Abstract
Objectives
Irrigation is the cornerstone of treating skeletal infection by eliminating pathogens in wounds. A previous study shows that irrigation with normal saline (0.9%) and ethylenediaminetetraacetic acid (EDTA) could improve the removal of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) compared with normal saline (NS) alone. However, it is still unclear whether EDTA solution is effective against infection with drug-resistant bacteria.
Methods
We established three wound infection models (skin defect, bone-exposed, implant-exposed) by inoculating the wounds with a variety of representative drug-resistant bacteria including methicillin-resistant S. aureus (MRSA), extended spectrum beta-lactamase-producing E. coli (ESBL-EC), multidrug-resistant Pseudomonas aeruginosa (MRPA), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Acinetobacter baumannii (MRAB), multidrug-resistant Enterobacter (MRE), and multidrug-resistant Proteus mirabilis (MRPM). Irrigation and debridement were repeated until the wound culture became negative. The operating times required to eliminate pathogens in wounds were compared through survival analysis.
Results
Compared with other groups (NS, castile soap, benzalkonium chloride, and bacitracin), the EDTA group required fewer debridement and irrigation operations to achieve pathogen eradication in all three models of wound infection.
Conclusion
Irrigation with EDTA solution was more effective than the other irrigation fluids used in the treatment of wound infections caused by drug-resistant pathogens.
Cite this article: Z. Deng, F. Liu, C. Li. Therapeutic effect of ethylenediaminetetraacetic acid irrigation solution against wound infection with drug-resistant bacteria in a rat model: an animal study. Bone Joint Res 2019;8:189–198. DOI: 10.1302/2046-3758.85.BJR-2018-0280.R3.
Keywords: Wound infection, Open fracture, Irrigation solution
Article focus
This study aimed to evaluate the efficacy of ethylenediaminetetraacetic acid (EDTA) irrigation solution in the eradication of drug-resistant bacteria in a rat wound infection model.
Key messages
EDTA solution did not negatively affect wound healing compared with normal saline.
Our study showed the superiority of EDTA solution over commonly used irrigation solutions in the eradication of drug-resistant pathogens from infected wounds.
Strengths and limitations
Our study used seven of the most commonly found bacterial species in fracture-related infections. The study was adequately powered (up to 50 rats for each group).
Our study used an in vivo rat model of wound infection; the findings presented here would still require validation in a human study prior to clinical application.
Introduction
Wound infections remain a challenging global surgical problem, despite advances in operative techniques and the widespread use of systemic antibiotic prophylaxis.1 The management of wound infection requires thorough irrigation and debridement to eliminate pathogens and promote healing.2,3 The main purpose of these procedures is to reduce the pathogen load in the wound. The potential advantages of irrigation include physical removal, destruction, and creating a hostile environment for contaminated bacteria. However, the optimal irrigation solution to use in these procedures is still unknown.1,4-9
Studies have demonstrated enhanced bacteria removal with surfactants compared with normal saline (0.9%) (NS).10-13 However, surfactants cause toxicity to host tissues and can adversely affect healing.14,15 Likewise, there is little evidence to support the effectiveness of bactericidal solutions, including bacitracin solution and benzalkonium chloride, as wound irrigation fluids. Additionally, there are concerns that associated toxicity to the host tissue with subsequent necrosis may in fact increase the risk of bacterial infection.16 The clinical use of such solutions is therefore not recommended.17 For those wounds with bacteria that are difficult to eradicate (e.g. prosthetic joint infection (PJI)), creating a pathogen-hostile environment might be effective by using chemicals including antibiotics and acetic acid.18-21 However, the effectiveness and safety profile of these solutions are yet to be definitively established in humans.22-24
A recent study demonstrated that NS supplemented with ethylenediaminetetraacetic acid (EDTA) increased the efficacy of removing Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), and caused no additional toxicity in an open fracture model, compared with NS alone.25 Bacterial adhesion requires adhesins, which are molecules located on the surface of pathogenic bacteria, to interact with host tissues.26 Most of the bacterial adhesins are cell-surface proteins that require the presence of ions including calcium, zinc, and magnesium to function.27-29 EDTA could competitively chelate these ions including calcium, zinc, and magnesium to form a complex and thereby remove bacteria.30,31 However, further study with other bacterial strains and species, especially drug-resistant ones, is still needed in order to determine the ability of EDTA to disrupt adhesion in different kinds of bacterial infections.32
Materials and Methods
Ethical approval
The study and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Beijing Changping Hospital (Beijing, China). All study methods were in accordance with China’s regulations on animal study.
Bacterial culture and preparation of inoculum
We chose seven species of bacteria that were responsible for over 90% of wound infections.33 The following strains of bacteria were obtained from the American Type Culture Collection (ATCC): methicillin-resistant Staphylococcus aureus (MRSA), BAA-1747; extended spectrum beta-lactamase-producing E. coli (ESBL-EC), BAA-196; multidrug-resistant Pseudomonas aeruginosa (MRPA), BAA-2108; vancomycin-resistant Enterococcus (VRE), 51559; multidrug-resistant Acinetobacter baumannii (MRAB), BAA-1605; multidrug-resistant Enterobacter (MRE), BAA-2468; and multidrug-resistant Proteus mirabilis (MRPM), BAA-2791. We maintained a stock culture of these strains of bacteria on tryptic soy agar (TSA) with 5% sheep blood (TSA II; BD GmbH, Heidelberg, Germany), and prepared fresh culture 24 hours before surgery at 37°C and in an aerobic medium (5% CO2). We prepared the inoculum by collecting the organisms on a cotton sterile swab, washing the cells three times in phosphate-buffered saline (PBS), and adjusting the cells to a concentration of 1 × 108 colony forming units (CFU) per millilitre according to a standard curve of optical density. Each wound would be inoculated with a 1 × 107 CFU bacteria in a 100 μl solution.
