Abstract
Lynch syndrome is caused by mutations in mismatch repair genes that lead to microsatellite instability (MSI). An increased number of mutation-associated neoantigens have been observed in patients with high-frequency MSI (MSI-H) cancer; in addition, membranous programmed death ligand-1 (PD-L1) tends to be expressed at higher levels in MSI-H cancers than in microsatellite-stable cancers. MSI-H cancer patients are therefore considered to be susceptible to immune checkpoint blockade. We herein report for the first time a case of lung adenocarcinoma with Lynch syndrome and the response to nivolumab.
Keywords: Lynch syndrome, MSI-H, nivolumab
Introduction
Lynch syndrome is an inherited disorder that increases the risk of many types of cancer. In this case report, we describe a patient with Lynch syndrome whose tumor proved susceptible to immune checkpoint blockade.
Case Report
A 68-year-old man with a 50-pack-year history of smoking had been followed since 1992 at our hospital. The patient's first cancer occurred in the ascending colon and was surgically resected in 1992. His second and third cancers occurred in the sigmoid colon and rectum, respectively, and were resected in 2012. The pathological diagnosis of both cancers was pStage I. His fourth cancer was squamous cell carcinoma in the lower lobe of the left lung (pT2bN0M0, pStage IIA); this tumor was resected in 2012. His fifth and sixth cancers occurred in the sigmoid colon and the rectum, respectively, and were endoscopically resected in 2013. The pathological diagnosis of both cancers was carcinoma in situ. His seventh cancer occurred in the prostate and was treated via retropubic radical prostatectomy in 2014 (pT3aN1M0, pStage IV).
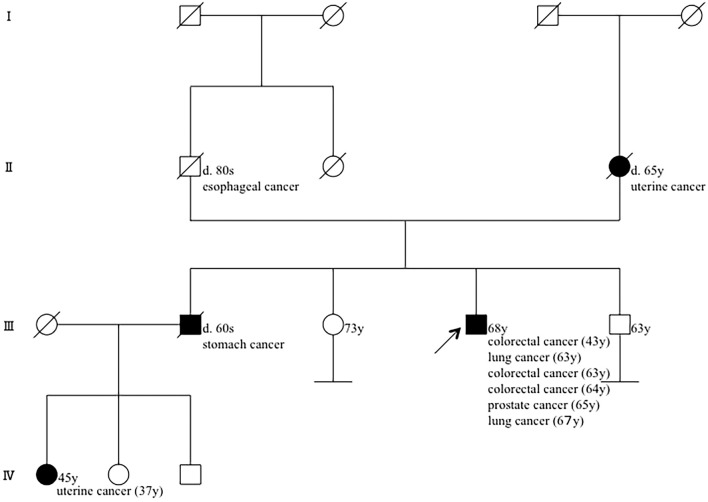
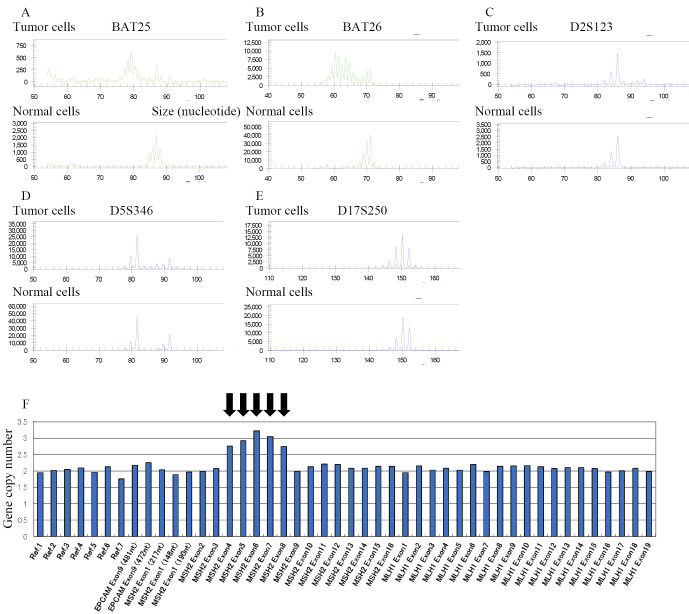
The patient's mother had had uterine cancer, and his brother had been diagnosed with stomach cancer at a young age (Fig. 1). Given this history, familial cancer syndrome was suspected. We provided counseling to him and his family according to the ethical guidelines of the Division of Clinical Genetic Oncology of our hospital; we then examined him for microsatellite instability (MSI). The loss of MSH2 and MSH6 was detected via immunohistochemistry (IHC) in the colon cancer resected in 2013, and two of five microsatellite markers (BAT25 and BAT26) showed replication error (Fig. 2A-E). Therefore, the patient's colon cancer was considered to be a high-frequency MSI (MSI-H) tumor. In addition, rearrangement in the MSH2 germline gene, which has previously been described in Lynch syndrome (1), was identified using a multiplex ligation-dependent probe amplification assay (Fig. 2F).
