result. The laboratory findings are summarized in Table 1. The angiotensin-converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R) levels were elevated. Pulmonary function testing revealed a normal respiratory function, and the arterial partial pressure of oxygen (PaO2) was 99.5 mmHg in a blood gas analysis. No abnormal findings were observed on electrocardiography or echocardiography.
Table 1.
Laboratory Data on Admission.
| Blood chemistry | Immunological variables | |||||
| Total protein | 7.2 | g/dL | T-SPOT. TB | Negative | ||
| Albumin | 3.6 | g/dL | Anti-MAC antibody | Negative | ||
| Aspartate aminotransferase | 30 | U/L | Angiotensin-converting enzyme | 28.0 | U/L | |
| Lactate dehydrogenase | 217 | U/L | Soluble IL-2 receptor | 1,630 | U/mL | |
| Urea nitrogen | 14 | mg/dL | Tumor necrosis factor-α | 3 | pg/mL | |
| Creatinine | 0.6 | mg/dL | ||||
| Calcium | 8.7 | mg/dL | Arterial blood gas analysis | |||
| Phosphorus | 4.1 | mg/dL | pH | 7.41 | ||
| C-reactive protein | 11.1 | mg/dL | PaCO2 | 39.3 | mm Hg | |
| Krebs von den Lungen-6 | 269 | U/mL | PaO2 | 99.5 | mm Hg | |
| Brain natriuretic peptide | 30.4 | pg/mL | ||||
| Procalcitonin | 0.09 | ng/mL | ||||
| Complete blood count | ||||||
| White blood cell count | 5,300 | /μL | ||||
| Red blood cell count | 446×104 | /μL | ||||
| Hemoglobin | 13.3 | g/dL | ||||
| Platelet count | 26.7×104 | /μL | ||||
PaCO2: partial pressure of arterial carbon dioxide, PaO2: partial pressure of arterial oxygen
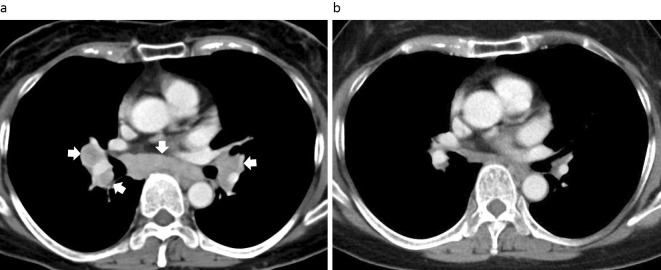
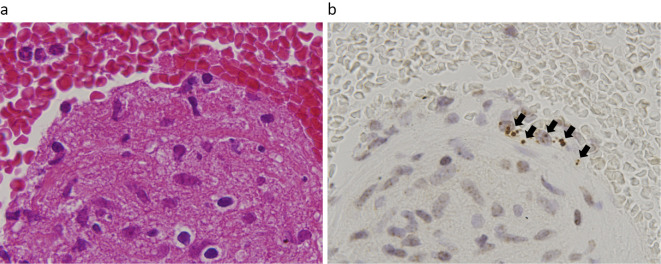
A chest radiograph showed bilateral hilar lymphadenopathy, and a contrast-enhanced chest computed tomography (CT) image showed mediastinal and hilar lymphadenopathy (Fig. 1a) without abnormal shadows in the lung fields. Positron emission tomography showed significant increases in the fluoro-2-deoxy-D-glucose uptake in the mediastinal and hilar lymph nodes. Bronchoscopy was performed to confirm the diagnosis. A bronchoalveolar lavage fluid analysis showed an elevated CD4/8 ratio (6.73) and high lymphocyte fraction (macrophages, 63%; lymphocytes, 31%; neutrophils, 5%; and eosinophils, 1%). A tissue specimen was obtained from a mediastinal lymph node (#7) by endobronchial ultrasonography-guided transbronchial needle aspiration (EBUS-TBNA), and a histopathologic analysis showed formation of a loose noncaseating epithelioid granulomas (Fig. 2a), without evidence of mycobacterium tuberculosis infection, as indicated by Ziehl-Neelsen staining. Immunohistochemistry using a specific monoclonal antibody against P. acnes lipoteichoic acid (PAB antibody) (11) revealed P. acnes in several granulomas in the tissue (Fig. 2b). These findings indicated a diagnosis of sarcoidosis, and etanercept treatment was implicated as the cause of the disease.
Figure 1.
Chest computed tomography (CT) images. (a) A chest contrast-enhanced CT image obtained on admission. The white arrows indicate mediastinal and hilar lymphadenopathy. (b) At 1 year after the discontinuation of etanercept, the size of the lymph nodes had decreased.
Figure 2.
Endobronchial ultrasound-guided transbronchial needle aspiration was used to collect a lymph node specimen. (a) Hematoxylin and Eosin staining shows a noncaseating epithelioid cell granuloma. (b) Immunohistochemical staining with a specific monoclonal antibody against Propionibacterium acnes lipoteichoic acid (PAB antibody) shows positive reaction products (black arrows) in the granuloma.
