Abstract
Objective
Extracorporeal life support (ECLS) is effective for improving the survival rate of patients with refractory cardiac arrest (rCA). As little data are available regarding the impact of ECLS on a favorable neurological outcome, the predictors of a favorable neurological outcome were evaluated in this study.
Methods
Between January 2007 and August 2016, we retrospectively recruited patients with rCA caused by cardiac events treated with ECLS in our institute. A favorable neurological outcome was defined as a Glasgow-Pittsburgh cerebral performance category score 1 at discharge. The study endpoint was the clinical outcomes and predictors of favorable neurologic patients at discharge.
Results
During the study period, 67 patients with CA caused by cardiac events (acute coronary syndrome, 57 patients; idiopathic ventricular fibrillation, 10 patients) were included. Of these, 20 patients (29.9%) were classified into the favorable neurological group. No marked difference was observed in the patient characteristics between those with and without a favorable outcome except for in the time from CA to starting ECLS (ECLS initiation time). A short ECLS initiation time resulted in a favorable outcome (37.8±28.1 minutes vs. 53.6±30.7 minutes, p=0.05). The cut-off time of ECLS initiation was 46 minutes, which was prolonged by the temporary return of spontaneous circulation before ECLS [odds ratio (OR), 3.69; 95% confidence interval (CI), 1.34-10.19; p=0.01] and transfer to the angiographic room (OR, 4.07; 95% CI, 1.44-11.53, p=0.008).
Conclusion
The early initiation of ECLS (within 46 minutes) might be associated with a favorable neurological outcome for patients with rCA caused by cardiac events.
Keywords: ECLS, cardiac arrest, neurological outcome
Introduction
Cardiac arrest (CA) caused by cardiac events, such as acute coronary syndrome (ACS) and ventricular fibrillation (VF), is a major cause of sudden death in developed countries and still has a low survival rate after cardiopulmonary resuscitation (CPR) (1). Despite strenuous efforts involving early CPR, many patients still fail to recover due to refractory CA (2-4). In patients with refractory CA, extracorporeal life support (ECLS) is an effective option for obtaining the return of spontaneous circulation (ROSC). The 2015 American Heart Association (AHA) guideline revealed that ECLS was considered a viable to conventional CPR for patients with refractory CA with potentially reversible CA etiology (5). Although recent studies have reported that the use of ECLS improved the survival rates compared with that of conventional CPR (6, 7), data on the association between ECLS and a favorable neurological outcome are scarce. Therefore, the present study evaluated the neurological outcomes of ECLS initiation in refractory CA patients. Factors affecting the initiation of ECLS were also determined.
Materials and Methods
This study included consecutive patients with refractory CA who required ECLS despite the continuous performance of conventional CPR, regardless of their CA location. Refractory CA is defined as the absence of ROSC after conventional CPR for more than two cycles of advanced cardiac life support (ACLS). Contraindications of ECLS for patients with CA are as follows: 1) terminal malignancies, 2) severe trauma, 3) uncontrollable hemorrhaging, 4) irreversible brain damage, 5) and signed consent for “do not resuscitate.” Given our focus on the effects of ECLS in emergency cases in the present study, we evaluated only the CA patients whose CA origin was ACS or VF, as these are common CA origins in the emergency room with similar CA situations (rapid change in the hemodynamics).
ECLS procedure
When ECLS is necessary for patients with refractory CA, the ECLS team should be called. The ECLS team consists of interventional cardiologists, a clinical engineer (perfusionist), a radiologist, and nurses. As the ECLS team has at least one cardiologist who stays in the hospital 24 hours a day, ECLS is started without waiting for other staff members. In principle, the initiation of ECLS is performed when CA occurs. The ECLS system consists of a centrifugal pump, polypropylene hollow-fiber membrane oxygenator, and circuit that is heparin-bonded (Terumo, Tokyo, Japan). Percutaneous cannulation of the femoral artery and vein is performed using the Seldinger technique (while conducting CPR) with 16.5-French catheter for arterial cannulation and 21-French catheter for venous cannulation. Diagnostic angiographies are mandatory unless prolonged asystole or pulseless electrical activity (PEA) is observed, regardless of the timing of ECLS initiation. Percutaneous coronary intervention (PCI) is attempted when the thrombolysis in myocardial infarction (TIMI) flow grade of the acute coronary syndrome-related artery is <3 or the culprit lesion is unstable.