Figure 1.
Pedigree of the patient as of current. His mother had a uterine cancer, and his brother had a stomach cancer. His niece also had a uterine cancer at a young age.
Figure 2.
Results of microsatellite instability (MSI) analysis of colorectal cancer. MSI status was assessed by the results of electrophoresis of polymerase chain reaction of five markers [BAT25 (A), BAT26 (B), D2S123 (C), D5S346 (D), and D17S250 (E)]. Instability of two markers [BAT25 (A) and BAT26 (B)] was observed and the patient’s colorectal cancer was identified as MSI-H cancer. Multiplex ligation-dependent probe amplification assay revealed increased copy number of exons 4-8 of the MSH2 gene in germline cells (F).
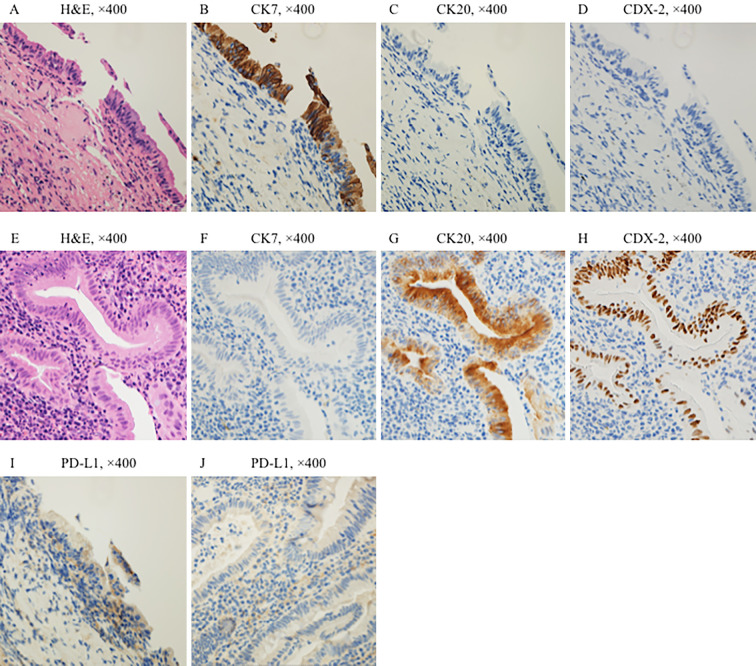
In April 2015, a consolidation shadow with a thick-walled cavity in the upper lobe of the left lung was identified on computed tomography. A transbronchial lung biopsy (TBLB) revealed organizing pneumonia. The shadow gradually expanded, and new lesions emerged in the right upper and lower lobes. Another TBLB performed in April 2016, revealed adenocarcinoma without epidermal growth factor receptor mutation or anaplastic lymphoma kinase rearrangements in the left upper lobe. We used IHC to confirm that the tumor was a primary lung cancer and not a metastatic tumor from known colorectal cancers (Fig. 3A-H). Furthermore, the results of additional IHC staining indicated that programmed death ligand-1 (PD-L1) expression was observed in about 3% of the tumor cells of lung biopsy tissue (Fig. 3I), and PD-L1 was not expressed in colorectal tumor cells (Fig. 3J).
Figure 3.