Etanercept treatment was stopped and a follow-up examination was performed. The serum levels of ACE and sIL-2R gradually decreased without treatment after the discontinuation of etanercept (Fig. 3). A chest CT image showed that the mediastinal and hilar lymph nodes were smaller after 1 year (Fig. 1b), which indicated improvement without therapeutic intervention.
Figure 3.
Changes in the serum biomarkers of sarcoidosis after discontinuation of etanercept. The angiotensin-converting enzyme (ACE) and soluble IL-2 receptor (sIL-2R) levels decreased after the cessation of etanercept discontinued.
Discussion
Sarcoidosis is diagnosed based on compatible clinical features, radiological findings, and histological evidence of noncaseating epithelioid granulomas (1). Our patient developed iritis followed by bilateral hilar lymphadenopathy. In addition, biomarkers, including ACE and sIL-2R, were markedly elevated at the disease onset, which are findings consistent with sarcoidosis. EBUS-TBNA confirmed the presence of noncaseating epithelioid cell granulomas in the mediastinal lymph nodes. The patient developed sarcoidosis during etanercept treatment and improved without therapeutic intervention after the cessation of etanercept. Thus, the final diagnosis was sarcoidosis induced by etanercept.
Previous studies found that TNF-α was important in granuloma formation in animal models of helminth infection (12-14) and in humans (15, 16). TNF-α is a key cytokine in the pathogenesis of sarcoidosis and a potential target of sarcoidosis treatment. In fact, based on small clinical trials, it has been reported that TNF-α inhibitors other than etanercept might be effective for sarcoidosis treatment (17-19). Interestingly, numerous recent studies have reported the development of sarcoidosis after TNF-α inhibitor treatment for systemic diseases. A literature search identified 63 cases of sarcoidosis due to TNF-α blocker treatment (Table 2). Most cases were caused by etanercept (68%), as occurred in our patient. Infliximab and adalimumab are monoclonal antibodies that recognize monomeric and trimeric soluble TNF and transmembrane TNF. In contrast, etanercept is the extracellular domain of the TNF receptor that is linked to the Fc and which only recognizes soluble trimeric TNF. Although the higher incidence of sarcoidosis due to etanercept treatment may be attributable to these different mechanisms of TNF-α inhibition, the pathogenesis of this condition has not been thoroughly studied.
Table 2.
Summary of Reported Cases of Sarcoidosis Development after Treatment with a TNF-α Inhibitor.
| No. of patients | TNF-α inhibitor E/I/A |
Time to onset (months) |
Underlying disease | Treatment | |
|---|---|---|---|---|---|
| Reported cases | 63 | 43/12/8 | 24±22 | RA 36, AS 11, PsA 10, JIA 4, SAPHO 1, Crohn 1 | Discontinuation 31 Corticosteroids 28 Not available 4 |
| Present case | 1 | Etanercept | 8 | RA | Discontinuation |
E: etanercept, I: infliximab, A: adalimumab, RA: rheumatoid arthritis, AS: ankylosing spondylitis, PsA: psoriatic arthritis, JIA: juvenile idiopathic arthritis, SAPHO: Sapho syndrome, Crohn: Crohn disease
Sarcoidosis is also induced by immune checkpoint inhibitors. Several recent studies reported that sarcoidosis or sarcoid-like granulomatosis developed in patients with malignant tumors treated by monoclonal antibodies against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed death 1 (PD-1) (4, 5). Braun et al. reported that the PD-1 pathway in CD4 T cells was upregulated in sarcoidosis (20). PD-1 is a marker of lymphocyte exhaustion; thus, chronic lymphocytic inflammation may be associated with the pathogenesis of sarcoidosis. Enhanced lymphocyte proliferation by blockade of co-inhibitory signalling by immune checkpoint inhibitors is thought to be related to disease development.
Sarcoidosis is believed to be caused by an immune response after exposure to environmental factors, including infectious agents, in genetically susceptible individuals. Accumulating evidence suggests that P. acnes is the causative organism of sarcoidosis (7-11), as it is the only microorganism that has been isolated from bacterial cultures of sarcoidosis granulomas (7). In addition, fragments of nucleic acids of P. acnes have been detected in sarcoid lymph nodes by quantitative polymerase chain reactions and in situ hybridization (8-10). In an immunohistochemical study using a PAB antibody, Negi et al. reported a high frequency and specificity of P. acnes in sarcoid granulomas (11). In their analysis, PAB antibodies did not react with non-sarcoid granulomas such as tuberculosis or in sarcoid reactions caused by malignant tumors. Furthermore, findings from case reports and small clinical trials suggest that antibiotics such as tetracyclines and macrolides are effective for treating sarcoidosis (21-23), perhaps because of their anti-inflammatory and anti-microbial effects against infectious microorganisms such as P. acnes.