Management
The ECLS flow rate in the present study was determined based on the blood pressure, urine output, and arterial blood gas. The mean blood pressure was maintained above 60 mmHg. Routine intraaortic balloon pump placement was recommended provided contraindications or an inability to initiate due to the presence of an arterial occlusion were not observed. The activated clotting time was maintained at 150 to 250 seconds with heparin administration. To avoid limb ischemia, an anterograde reperfusion catheter for distal limb perfusion was inserted if necessary after achieving hemodynamic stability. Minimal lung ventilation was maintained to avoid pulmonary collapse during ECLS. Details concerning the ECLS technique and its management have been presented in previous studies (8, 9).
Study endpoint
The study endpoints were the clinical outcomes and predictors of a favorable neurologic outcome at discharge following ECLS treatment. The ECLS-related complications were also evaluated, including cannula site complications (bleeding requiring a clinically hemostatic technique and ECLS access infection), retroperitoneal hemorrhaging, lower-limb ischemia, and cerebral hemorrhaging. Fatal complications were defined as ECLS-related complications resulting in death.
The cut-off time of ECLS initiation, which is the most important factor, was evaluated. The initiation time of ECLS was defined as the time from first CA to the initiation of ECLS, regardless of temporary ROSC. The predictors of a delayed ECLS initiation were then investigated using the cut-off time.
A Glasgow-Pittsburgh cerebral performance category (CPC) score of 1 (good recovery) was considered a favorable neurologic outcome, while CPC scores of 2 (moderate disability), 3 (severe disability), 4 (coma or vegetative state), or 5 (brain death) were regarded as an unfavorable neurologic outcome. Patients who did not successfully recover from ECLS initiation were classified into the unfavorable neurological group. The CPC score was calculated based on the discharge summary abstracts or documentation in our hospital records. All data were taken from our hospital records. The review boards in our institute approved the study protocol.
Statistical analysis
Comparisons of clinical and baseline factors or procedure-related characteristics were performed using Student's t-test, Wilcoxon's rank-sum test (continuous variables), or the chi-square test (categorical) according to the favorable neurological outcome. Receiver operator characteristic (ROC) curves and the corresponding area under the curve (AUC) regarded as a favorable neurological outcome were used to evaluate the cut-off time for ECLS initiation. The ROC curve is a graphical representation of test characteristics with sensitivity on the y-axis and specificity on the x-axis, the overall possible cut points for defining positive and negative test results. We determined the cut-off time of the ECLS initiation according to the area under the ROC curve. After dividing all evaluated patients into the two groups based on whether the-ECLS initiation time was above or below the cut-off value, we performed comparisons again. Analyses were conducted using the IBM SPSS software program for Windows, version 21.0 (IBM SPSS, Chicago, USA).
Results
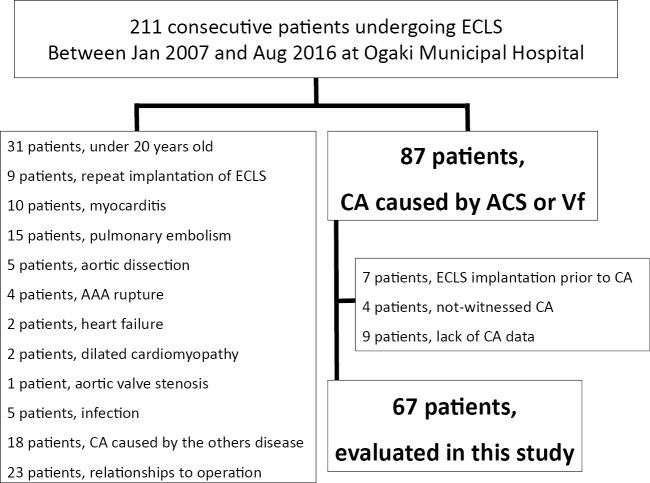
Between January 2007 and August 2016, 211 consecutive patients were treated with ECLS for either in-hospital CA (IHCA) or out-of-hospital CA (OHCA) in our institute. Of these, we excluded patients with 1 or more of the following conditions: 1) age below 20 years, 2) ECLS re-initiation, 3) CA origins other than ACS or VF, 4) CA origins related to operations, 5) ECLS initiation prior to CA, and 6) lack of CA data. Of the initial 211 patients considered, 67 (ACS, 57 patients; VF, 10 patients) were enrolled in this study (Fig. 1). A favorable neurological outcome was observed in 20 of the 67 (29.8%) patients. The baseline procedural characteristics of our population are shown in Tables 1 and 2. Although no significant difference in the initial rhythm or pH was observed, the initiation time from CA to ECLS seemed to be shorter in the favorable group (37.8±28.1 minutes vs. 53.6±30.7 minutes, p=0.05) than in the unfavorable group. During ECLS, bleeding complications related to ECLS occurred in 22 patients (access site bleeding, 8 patients; retroperitoneal bleeding, 2 patients). Ischemia of the lower limb occurred in 6 patients. No significant differences in the ECLS-related complications between the two groups, except for fatal bleeding complications were observed (Table 3). The area under the ROC curve of the ECLS initiation time was 0.69 [95% confidence interval (CI) 0.55-0.82; p=0.02], and the cut-off time of 46 minutes had the highest combined sensitivity and specificity (Fig. 2). In addition, a change in the location during CPR and temporary ROSC before ECLS were significantly associated with ECLS initiation within 46 minutes (Table 4).