Histological and immunohistochemical findings comparing lung tumor and colorectal tumor tissues. Hematoxylin and Eosin staining of the lung tumor (A) and colorectal tumor (E) are shown. Expression of cytokeratin (CK) 7, a marker of lung cancer, was observed in the tumor cells of lung biopsy tissue (B). The expression of CK 20 and caudal-type homeobox protein 2 (CDX-2), which are markers of colorectal cancer, was not observed in the tumor cells of lung biopsy tissue (C, D). In contrast, expression of CK 7 was not observed in the tumor cells of colorectal tissue (F). The expression of CK 20 and CDX-2 was observed in the tumor cells of colorectal tissue (G, H). Both lung cancer and colorectal cancer were considered as primary cancers because the IHC results were exclusive. PD-L1 expression was observed in the tumor cells of lung biopsy tissue (I). CK7 (clone OVTL12/30, DAKO), CK20 (clone IT-Ks20.8, POG), CDX-2 (clone DAK-CDX2, DAKO), PD-L1 (clone 28-8, abcam).
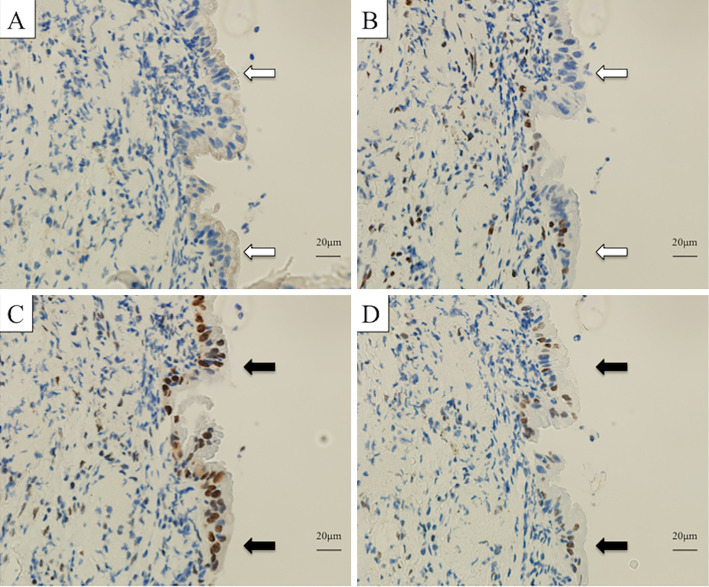
Examinations of MSI in lung biopsy tissue were performed via IHC and MSI analyses. IHC revealed the loss of MSH2 and MSH6 in the lung cancer (Fig. 4). However, MSI-H was not detected in the MSI analysis.
Figure 4.
A comparison of histology and immunohistochemistry (IHC) findings for lung tumor and colorectal tumor tissues. MSH2 (A) and MSH6 (B) were not expressed in the nuclei of tumor cells (white arrows), whereas MLH1 (C) and PMS2 (D) were expressed in tumor cell nuclei (black arrows). MSH2 (clone G219-1129, BD Biosciences), MSH6 (clone EPR3945, Gene Tex), MLH1 (clone ES05, Leica), PMS2 (clone A16-4, BD Pharmingen).
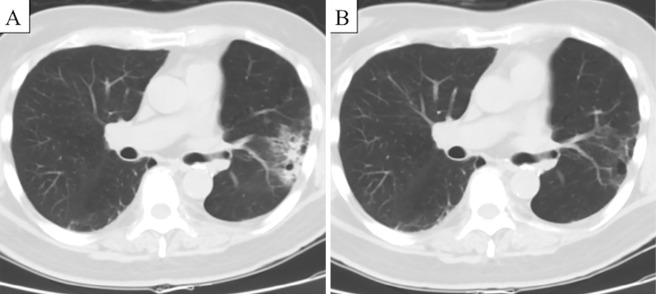
In summary, the patient was diagnosed with primary lung cancer of the left upper lobe, which was pathologically proven to be adenocarcinoma, as well as MSI with radiologically multiple pulmonary metastases of the right upper and lower lobes (cT3N0M1a, cStage IV). He began receiving cisplatin and pemetrexed therapy in June 2016 and was subsequently treated with maintenance pemetrexed. The lung tumors exhibited a partial response, but the bilateral lung tumors progressed after five months (Fig. 5A). The patient began receiving nivolumab (3 mg/kg intravenously every 2 weeks) in November 2016. The lung tumors dramatically decreased in size within 2 months. However, the patient discontinued nivolumab due to drug-induced interstitial lung disease and started treatment with corticosteroids in March 2017. Surprisingly, tumor shrinkage continues to be obtained without anti-cancer treatment as of September 2018, when we drafted this report (Fig. 5B).
Figure 5.