Rat wound infection model
To create models of wound infection, we established three modified models described in a previous study (skin defect, bone-exposed, and implant-exposed models).13,25 In brief, after the backs were shaved, povidone iodine solution was applied. For the skin defect model, a full-thickness skin defect (18 mm in diameter to avoid rapid healing) was created and then inoculated with MRSA, ESBL-EC, MRPA, VRE, MRAB, MRE, and MRPM, respectively. For the bone-exposed model, the spinous process was exposed in addition to the 18 mm skin defect. Furthermore, we created the implant-exposed model by drilling one hole in the exposed spinous process, through which we inserted a 5 mm stainless wire. The rats were then randomly assigned to each treatment group. Treatment was withheld for the initial 48 hours in order to allow the wound infection to be established.
Preparation of irrigation solution
There were five treatment groups in this study: EDTA solution, castile soap, benzalkonium chloride, bacitracin, and NS solution. The EDTA solution was made by dissolving 1 mmol of EDTA into one litre of NS. After that, the pH was adjusted to 7.4. The soap solution was prepared with 0.45% of castile soap in NS. The benzalkonium chloride solution was prepared by injecting 1.76 ml of 17% benzalkonium chloride into a litre of NS solution. The bacitracin solution was prepared by injecting 40 000 U into a litre of NS solution.
Irrigation and debridement procedures
Blinding was not possible as the solutions could not be masked from the investigators. The castile soap is slightly opaque and with foam; benzalkonium chloride is transparent, yellow, and with foam; bacitracin is transparent, colourless, and with foam; EDTA solution and NS are transparent, colourless, and without foam. Following irrigation and debridement of the wound, sample for bacterial culture was obtained with a cotton sterile swab and the wound was then covered by sterile dressings. We repeated the irrigation and debridement every 48 hours until the cultures obtained after previous irrigation and debridement were negative. The primary outcome was negative bacterial culture, as this has been shown to be an important indication for delayed wound closure.34 The interval was recommended in clinical practice and adopted by previous animal studies.34,35
All of the rats were given an intraperitoneal injection of 450 mg/kg chloral hydrate for general anaesthesia before the procedures. The rats then received thorough irrigation and debridement with 400 ml of the test irrigation solution using a syringe. Irrigation pressure was manually controlled. The basic principle for debridement was to establish margins of viable tissue with perfusion. After the above procedures, all of the rats in the three groups received an additional irrigation with 100 ml NS to remove residual additives or to act as a control.
Bacterial culture of wound specimens
All specimens obtained during surgery were incubated on TSA with 5% sheep blood (TSA II; BD GmbH) for 48 hours (37°C, aerobic 5% CO2). A positive result was indicated by the formation of at least one colony. For further confirmation, bacterial identification was conducted with 16S ribosomal DNA sequencing by using the MicroSeq 500 microbial identification system (Thermo Fisher Scientific, Waltham, Massachusetts).
Statistical analysis
Kaplan–Meier curves were computed and compared using the log-rank test. SPSS software (IBM Corp., Armonk, New York) was used for statistical analysis. Significant differences between groups for numerical and non-numerical variables were evaluated using Student’s t-test and chi-squared test, respectively.
Results
The direct effects of different solutions on wound healing
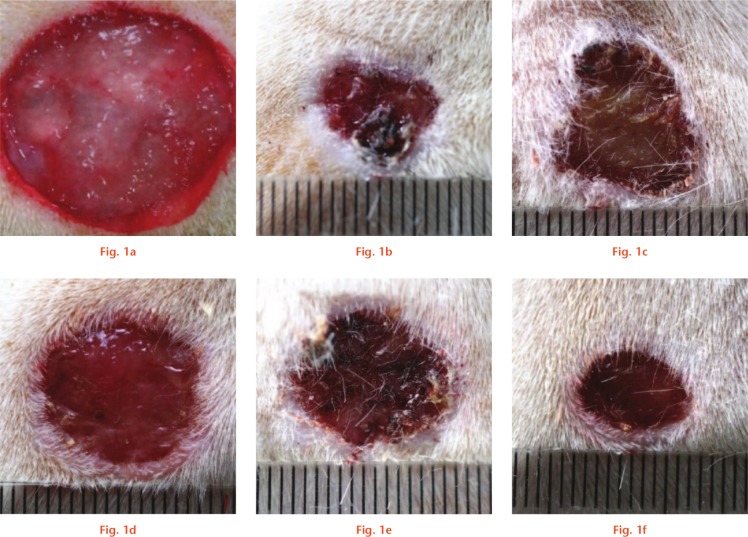
We first assessed the direct effects of different solutions on wound healing by creating a 5 mm wound and irrigating the wound daily. We then compared the time needed to achieve complete healing of the wound. The results are summarized in Table I. Compared with NS and EDTA solution, castile soap, benzalkonium chloride, and bacitracin showed a delay in wound healing. In addition, we repeated this daily irrigation experiment, but with 18 mm wounds. Images of the wounds are shown in Figure 1. Wound healing was delayed following irrigation with castile soap, benzalkonium chloride, or bacitracin.
Table I.
Direct effects of different irrigation solutions on wound healing
| Solution | Mean time for complete healing, days (sd) | p-value* |
|---|---|---|
| Normal saline | 7.82 (2.13) | N/A |
| Castile soap | 10.98 (3.54) | < 0.001 |
| Benzalkonium chloride | 12.16 (3.77) | < 0.001 |
| Bacitracin | 11.92 (4.11) | < 0.001 |
| EDTA | 7.62 (2.37) | 0.562 |
Significant differences (p < 0.05) versus normal saline were calculated using Student’s t-test
N/A, not applicable; EDTA, ethylenediaminetetraacetic acid
Fig. 1.