Figure 1.
Study profile between January 2007 and August 2016, 211 consecutive patients were treated with ECLS in our institute. We excluded patients who met any of the exclusion criteria. Ultimately, 67 patients were enrolled in this study.
Table 1.
Baseline Clinical Characteristics of Cardiac Arrest Patients Treated with Extracorporeal Life Support.
| Patients: n (%) | Favorable neurological outcome (n=20) |
Unfavorable neurological outcome (n=47) |
p value |
|---|---|---|---|
| Age (years) | 62.35±13.50 | 65.11±14.32 | 0.47 |
| Male gender | 14 (70.0) | 36 (76.6) | 0.56 |
| Body mass index (kg/m2) | 23.9±3.39 | 23.5±3.67 | 0.62 |
| Cardiovascular risk factor | |||
| Hypertension | 13 (65.0) | 26 (55.3) | 0.59 |
| Smoker | 12 (60.0) | 23 (48.9) | 0.44 |
| Diabetes | 7 (35.0) | 17 (36.2) | >0.99 |
| Dyslipidemia | 16 (80.0) | 35 (74.5) | 0.76 |
| CKD (e-GFR<60) | 4 (20.0) | 16 (34.0) | 0.38 |
| Reason for CPR | |||
| Acute coronary syndrome | 19 (95.0) | 38 (80.9) | 0.26 |
| Left main trunk | 3 (15.0) | 6 (12.8) | >0.99 |
| Myocardial rupture | 0 (0.0) | 8 (17.0) | 0.10 |
| Non-ACS ventricular fibrillation | 1 (5.0) | 9 (19.1) | 0.26 |
| OHCA | 3 (15.0) | 13 (27.7) | 0.36 |
| Transport time (min) | 21.3±9.61 | 27.6±16.46 | 0.54 |
| IHCA | 17 (85.0) | 34 (72.3) | 0.36 |
| Initial rhythm at CA | |||
| Asystole | 3 (15.0) | 8 (17.0) | >0.99 |
| PEA | 2 (10.0) | 9 (19.1) | 0.48 |
| VT/VF | 14 (70.0) | 26 (55.3) | 0.29 |
| Not reported | 1 (5.0) | 4 (8.5) | >0.99 |
| Initial pH | 7.25±0.24 | 7.20±0.20 | 0.43 |
Data are presented as percentages and absolute numbers or means±standard deviation, unless otherwise specified.
e-GFR: estimated glomerular filtration rate, CPR: cardiopulmonary resuscitation, CA: cardiac arrest, OHCA: out-of-hospital cardiac arrest, IHCA: in-hospital cardiac arrest, PEA: pulseless electrical activity, VT: ventricular tachycardia, VF: ventricular fibrillation
Table 2.
The Procedural Characteristics of Cardiac Arrest Patients Treated with Extracorporeal Life Support.
| Patients: n (%) | Favorable neurological outcome (n=20) |
Unfavorable neurological outcome (n=47) |
p value |
|---|---|---|---|
| ECLS implantation time (min) | 37.8±28.1 | 53.6±30.7 | 0.05 |
| Duration ECLS treatment (days) | 3.85±3.65 | 3.09±2.76 | 0.35 |
| Temporary ROSC before ECLS | 10 (50.0) | 23 (48.9) | >0.99 |
| Location change during CPR | 5 (25.0) | 21 (44.7) | 0.17 |
| Transfusions | |||
| Red blood cell concentrate (IU) | 28.2±22.6 | 21.9±14.4 | 0.26 |
| Platelet concentrate (IU) | 20.8±31.8 | 8.9±14.0 | 0.12 |
| Fresh frozen plasma (IU) | 6.3±5.8 | 5.0±3.9 | 0.30 |
| Renal replacement therapy | 11 (55.0) | 23 (48.9) | 0.79 |
| Therapeutic hypothermia | 7 (35.0) | 16 (34.0) | >0.99 |
Data are presented as percentages and absolute numbers or means±standard deviation, unless otherwise specified.