Computed tomography findings for the patient. Consolidation shadows with ground glass opacities were observed in the left upper and right lower lobes before nivolumab therapy (A). Tumor shadows were greatly reduced in size after 2 months of nivolumab therapy (B).
Discussion
Lynch syndrome, which is also known as hereditary non-polyposis colorectal cancer, is caused by mutations in mismatch repair genes, such as MSH2, MLH1, MSH6, PMS1, and PMS2. These mutations lead to MSI. Patients with Lynch syndrome are at a high risk of synchronously and metachronously developing colorectal, uterine, ovarian, stomach, small bowel, pancreatic, kidney, and brain cancers; however, the risk of lung cancer is considered the same as that of the general population (2). Over a 25-year period, the patient described here developed a total of 5 colorectal cancers; 2 lung cancers, including squamous cell carcinoma and adenocarcinoma; and 1 prostate cancer. The patient's condition satisfied the Amsterdam II criteria and Bethesda guidelines for Lynch syndrome in all respects (3). Because the loss of MSH2 and MSH6 was detected by IHC in the colon and lung cancers, we regarded both of these cancers as attributable to Lynch syndrome. MSI-H was observed in the colon cancer specimen but not in the lung cancer specimen, although there may have been insufficient tumor cells in the lung biopsy tissue for the detection of MSI-H via the MSI analysis.
Programmed death-1 (PD-1)/PD-L1 inhibitors, such as nivolumab and pembrolizumab, are dramatically changing the treatment of various cancers, including lung cancer. However, the response rate to these agents is not high (nearly 20% in non-selected lung cancer patients and 3% in non-selected colon cancer patients) (4). Thus, the identification of a predictive biomarker of response to PD-1 inhibitors is desirable.
The PD-L1 expression in lung cancer and mismatch-repair status in colon cancer may be correlated with the efficacy of PD-1 inhibitors. In a phase II study of pembrolizumab, seven patients with mismatch repair-deficient non-colorectal cancers were treated, and the overall response rate was 71% (1 patient with a complete response and 4 with a partial response) (4). However, lung cancer was not among the non-colorectal cancers examined in this study. There are no previous reports describing the anti-PD-1 antibody treatment of lung cancer in patients with Lynch syndrome. One possible reason for differences in the response to anti-PD-1 antibody is that an increased number of mutation-associated neoantigens have been observed in patients with MSI-H cancer compared with microsatellite-stable cancers; in addition, membranous PD-L1 tends to be expressed at higher levels in MSI-H cancers than in microsatellite-stable cancers (5). In the case described here, IHC staining of PD-L1 was not strongly observed in the patient's lung tumor cells. This suggests that other factors may influence the response to nivolumab aside from the PD-L1 expression.
The findings in the present case suggest that rare phenotypes of cancers such as lung adenocarcinoma in patients with Lynch syndrome may be susceptible to immune checkpoint blockade.
Written informed consent was obtained from the patient for publishing this case report and accompanying images.
Author's disclosure of potential Conflicts of Interest (COI).
Noriko Yanagitani: Advisory role, Chugai Pharmaceutical. Atsushi Horiike A: Honoraria, Pfizer, Chugai Pharmaceutical, Eli Lilly and AstraZeneca; Research funding, Chugai Pharmaceutical, Quintiles, MSD and Daiichi Sankyo. Makoto Nishio: Honoraria, Pfizer, Bristol-Myers Squibb, ONO Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, TAIHO Pharmaceutical and AstraZeneca; Research funding, Novartis, ONO Pharmaceutical, Chugai Pharmaceutical, Bristol-Myers Squibb, TAIHO Pharmaceutical, Eli Lilly, Pfizer, Astellas Pharma and AstraZeneca.
References
- 1.Nakagawa H, Hampel H, de la Chapelle A. Identification and characterization of genomic rearrangements of MSH2 and MLH1 in Lynch syndrome (HNPCC) by novel techniques. Hum Mutat 22: 258, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Win AK, Lindor NM, Young JP, et al. . Risks of primary extracolonic cancers following colorectal cancer in Lynch syndrome. J Nat Cancer Inst 104: 1363-1372, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasen HF, Moslein G, Alonso A, et al. . Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 44: 353-362, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, et al. . PD-1 blockade in tumors with mismatch-repair deficiency. N Eng J Med 372: 2509-2520, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev 106: 302-323, 2006. [DOI] [PubMed] [Google Scholar]