Images of wounds before and after two weeks. a) The original wound was created in an 18 mm size. The wounds were then irrigated daily for two weeks with: b) normal saline; c) castile soap; d) benzalkonium chloride; e) bacitracin; and f) ethylenediaminetetraacetic acid (EDTA).
EDTA irrigation in skin defect wound model
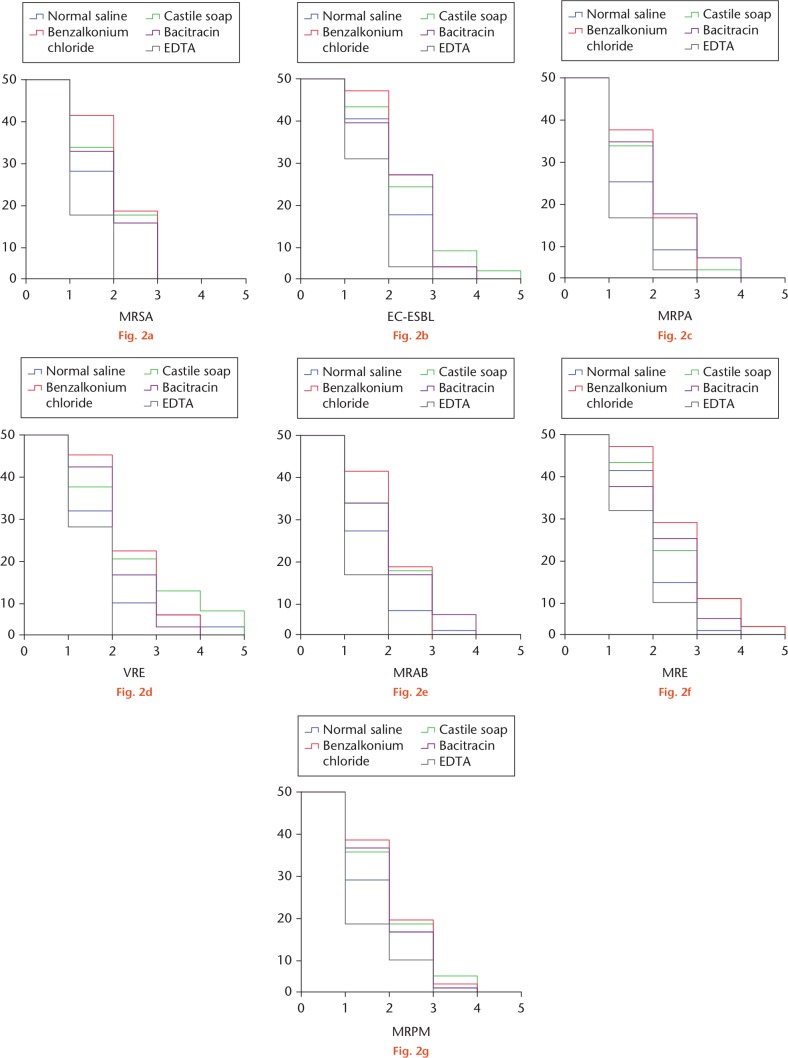
We compared the number of operations required to achieve a culture-negative wound among the different treatment groups. A survival analysis of culture-positive wounds over operation times was conducted. The Kaplan–Meier survival curves are shown in Figure 2. The EDTA group showed the most superior results in the treatment of wound infections for all bacterial strains. The use of EDTA irrigation required the least number of procedures in order to produce a culture-negative wound. The mean and median number of operations to achieve culture-negative wounds are summarized in Table II, along with the results of log-rank analysis of the Kaplan–Meier survival curves.
Fig. 2.
Kaplan–Meier survival curves of skin defect wounds with positive culture of: a) methicillin-resistant Staphylococcus aureus (MRSA); b) extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC); c) multidrug-resistant Pseudomonas aeruginosa (MRPA); d) vancomycin-resistant Enterococcus (VRE); e) multidrug-resistant Acinetobacter baumannii (MRAB); f) multidrug-resistant Enterobacter (MRE); and g) multidrug-resistant Proteus mirabilis (MRPM). EDTA, ethylenediaminetetraacetic acid.
Table II.