ECLS: extracorporeal life support, ROSC: return of spontaneous circulation, CPR: cardiopulmonary resuscitation
Table 3.
The ECLS-related Complications in Cardiac Arrest Patients Treated with ECLS.
| Patients: n (%) | Favorable neurological outcome (n=20) |
Unfavorable neurological outcome (n=47) |
p value |
|---|---|---|---|
| Bleeding complication | 8 (40.0) | 14 (29.8) | 0.57 |
| Access site bleeding | 4 (20.0) | 4 (8.5) | 0.23 |
| Retroperitoneal bleeding | 0 (0.0) | 2 (4.3) | >0.99 |
| Other bleeding | 4 (20.0) | 8 (17.0) | 0.74 |
| Fatal bleeding complication | 0 (0.0) | 9 (19.1) | 0.05 |
| Ischemia complication | 1 (5.0) | 5 (10.6) | 0.66 |
Data are presented as percentages and absolute numbers or means±standard deviation, unless otherwise specified.
ECLS: extracorporeal life support
Figure 2.
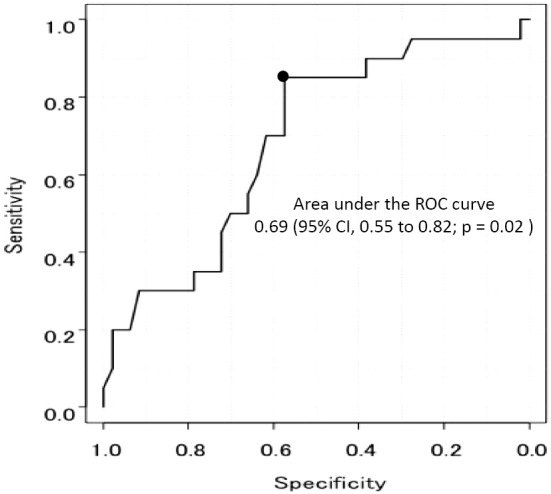
Receiver-operating-characteristics (ROC) curves for the cut-off of the ECLS initiation time to differentiate favorable and unfavorable neurologic outcomes at hospital discharge.
Table 4.
Baseline and Procedural Clinical Characteristics of Cardiac Arrest Patients Treated with Extracorporeal Life Support.
| Patients: n (%) | ECLS implantation time ≤ 46 min (n=37) |
ECLS implantation time > 46 min (n=30) |
p value |
|---|---|---|---|
| Age (years) | 65.4±13.0 | 62.9±15.4 | 0.47 |
| Male gender | 26 (70.3) | 24 (80.0) | 0.41 |
| Body mass index (kg/m2) | 23.9±4.12 | 23.3±2.68 | 0.48 |
| Cardiovascular risk factor | |||
| Hypertension | 21 (56.8) | 18 (60.0) | 0.81 |
| Smoker | 19 (51.4) | 16 (53.3) | >0.99 |
| Diabetes | 14 (37.8) | 10 (33.3) | 0.80 |
| Dyslipidemia | 30 (81.1) | 21 (70.0) | 0.39 |
| CKD (e-GFR<60) | 12 (32.4) | 8 (26.7) | 0.79 |
| Reason for CPR | |||
| Acute coronary syndrome | 34 (94.6) | 22 (73.3) | 0.03 |
| Left main trunk | 6 (16.2) | 3 (10.0) | 0.72 |
| Myocardial rupture | 5 (13.5) | 3 (10.0) | 0.72 |
| Non-ACS ventricular fibrillation | 2 (5.4) | 8 (26.7) | 0.34 |
| OHCA | 4 (10.8) | 12 (40.0) | 0.01 |
| Transport time (min) | 18.8±11.4 | 29.0±16.0 | 0.26 |
| IHCA | 32 (89.2) | 18 (60.0) | 0.01 |
| Initial rhythm at CA | |||
| Asystole | 7 (18.9) | 4 (13.3) | 0.74 |
| PEA | 4 (10.8) | 7 (23.3) | 0.20 |
| VT/Vf | 22 (59.5) | 18 (60.0) | >0.99 |
| Not reported | 4 (10.8) | 1 (3.3) | 0.37 |
| Initial pH | 7.24±0.21 | 7.18±0.20 | 0.24 |
| Duration ECLS treatment (days) | 3.73±3.60 | 2.80±2.10 | 0.19 |
| Temporary ROSC before ECLS | 13 (35.1) | 20 (66.7) | 0.01 |
| Location change during CPR | 9 (24.3) | 17 (56.7) | 0.01 |
Data are presented as percentages and absolute numbers or means±standard deviation, unless otherwise specified.