In our patient, P. acnes was identified in noncaseating granulomas of sarcoidosis induced by TNF-α inhibitors. It is hypothesized that latent P. acnes infection in the lungs and lymph nodes is activated by environmental factors, which triggers an immune response in patients with P. acnes allergy (24). Thus, we hypothesize that among individuals with systemic immunological disorders and malignant tumors, those with latent P. acnes infection may show aberrant responses to drugs such as TNF-α inhibitors and immune checkpoint inhibitors and consequently develop drug-induced sarcoidosis. This hypothesis suggests that DISRs and sarcoidosis share a pathogenetic mechanism and differ only in the exogenous stimuli that trigger their development. The extrinsic factors that activate the host are distinct in DISRs and are unclear in some patients with sarcoidosis.
Conclusions
To the best of our knowledge, this is the first case in which P. acnes was detected in the granulomas of a patient with sarcoidosis induced by a TNF-α inhibitor. These findings suggest that P. acnes is a cause of sarcoidosis, even in cases induced by drugs such as TNF-α inhibitors.
Author's disclosure of potential Conflicts of Interest (COI).
Kazutoshi Shibuya: Research funding, Dainippon Sumitomo Pharma. Sakae Homma: Research funding, Nippon Boehringer Ingerheim.
Acknowledgement
The authors thank David Kipler for editing the language of the article.
References
- 1.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/Wrold Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 16: 149-173, 1999. [PubMed] [Google Scholar]
- 2.Sawahata M, Sugiyama Y, Yamasawa H, et al. Sarcoidosis during etanercept treatment for rheumatoid arthritis in women with a history of bilateral oophorectomy. Sarcoidosis Vasc Diffuse Lung Dis 33: 178-181, 2016. [PubMed] [Google Scholar]
- 3.Daien CL, Monnier A, Claudepierre P, et al. Sarcoid-like granulomatosis in patients with tumor necrosis factor blockers: 10 cases. Rheumatology 48: 883-886, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Danlos FX, Pages C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest 149: e133-e136, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Reuss JE, Kunk PR, Stowman AM, et al. Sarcoidosis in the settings of combination ipilimumab and nivolumab immunotherapy: a case report & review of the literature. J immunother Cancer 4: 94, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra A, Nautiyal A, Kalkanis A, et al. Drug-induced sarcoidosis-like reactions. Chest 154: 664-677, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Homma JY, Abe C, Chosa H, et al. Bacterial investigation on biopsy specimens from patients with sarcoidosis. Jpn J Exp Med 48: 251-255, 1978. [PubMed] [Google Scholar]
- 8.Ishige I, Usui Y, Takemura T, et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymphoid nodes of Japanese patients with sarcoidosis. Lancet 354: 120-123, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 40: 198-204, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol 198: 541-547, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Negi M, Takemura T, Guzman J, et al. Localization of Propionibacterium acnes in granulomas supports a possible etiologic link between sarcoidosis and the bacterium. Mod Pathol 25: 1284-1297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiri P, Locksley RM, Paraslow TG, et al. Tumor necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356: 604-607, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Joseph AL, Boros DL. Tumor necrosis fator plays a role in Schistosoma mansoni egg-induced granulomatous inflammation. J Immunol 151: 5461-5471, 1993. [PubMed] [Google Scholar]
- 14.Lukacs NW, Chensue SW, Strieter RM, et al. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol 152: 5883-5889, 1994. [PubMed] [Google Scholar]
- 15.Prior C, Knight RA, Herold M, et al. Pulmonary sarcoidosis: patterns in cytokines production. Eur Respir J 9: 47-53, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Ziegenhagen MW, Bender UK, Zissel G, et al. Sarcoidosis: TNF-α release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med 156: 1586-1592, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 174: 795-802, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kamphuis LS, Lam-Tse WK, Dik WA, et al. Efficacy of adalimumab in chronically active and symptomatic patients with sarcoidosis. Am J Respir Crit Care Med 184: 1214-1216, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Ultz JP, Limper AH, Kalra S, et al. Etanercept for the treatment of stage 2 and 3 progressive pulmonary sarcoidosis. Chest 124: 177-185, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Braun NA, Celada LJ, Herazo-Maya JD, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med 190: 560-571, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki E, Ando M, Fukami T, et al. Minocycline for the treatment of sarcoidosis: is the mechanism of action immunomodulating or antimicrobial effect? Clin Rheumatol 27: 1195-1197, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Takemori N, Nakamura M, Kojima M, et al. Successful treatment in a case of Propionibacterium acnes-associated sarcoidosis with clarithromycin administration: a case report. J Med Case Rept 8: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachelez H, Senet P, Cadranel J, et al. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol 137: 69-73, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Eishi Y. Etiologic aspect of sarcoidosis as an allergic endogenous infection caused by Propionibacterium acnes. Biomed Res Int (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]