Operation times to achieve culture-negative wounds (skin defect wound)
| Strains / treatment | Mean operations, n (sd) | 95% CI | Median operations, n (95% CI) | p-value versus EDTA (log-rank) |
|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | ||||
| Normal saline | 1.82 (0.12) | 1.58 to 2.06 | 2.00 (N/A) | < 0.001 |
| Castile soap | 1.98 (0.12) | 1.75 to 2.21 | 2.00 (1.62 to 2.38) | < 0.001 |
| Benzalkonium chloride | 2.16 (0.10) | 1.96 to 2.36 | 2.00 (1.73 to 2.27) | < 0.001 |
| Bacitracin | 1.92 (0.11) | 1.70 to 2.14 | 2.00 (1.65 to 2.35) | < 0.001 |
| EDTA* | 1.32 (0.07) | 1.19 to 1.45 | 1.00 (N/A) | N/A |
| Extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) | ||||
| Normal saline | 2.12 (0.10) | 1.92 to 2.32 | 2.00 (1.73 to 2.27) | 0.001 |
| Castile soap* | 2.50 (0.14) | 2.22 to 2.78 | 2.00 (1.62 to 2.38) | < 0.001 |
| Benzalkonium chloride* | 2.52 (0.10) | 2.32 to 2.72 | 3.00 (2.85 to 3.15) | < 0.001 |
| Bacitracin | 2.36 (0.13) | 2.11 to 2.61 | 3.00 (2.82 to 3.18) | < 0.001 |
| EDTA* | 1.66 (0.08) | 1.50 to 1.82 | 2.00 (1.88 to 2.12) | N/A |
| Multidrug-resistant Pseudomonas aeruginosa (MRPA) | ||||
| Normal saline | 1.62 (0.10) | 1.42 to 1.82 | 1.00 (N/A) | 0.032 |
| Castile soap* | 2.00 (0.13) | 1.76 to 2.24 | 2.00 (1.65 to 2.35) | < 0.001 |
| Benzalkonium chloride* | 2.04 (0.11) | 1.83 to 2.25 | 2.00 (1.71 to 2.29) | < 0.001 |
| Bacitracin* | 2.10 (0.14) | 1.83 to 2.37 | 2.00 (1.64 to 2.36) | < 0.001 |
| EDTA* | 1.34 (0.08) | 1.19 to 1.49 | 1.00 (N/A) | N/A |
| Vancomycin-resistant Enterococcus (VRE) | ||||
| Normal saline | 1.92 (0.15) | 1.64 to 2.20 | 2.00 (1.78 to 2.22) | 0.042 |
| Castile soap* | 2.46 (0.19) | 2.10 to 2.82 | 2.00 (1.63 to 2.37) | < 0.001 |
| Benzalkonium chloride* | 2.42 (0.12) | 2.20 to 2.64 | 2.00 (1.72 to 2.28) | < 0.001 |
| Bacitracin | 2.18 (0.11) | 1.97 to 2.39 | 2.00 (1.76 to 2.24) | < 0.001 |
| EDTA* | 1.54 (0.07) | 1.40 to 1.68 | 1.00 (N/A) | N/A |
| Multidrug-resistant Acinetobacter baumannii (MRAB) | ||||
| Normal saline | 1.66 (0.11) | 1.45 to 1.87 | 2.00 (N/A) | 0.004 |
| Castile soap* | 1.98 (0.12) | 1.75 to 2.21 | 2.00 (1.62 to 2.38) | < 0.001 |
| Benzalkonium chloride* | 2.16 (0.10) | 1.96 to 2.36 | 2.00 (1.73 to 2.27) | < 0.001 |
| Bacitracin* | 2.06 (0.14) | 1.79 to 2.33 | 2.00 (1.65 to 2.35) | < 0.001 |
| EDTA* | 1.30 (0.07) | 1.17 to 1.43 | 1.00 (N/A) | N/A |
| Multidrug-resistant Enterobacter (MRE) | ||||
| Normal saline | 2.10 (0.10) | 1.90 to 2.30 | 2.00 (1.78 to 2.22) | 0.033 |
| Castile soap* | 2.50 (0.15) | 2.21 to 2.79 | 2.00 (1.69 to 2.31) | < 0.001 |
| Benzalkonium chloride* | 2.72 (0.13) | 2.46 to 2.98 | 3.00 (2.72 to 3.28) | < 0.001 |
| Bacitracin | 2.30 (0.14) | 2.04 to 2.56 | 2.00 (1.58 to 2.42) | 0.001 |
| EDTA* | 1.78 (0.10) | 1.58 to 1.98 | 2.00 (1.78 to 2.22) | N/A |
| Multidrug-resistant Proteus mirabilis (MRPM) | ||||
| Normal saline | 1.88 (0.13) | 1.63 to 2.13 | 2.00 (1.51 to 2.49) | 0.031 |
| Castile soap | 2.12 (0.13) | 1.86 to 2.38 | 2.00 (1.64 to 2.36) | 0.001 |
| Benzalkonium chloride | 2.16 (0.12) | 1.93 to 2.39 | 2.00 (1.67 to 2.33) | < 0.001 |
| Bacitracin | 2.04 (0.11) | 1.82 to 2.26 | 2.00 (1.70 to 2.30) | 0.003 |
| EDTA* | 1.50 (0.11) | 1.29 to 1.71 | 1.00 (N/A) | N/A |
Significant differences (p < 0.05) versus normal saline were calculated using the log-rank statistic based on Kaplan–Meier curves
CI, confidence interval; EDTA, ethylenediaminetetraacetic acid; N/A, not applicable
EDTA irrigation in bone-exposed wound model
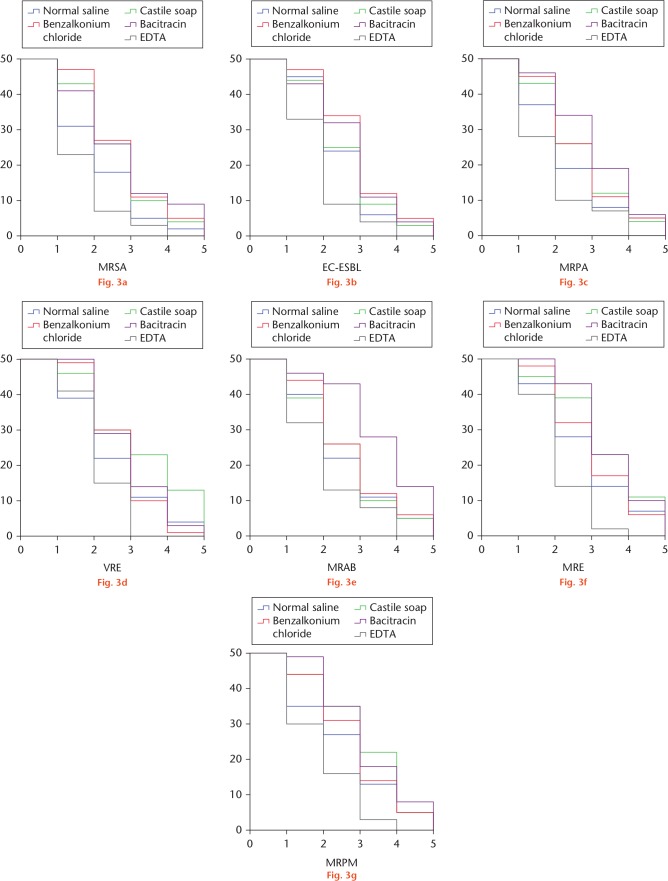
The results of skin defect wound model were similarly consistent and the survival analysis of culture-positive wounds over operation times has revealed a great advantage of EDTA solution over other irrigation solutions (Fig. 3). Likewise, the mean and median number of operations to achieve culture-negative wounds are summarized in Table III, along with the Kaplan–Meier curves.