e-GFR: estimated Glomerular Filtration Rate, CPR: Cardiopulmonary Resuscitation, CA: Cardiac Arrest, OHCA: out-of-hospital cardiac arrest, IHCA: in-hospital cardiac arrest, PEA: Pulseless Electrical Activity, VT: Ventricular tachycardia, Vf: Ventricular fibrillation
Discussion
The main findings of our study were as follows: 1) although ECLS-related fatal complications occurred in 19.1% of patients, a favorable neurological outcome was observed in 29.8% of the patients following ECLS initiation for refractory cardiac arrest caused by cardiac events; 2) a shorter initiation time of ECLS alone is associated with a favorable neurological outcome, and a cut-off time of 46 minutes for ECLS initiation should be obtained in order to ensure a favorable neurological outcome; and 3) changing the location during CPR and temporary ROSC before ECLS were significant inhibitors of the initiation of ECLS within 46 minutes.
In the present study, the most important finding was that a short initiation time of ECLS was significantly associated with favorable neurological outcomes, and this finding is in accordance with those of recent studies showing that a delay in ROSC is the only independent predictor of the survival after CPR (10-12). Among these reports, Kagawa et al. demonstrated that higher survival rates after ECLS treatment were observed in IHCA patients than in OHCA patients, but this disadvantage in OHCA patients disappeared after modifying the initiation time of ECLS (13), which indicated that the initiation time of ECLS itself was an important factor for a better survival rate. Based on the favorable neurological outcomes, Hase et al. showed that the early recovery of the circulation within 35 minutes using on-site counter shock or ECLS might result in a favorable recovery (14). In their report, however, only 38 out of 100 OHCA patients were treated with ECLS. It is therefore reasonable that the initiation of ECLS within 46 minutes was significantly associated with a favorable neurological outcome in refractory CA patients who were treated with ECLS in our study.
Considering that ECLS preparation takes a few minutes, we only have a short time to determine the ECLS indication if we are to initiate ECLS within 46 minutes after the onset of CA. Therefore, the indication of ECLS initiation should be determined immediately after several cycles of ACLS fail to recover the circulation in CA patients. In our study, OHCA patients arrived a hospital after approximately 27.3 minutes on average by ambulance, which is in accordance with the findings of Nagao's report (15). Therefore, ECLS initiation should not be delayed in OHCA patients because of prolonged conventional resuscitation. Indeed, Nagao et al. showed initiating ECLS from CA within 55.5 minutes for OHCA patients resulted in a favorable neurological outcome after early hypothermia induction (15). Considering that the early induction of hypothermia was not routinely performed in our study due to the long enrollment period, the improved favorable neurological outcome was considered to be the result of the routine early induction of hypothermia for refractory CA patients.
Notably, changing the location during conventional CPR was a significant inhibitor of achieving ECLS initiation within 46 minutes. As such, ECLS should be started at the place where CA occurs or in the emergency room in order to avoid delays and transfer to the angiographic room. X-rays were found to be useful for confirming the wire into the ascending aorta and the other wire into the superior or inferior vena cava, respectively, when the position of the inserted wire is uncertain (Fig. 3). Such an X-ray check may make emergent ECLS initiation easier and safer, regardless of the location.
Figure 3.
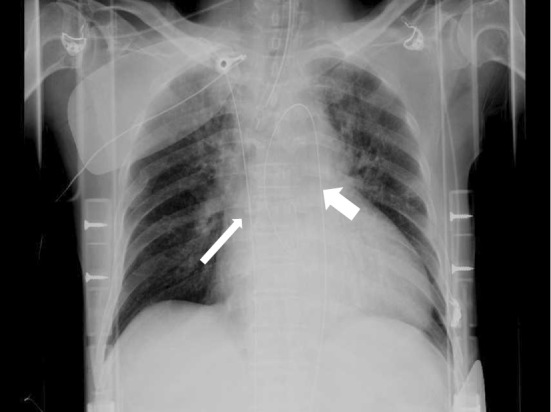
Chest X-ray after the initiation of ECLS. The thick arrow shows the wire inserted into the ascending aorta, and the thin arrow shows the wire inserted into the inferior vena cava.