Fig. 3.
Kaplan–Meier survival curves of bone-exposed wounds with positive culture of: a) methicillin-resistant Staphylococcus aureus (MRSA); b) extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC); c) multidrug-resistant Pseudomonas aeruginosa (MRPA); d) vancomycin-resistant Enterococcus (VRE); e) multidrug-resistant Acinetobacter baumannii (MRAB); f) multidrug-resistant Enterobacter (MRE); and g) multidrug-resistant Proteus mirabilis (MRPM). EDTA, ethylenediaminetetraacetic acid.
Table III.
Operation times to achieve culture-negative wounds (bone-exposed wound)
| Strains / treatment | Mean operations, n (sd) | 95% CI | Median operations, n (95% CI) | p-value versus EDTA (log-rank) |
|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | ||||
| Normal saline | 2.12 (0.16) | 1.81 to 2.43 | 2.00 (1.49 to 2.51) | 0.022 |
| Castile soap* | 2.66 (0.16) | 2.35 to 2.97 | 3.00 (2.66 to 3.34) | < 0.001 |
| Benzalkonium chloride* | 2.80 (0.15) | 2.50 to 3.10 | 3.00 (2.68 to 3.32) | < 0.001 |
| Bacitracin* | 2.76 (0.19) | 2.39 to 3.13 | 3.00 (2.59 to 3.41) | < 0.001 |
| EDTA* | 1.66 (0.12) | 1.42 to 1.90 | 1.00 (N/A) | N/A |
| Extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) | ||||
| Normal saline | 2.56 (0.14) | 2.29 to 2.83 | 2.00 (1.64 to 2.36) | 0.001 |
| Castile soap | 2.62 (0.15) | 2.33 to 2.91 | 2.00 (1.60 to 2.40) | 0.001 |
| Benzalkonium chloride | 2.96 (0.15) | 2.68 to 3.24 | 3.00 (2.73 to 3.27) | < 0.001 |
| Bacitracin | 2.80 (0.16) | 2.49 to 3.11 | 3.00 (2.73 to 3.27) | < 0.001 |
| EDTA* | 1.92 (0.12) | 1.68 to 2.16 | 2.00 (1.78 to 2.22) | N/A |
| Multidrug-resistant Pseudomonas aeruginosa (MRPA) | ||||
| Normal saline | 2.38 (0.17) | 2.04 to 2.72 | 2.00 (1.63 to 2.37) | 0.033 |
| Castile soap | 2.70 (0.16) | 2.38 to 3.02 | 3.00 (2.62 to 3.38) | 0.001 |
| Benzalkonium chloride | 2.74 (0.16) | 2.43 to 3.05 | 3.00 (2.66 to 3.34) | < 0.001 |
| Bacitracin* | 3.10 (0.16) | 2.78 to 3.42 | 3.00 (2.55 to 3.45) | < 0.001 |
| EDTA* | 1.90 (0.15) | 1.61 to 2.19 | 2.00 (1.69 to 2.31) | N/A |
| Vancomycin-resistant Enterococcus (VRE) | ||||
| Normal saline | 2.52 (0.17) | 2.18 to 2.86 | 2.00 (1.60 to 2.40) | 0.025 |
| Castile soap* | 3.24 (0.19) | 2.86 to 3.62 | 3.00 (2.19 to 3.81) | < 0.001 |
| Benzalkonium chloride | 2.80 (0.12) | 2.57 to 3.03 | 3.00 (2.72 to 3.28) | < 0.001 |
| Bacitracin | 2.92 (0.13) | 2.66 to 3.18 | 3.00 (2.58 to 3.42) | < 0.001 |
| EDTA* | 2.12 (0.10) | 1.93 to 2.31 | 2.00 (1.76 to 2.24) | N/A |
| Multidrug-resistant Acinetobacter baumannii (MRAB) | ||||
| Normal saline | 2.56 (0.17) | 2.22 to 2.90 | 2.00 (1.62 to 2.38) | 0.030 |
| Castile soap | 2.60 (0.17) | 2.26 to 2.94 | 3.00 (2.62 to 3.38) | 0.020 |
| Benzalkonium chloride | 2.76 (0.17) | 2.43 to 3.09 | 3.00 (2.63 to 3.37) | 0.003 |
| Bacitracin* | 3.62 (0.17) | 3.29 to 3.95 | 4.00 (3.56 to 4.44) | < 0.001 |
| EDTA* | 2.06 (0.15) | 1.76 to 2.36 | 2.00 (1.68 to 2.32) | N/A |
| Multidrug-resistant Enterobacter (MRE) | ||||
| Normal saline | 2.84 (0.18) | 2.49 to 3.19 | 3.00 (2.56 to 3.44) | < 0.001 |
| Castile soap | 3.36 (0.18) | 3.02 to 3.70 | 3.00 (2.51 to 3.49) | < 0.001 |
| Benzalkonium chloride | 3.06 (0.16) | 2.76 to 3.36 | 3.00 (2.56 to 3.44) | < 0.001 |
| Bacitracin* | 3.52 (0.14) | 3.25 to 3.79 | 3.00 (2.58 to 3.42) | < 0.001 |
| EDTA* | 2.12 (0.11) | 1.91 to 2.33 | 2.00 (1.76 to 2.24) | N/A |
| Multidrug-resistant Proteus mirabilis (MRPM) | ||||
| Normal saline | 2.60 (0.19) | 2.23 to 2.97 | 3.00 (2.45 to 3.55) | 0.004 |
| Castile soap | 3.12 (0.17) | 2.79 to 3.45 | 3.00 (2.47 to 3.53) | < 0.001 |
| Benzalkonium chloride | 2.88 (0.16) | 2.56 to 3.20 | 3.00 (2.63 to 3.37) | < 0.001 |
| Bacitracin | 3.20 (0.15) | 2.90 to 3.50 | 3.00 (2.61 to 3.39) | < 0.001 |
| EDTA* | 1.98 (0.14) | 1.71 to 2.25 | 2.00 (1.53 to 2.47) | N/A |
Significant differences (p < 0.05) versus normal saline were calculated using the log-rank statistic based on Kaplan–Meier curves
CI, confidence interval; EDTA, ethylenediaminetetraacetic acid; N/A, not applicable
EDTA irrigation in implant-exposed wound model
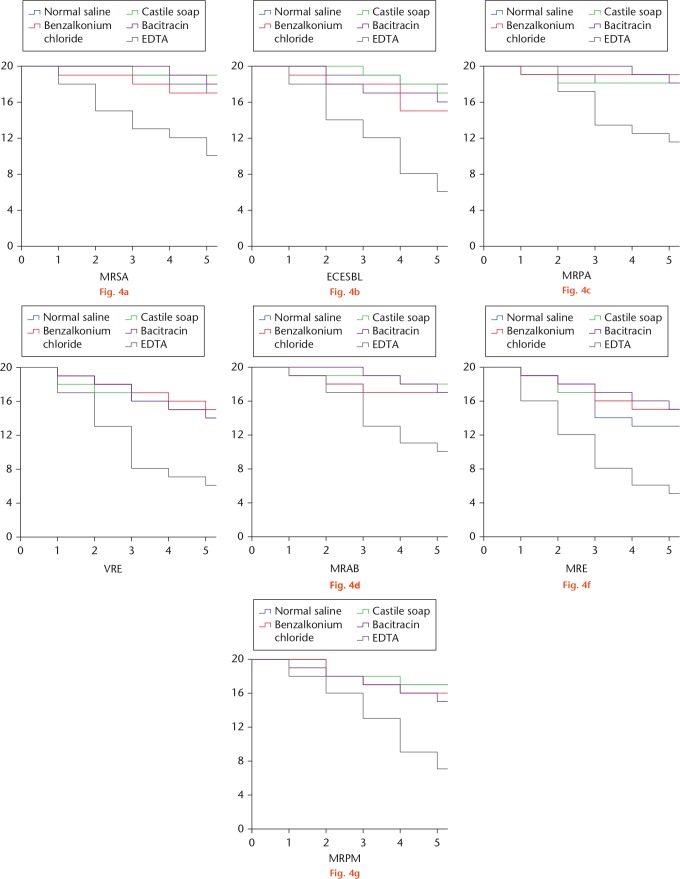
The survival analysis of culture-positive wounds over operation times was conducted and the results are shown in Figure 4. The number of culture-positive wounds after five operations was compared and is summarized in Table IV. Interestingly, only EDTA solution showed a potent effect against pathogens in implant-exposed wounds.
Fig. 4.
Kaplan–Meier survival curves of implant-exposed wounds with positive culture of: a) methicillin-resistant Staphylococcus aureus (MRSA); b) extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC); c) multidrug-resistant Pseudomonas aeruginosa (MRPA); d) vancomycin-resistant Enterococcus (VRE); e) multidrug-resistant Acinetobacter baumannii (MRAB); f) multidrug-resistant Enterobacter (MRE); and g) multidrug-resistant Proteus mirabilis (MRPM). EDTA, ethylenediaminetetraacetic acid.
Table IV.
Wound culture after five procedures of irrigation and debridement (implant-exposed wound)
| Strains / treatment | Positive wounds, n | Negative wounds, n | p-value versus EDTA (log-rank) |
|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | |||
| Normal saline | 17 | 3 | 0.014 |
| Castile soap | 19 | 1 | 0.001 |
| Benzalkonium chloride | 17 | 3 | 0.020 |
| Bacitracin | 18 | 2 | 0.004 |
| EDTA* | 10 | 10 | N/A |
| Extended spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) | |||
| Normal saline | 18 | 2 | < 0.001 |
| Castile soap | 17 | 3 | < 0.001 |
| Benzalkonium chloride | 15 | 5 | 0.005 |
| Bacitracin | 16 | 4 | 0.002 |
| EDTA* | 6 | 14 | N/A |
| Multidrug-resistant Pseudomonas aeruginosa (MRPA) | |||
| Normal saline | 18 | 2 | 0.018 |
| Castile soap | 18 | 2 | 0.021 |
| Benzalkonium chloride | 19 | 1 | 0.005 |
| Bacitracin | 18 | 2 | 0.010 |
| EDTA* | 11 | 9 | N/A |
| Vancomycin-resistant Enterococcus (VRE) | |||
| Normal saline | 15 | 5 | 0.004 |
| Castile soap | 14 | 6 | 0.012 |
| Benzalkonium chloride | 15 | 5 | 0.004 |
| Bacitracin | 14 | 6 | 0.009 |
| EDTA* | 6 | 14 | N/A |
| Multidrug-resistant Acinetobacter baumannii (MRAB) | |||
| Normal saline | 17 | 3 | 0.028 |
| Castile soap | 18 | 2 | 0.007 |
| Benzalkonium chloride | 17 | 3 | 0.028 |
| Bacitracin | 17 | 3 | 0.014 |
| EDTA* | 10 | 10 | N/A |
| Multidrug-resistant Enterobacter (MRE) | |||
| Normal saline | 13 | 7 | 0.011 |
| Castile soap | 15 | 5 | 0.002 |
| Benzalkonium chloride | 15 | 5 | 0.001 |
| Bacitracin | 15 | 5 | 0.001 |
| EDTA* | 5 | 15 | N/A |
| Multidrug-resistant Proteus mirabilis (MRPM) | |||
| Normal saline | 17 | 3 | 0.002 |
| Castile soap | 17 | 3 | 0.002 |
| Benzalkonium chloride | 16 | 4 | 0.006 |
| Bacitracin | 15 | 5 | 0.014 |
| EDTA* | 7 | 13 | N/A |
Significant differences (p < 0.05) versus normal saline or EDTA were calculated using Pearson’s chi-squared test
EDTA, ethylenediaminetetraacetic acid; N/A, not applicable
The strain of bacteria cultured from the wound was identical to the bacteria inoculated in the whole study. In other words, no contaminated bacteria were detected throughout the study.