Interestingly, our data demonstrated that temporary ROSC before ECLS initiation, which was often observed in daily practice, was also an inhibitor for initiating ECLS within 46 minutes. Therefore, prophylactic ECLS initiation might be considered when the hemodynamic status is unstable after ROSC.
ECLS-related complications occurred in 28 of 67 (41.7%) patients in our study, which corresponds with the rates noted in previous studies (30-40%) (16, 17). As Thiagarajan et al. demonstrated that percutaneous insertion was associated with a better hospital survival than surgical insertion (18), almost all patients were percutaneously treated. Indeed, the rate of ECLS-related complications did not increase when we started ECLS in a room where an angiography system was not available. The reasons for this result are as follows: 1) the operators who performed the percutaneous insertion of ECLS were mainly experienced interventional cardiologists; 2) an anterograde reperfusion catheter was routinely used for distal limb perfusion to avoid lower-limb ischemia, as was noted in the study conducted by Leick et al. (19); and 3) if needed, ultrasound-guided cannulation was attempted to reduce the risk of complications at the puncture site (20).
Limitation
Several limitations associated with the present study warrant mention. First this retrospective study included a small number of patients from a single center. A dedicated multicenter study is necessary in order to confirm the findings of this study. Second, both OHCA and IHCA patients were enrolled in this study, hampering the interpretation of our findings. In addition, the quality of CPR before ECLS initiation differed among patients. The imbalance in the quality of CPR, especially in OHCA patients, is thus a limitation of our study. Third, although we assessed the neurologic outcome, we lacked sufficient data on the blood flow to the head for the included patients, and therapeutic hypothermia was not performed in all CA patients because some were enrolled in a 2007 study when hypothermia was not mandatory for CA patients. Therefore, the importance of time in relation to a favorable neurological outcome might have been underestimated in our study. In the present study, we emphasized that an ECLS initiation time within 46 minutes is associated with a favorable neurologic outcome. However, we must bear in mind that ensuring a good outcome requires a stable quality of CPR and a reduction in the rate of fatal complications associated with ECLS.
Conclusion
The early initiation of ECLS (within 46 minutes) was found to be associated with a favorable neurological outcome in patients suffering from CA caused by cardiac events. ECLS can be initiated within 46 minutes of the event if it is performed when and where the CA occurred.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med 344: 1304-1313, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 58: 297-308, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Hajbaghery MA, Mousavi G, Akbari H. Factors influencing survival after in-hospital cardiopulmonary resuscitation. Resuscitation 66: 317-321, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Shih CL, Lu TC, Jerng JS, et al. A web-based Utstein style registry system of in-hospital cardiopulmonary resuscitation in Taiwan. Resuscitation 72: 394-403, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132: S444-S464, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372: 554-561, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto T, Morimura N, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation 85: 762-768, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Chen YS, Yu HY, Huang SC, et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med 36: 2529-2535, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Beckmann A, Benk C, Beyersdorf F, et al. Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg 40: 676-680, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Skrifvars MB, Varghese B, Parr MJ. Survival and outcome prediction using the Apache III and the out-of-hospital cardiac arrest (OHCA) score in patients treated in the intensive care unit (ICU) following out-of-hospital, in-hospital or ICU cardiac arrest. Resuscitation 83: 728-733, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari M, Hekmat K, Jung C, et al. Better outcome after cardiopulmonary resuscitation using percutaneous emergency circulatory support in non-coronary patients compared to those with myocardial infarction. Acute Cardiac Care 13: 30-34, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Morimura N, Sakamoto T, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a review of the Japanese literature. Resuscitation 82: 10-14, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Kagawa E, Inoue I, Kawagoe T, et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 81: 968-973, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Hase M, Tsuchihashi K, Fujii N, et al. Early defibrillation and circulatory support can provide better long-term outcomes through favorable neurological recovery in patients with out-of-hospital cardiac arrest of cardiac origin. Circ J 69: 1302-1307, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Nagao K, Kikushima K, Watanabe K, et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circulation Journal 74: 77-85, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto S, Taniguchi N, Nakajima S, Takahashi A. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg 94: 1-7, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Haneya A, Philipp A, Diez C, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation 83: 1331-1337, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg 87: 778-785, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Leick J, Liebetrau C, Szardien S, et al. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol 102: 661-669, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Massetti M, Tasle M, Le Page O, et al. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg 79: 178-183, discussion 83-84, 2005. [DOI] [PubMed] [Google Scholar]