Discussion
Infections caused by multidrug-resistant pathogens represent a major threat worldwide.36 Pathogens such as MRSA, ESBL-EC, MRPA, VRE, MRAB, MRE, and MRPM have been found to be the most frequent drug-resistant strains isolated from infected open fractures.33 Although many novel antimicrobials have been established in recent decades, management of wound infection by these bacteria has been increasingly challenging.37 Irrigation and debridement of wounds remains the cornerstone to eliminating such infections.38,39
There has been a substantial number of basic and translational research projects focused on alternative irrigation solutions, but evidence from high-quality clinical trials is still scarce. As a result, the choice of irrigation solution remains controversial. A recent study demonstrated that NS as an irrigation solution was superior to surfactant-additive saline, citing concerns about toxicity and adverse effects on healing.14 Likewise, even more wound-healing problems after bacitracin irrigation were observed.8
We believe that this study will be an important stepping stone for the clinical translation of EDTA irrigation solution. In this study, we answered a straightforward question: “What is the effect of EDTA solution on bacteria, especially those with multidrug resistance?” We further confirmed the previous observation conducted with S. aureus and E. coli.25 EDTA was more effective than NS solution in a rat wound model infected with seven of the most commonly isolated pathogens, which represent more than 90% of causative strains.33 Furthermore, we showed that EDTA solution was also superior to castile soap, benzalkonium chloride, and bacitracin. PJI remains a great challenge to the orthopaedic community.40 In this study, we showed that a substantial proportion of implant-exposed infection was eradicated after irrigation with EDTA. This result was particularly notable because of the difficulty of breaking a biofilm on an implant.41
As with all studies that use animal models, the current work does have limitations. First, direct inoculation of swabs on agar is associated with a reduction in bacterial detection.42 Because the detection method was identically applied in all groups, any major conclusion would not be affected. Nevertheless, this would be important for future evaluation in humans. Second, we cannot determine whether these results will make a difference in a clinical scenario. A larger model should be chosen in any future studies as it more closely mimics the clinical scenario than does a smaller animal model. Finally, if those prove similarly promising, the safety of EDTA solution should be assessed in a small-scale clinical study. It is likely that EDTA would be safe because of the long-standing usage of EDTA for medical purposes.43 Then, perhaps a clinical study designed similarly to the fluid lavage of open wounds (FLOW) study14 may be performed that, hopefully, would translate this important finding into clinical practice. Notably, the effective concentration might be slightly different when translating into practice among human subjects.
Footnotes
Author contributions: Z. Deng: Conducted the experiments.
F. Liu: Designed the study, Analyzed the data.
C. Li: Designed the study, Wrote the manuscript.
Ethical review statement: The study and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Beijing Changping Hospital (Beijing, China).
Follow us @BoneJointRes
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Sprague S, Petrisor B, Jeray K, et al. Wound irrigation does not affect health-related quality of life after open fractures: results of a randomized controlled trial. Bone Joint J 2018;100-B:88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nota SP, Braun Y, Ring D, Schwab JH. Incidence of surgical site infection after spine surgery: what is the impact of the definition of infection? Clin Orthop Relat Res 2015;473:1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haddad S, Nunez-Pereira S, Pigrau C, et al. The impact of deep surgical site infection on surgical outcomes after posterior adult spinal deformity surgery: a matched control study. Eur Spine J 2018;27:2518-2528. [DOI] [PubMed] [Google Scholar]
- 4. Petrisor B, Jeray K, Schemitsch E, et al. Fluid Lavage in patients with Open fracture Wounds (FLOW): an international survey of 984 surgeons. BMC Musculoskelet Disord 2008;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crowley DJ, Kanakaris NK, Giannoudis PV. Irrigation of the wounds in open fractures. J Bone Joint Surg [Br] 2007;89-B:580-585. [DOI] [PubMed] [Google Scholar]
- 6. Barnes S, Spencer M, Graham D, Johnson HB. Surgical wound irrigation: a call for evidence-based standardization of practice. Am J Infect Control 2014;42:525-529. [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee JS. A critical review of irrigation techniques in acute wounds. Int Wound J 2005;2:258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anglen JO. Comparison of soap and antibiotic solutions for irrigation of lower-limb open fracture wounds. A prospective, randomized study. J Bone Joint Surg [Am] 2005;87-A:1415-1422. [DOI] [PubMed] [Google Scholar]
- 9. Gustilo RB. Current concepts in the management of open fractures. Instr Course Lect 1987;36:359-366. [PubMed] [Google Scholar]
- 10. Falconer B, Liljedahl SO, Olovson T. On the effect of treatment of traumatic wounds with soap solution; an experimental study. Acta Chir Scand 1952;103:222-235. [PubMed] [Google Scholar]
- 11. Anglen J, Apostoles PS, Christensen G, Gainor B, Lane J. Removal of surface bacteria by irrigation. J Orthop Res 1996;14:251-254. [DOI] [PubMed] [Google Scholar]
- 12. Moussa FW, Gainor BJ, Anglen JO, Christensen G, Simpson WA. Disinfecting agents for removing adherent bacteria from orthopaedic hardware. Clin Orthop Relat Res 1996;329:255-262. [DOI] [PubMed] [Google Scholar]
- 13. Huyette DR, Simpson WA, Walsh R, et al. Eradication by surfactant irrigation of Staphylococcus aureus from infected complex wounds. Clin Orthop Relat Res 2004;427:28-36. [DOI] [PubMed] [Google Scholar]
- 14. Bhandari M, Jeray KJ, Petrisor BA, et al. A trial of wound irrigation in the initial management of open fracture wounds. N Engl J Med 2015;373:2629-2641. [DOI] [PubMed] [Google Scholar]
- 15. Crawford C, Zirwas MJ. Laundry detergents and skin irritancy–a comprehensive review. Skinmed 2014;12:23-31. [PubMed] [Google Scholar]
- 16. Owens BD, White DW, Wenke JC. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg [Am] 2009;91-A:92-98. [DOI] [PubMed] [Google Scholar]
- 17. Anglen JO. Wound irrigation in musculoskeletal injury. J Am Acad Orthop Surg 2001;9:219-226. [DOI] [PubMed] [Google Scholar]
- 18. Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 2013;95-B:1450-1452. [DOI] [PubMed] [Google Scholar]
- 19. Tsang STJ, Gwynne PJ, Gallagher MP, Simpson A. The biofilm eradication activity of acetic acid in the management of periprosthetic joint infection. Bone Joint Res 2018;7:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams RL, Ayre WN, Khan WS, Mehta A, Morgan-Jones R. Acetic acid as part of a debridement protocol during revision total knee arthroplasty. J Arthroplasty 2017;32:953-957. [DOI] [PubMed] [Google Scholar]
- 21. Ernest EP, Machi AS, Karolcik BA, LaSala PR, Dietz MJ. Topical adjuvants incompletely remove adherent Staphylococcus aureus from implant materials. J Orthop Res 2018;36:1599-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edelstein AI, Weiner JA, Cook RW, et al. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg [Am] 2017;99-A:232-238. [DOI] [PubMed] [Google Scholar]
- 23. Whiteside LA. Prophylactic peri-operative local antibiotic irrigation. Bone Joint J 2016;98-B(1 Suppl A):23-26. [DOI] [PubMed] [Google Scholar]
- 24. Norman G, Atkinson RA, Smith TA, et al. Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev 2017;10:CD012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu H, Bao B, Zheng X. Is NS-EDTA effective in clearing bacteria from infected wounds in a rat model? Clin Orthop Relat Res 2018;476:1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carvalho E, Ching Ching AT, Estima Abreu PA, Ho PL, Barbosa AS. Breaking the bond: recent patents on bacterial adhesins. Recent Pat DNA Gene Seq 2012;6:160-171. [DOI] [PubMed] [Google Scholar]
- 27. Vengadesan K, Narayana SV. Structural biology of Gram-positive bacterial adhesins. Protein Sci 2011;20:759-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krishnan V, Narayana SV. Crystallography of gram-positive bacterial adhesins. Adv Exp Med Biol 2011;715:175-195. [DOI] [PubMed] [Google Scholar]
- 29. Kline KA, Falker S, Dahlberg S, Normark S, Henriques-Normark B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009;5:580-592. [DOI] [PubMed] [Google Scholar]
- 30. Ouyang P, Gottlieb SH, Culotta VL, Navas-Acien A. EDTA chelation therapy to reduce cardiovascular events in persons with diabetes. Curr Cardiol Rep 2015;17:96. [DOI] [PubMed] [Google Scholar]
- 31. Chappell LT. Should EDTA chelation therapy be used instead of long-term clopidogrel plus aspirin to treat patients at risk from drug-eluting stents? Altern Med Rev 2007;12:152-158. [PubMed] [Google Scholar]
- 32. Kennedy SA. CORR Insights(R): is NS-EDTA effective in clearing bacteria from infected wounds in a rat model? Clin Orthop Relat Res 2018;476:1091-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu H, Li X, Zheng X. A descriptive study of open fractures contaminated by seawater: infection, pathogens, and antibiotic resistance. BioMed Res Int 2017;2017:2796054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lenarz CJ, Watson JT, Moed BR, et al. Timing of wound closure in open fractures based on cultures obtained after debridement. J Bone Joint Surg [Am] 2010;92-A:1921-1926. [DOI] [PubMed] [Google Scholar]
- 35. Shahan MH, Chuang AH, Brennan WA, et al. The effect of chlorhexidine irrigation on tensile wound strength. J Periodontol 1993;64:719-722. [DOI] [PubMed] [Google Scholar]
- 36. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-281. [DOI] [PubMed] [Google Scholar]
- 37. Krychowiak M, Grinholc M, Banasiuk R, et al. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS One 2014;9:e115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dau AA, Tloba S, Daw MA. Characterization of wound infections among patients injured during the 2011 Libyan conflict. East Mediterr Health J 2013;19:356-361. [PubMed] [Google Scholar]
- 39. Rajpaul K. Biofilm in wound care. Br J Community Nurs 2015;Suppl Wound Care:S6, S8, S10-S11. [DOI] [PubMed] [Google Scholar]
- 40. Zmistowski B, Della Valle C, Bauer TW, et al. Diagnosis of periprosthetic joint infection. J Orthop Res 2014;32(Suppl 1):S98-S107. [DOI] [PubMed] [Google Scholar]
- 41. Lee YS, Koo KH, Kim HJ, et al. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg [Am] 2017;99-A:2077-2084. [DOI] [PubMed] [Google Scholar]
- 42. Tsang STJ, McHugh MP, Guerendiain D, et al. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques: one third of carriers missed. Bone Joint Res 2018;7:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Finnegan S, Percival SL. EDTA: an antimicrobial and antibiofilm agent for use in wound care. Adv Wound Care 2015;4:415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]