Abstract
Regulation of vascular permeability plays a major role in the pathophysiology of visually threatening conditions such as retinal vein occlusion and diabetic retinopathy. Principally, several factors such as vascular endothelial growth factor (VEGF), are up-regulated or induced in response to hypoxia thus adversely affecting the blood-retinal barrier (BRB), resulting in retinal edema and neovascularisation. Furthermore, current evidence supports a dysregulation of the inner retinal neural-vascular integrity as a critical factor driving retinal ganglion cell (RGC) death and visual loss. The principal objective of this study was to interrogate whether Substance P (SP), a constitutive neurotransmitter of amacrine and ganglion cells, may protect against N-methyl-d-aspartate (NMDA)-induced excitotoxic apoptosis of ganglion cells and VEGF-induced vessel leakage in the retina. Tight junctional protein expression and a Vascular Permeability Image Assay were used to determine vascular integrity in vitro. The protective effect of SP on RGC was established in ex vivo retinal explants and in vivo murine models. After NMDA administration, a reduction in TUNEL+ cells and a maintained number of Brn-3a+ cells were found, indicating an inhibition of RGC apoptosis mediated by SP. Additionally, SP maintained endothelial tight junctions and decreased VEGF-induced vascular permeability. In conclusion, administration of SP protects against NMDA apoptosis of RGC and VEGF-induced endothelial barrier breakdown.
Keywords: Substance P, Retinal ganglion cell, Vascular permeability, ZO-1
1. Introduction
The retina is one of the most metabolically active tissues and its demand for oxygen is higher than many other tissues including the brain [1,2]. Optimal function of the retina is dependent on a continuous supply of oxygen from the circulation [3]. A number of retinal disorders are propagated as a result of loss of retinal vascular integrity and subsequent retinal cell apoptosis. For example, in conditions such as retinal artery or vein occlusions and diabetic retinopathy, ischemia causes an up-regulation hypoxia-inducible factors such as HIF-1 alpha which elevate pro-angiogenic factors including vascular endothelial growth factor (VEGF). As a result the blood-retinal barrier (BRB) is disrupted which leads to increased vascular permeability. With respect to the inner retinal barrier the resulting leakage of fluid into the inner retina is a critical response implicated in retinal ganglion cell (RGC) death and loss of vision [4]. RGC are integral to processing of visual information and thus critical for vision [5]. Due to RGCs and other central nervous system neurons failure to regenerate after injury, the loss of RGCs observed in many retinal diseases further contribute to visual loss [6]. Chronic diseases such as glaucoma and diabetic retinopathy are associated with RGC death [7,8] and associated with persistent ischemia in glaucoma [9,10], either by mechanical compression of retinal blood vessels around the optic nerve head caused by increased intraocular pressure or by ineffective vascular autoregulation [11]. The implication is that additional therapeutic options should be considered addressing the retinal neurovascular unit [12]. Consequently, therapeutic strategies for enhancing RGC viability (neuroprotection) and preventing or reversing retinal neuronal dysfunction as well as vascular leakage remain a goal of basic and translational research.
Substance P (SP) is a neuropeptide secreted by neurons and is involved in many biological processes via specific receptors [13]. More than 10 neuropeptides are expressed in the inner nuclear layer and the ganglion cell layer of the retina, of which SP is the most abundant [14,15,16]. SP modulates a diverse set of pathways, including those involved in vasodilatation, cell proliferation, apoptosis, and inflammation [17], as well as promoting the migration and differentiation of vascular endothelial cells [18]. Animal models have provided insights into the biology of this peptide, and compelling evidence highlights the importance of SP in cell-to-cell communication by either paracrine or endocrine signaling [13]. Moreover, higher levels of SP are observed in retina in response to acute stress [19]. A corollary observed is that SP is markedly reduced in diabetes [20,21], supporting a notion that SP may be important for the retinal health and its loss contributes to disease progression.
Based on these functions, we hypothesized that SP is neuroprotective, attenuating retinal neural apoptosis and maintains inner retinal vascular integrity. In this study, the effect of SP was assessed by evaluating endothelial and ganglion cell viability, apoptosis, and vascular permeability. ex vivo, as well as in an animal model in vivo.
2. Materials and methods
2.1. Intravitreal administration of VEGF and N-methyl-d-aspartate (NMDA)
All procedures were conducted under the regulation of the United Kingdom Home Office Animals (Scientific Procedures) Act 1986, and were in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research. The methods were carried out in accordance with the approved University of Bristol institutional guidelines and all experimental protocols under Home Office Project Licence 30/3045 and 30/3281 were approved by the University of Bristol Ethical Review Group.
In vivo retinal vascular permeability was assessed as previously described [22]. Eight-week-old C57BL/6 mice were purchased from Charles River. Briefly, adult mice were deeply anesthetized by intraperitoneal injection of Vetelar (ketamine hydrochloride 100 mg/ml, Pfizer, UK) and Rompun (xylazine hydrochloride 20 mg/mL, Bayer, UK) mixed with sterile water in the ratio 0.6:1:84, and the pupils of mice were dilated using topical 1% tropicamide (0.5% w/v, Bausch & Lomb, Aubenas, France), before induction of anesthesia. Mice received the following intravitreal injections (2 μl) with a 33-gauge needle into one eye: VEGF (v7259, Sigma-Aldrich, in distilled water) (100 ng); SP (ab120170, Abcam, in distilled water) (20 nmol); VEGF plus SP together; 0.9% saline vehicle. 48 h post the first injection mice received tail vein injections of Evans Blue (200 μl, 2%). Mice were killed 10 min later and eyes were fixed with 4% paraformaldehyde (PFA) for 2 h. Retinal flatmounts were mounted in antifading medium (Vector Laboratories, Inc., Burlingame, CA, UAS), and images were captured by Leica SP5-AOBS confocal laser microscope.
NMDA (M3262, Sigma-Aldrich, in distilled water) was used to induce excitotoxicity of RGC in the retina, under experimental conditions described above. Mice received the following intravitreal injections (2 μl) with a 33-gauge needle into one eye: NMDA (10 nmol); SP (20 nmol); NMDA with SP together; 0.9% saline vehicle control. Mice were killed 24 h following NMDA injection, and retinal flatmounts from eyes prepared. Eyes were fixed in 2% PFA for 2 h for TUNEL labeling as well as immunofluorescence staining.
2.2. Retinal explant culture ex vivo model
Retinal explant culture was performed as per protocol [23], Han Wistar rat (20-old-day) with healthy, untreated eyes were killed by exposure to a rising concentration of carbon dioxide followed by cervical dislocation. The retina was dissected radially into four equal-sized pieces, and individual explants positioned onto 12-mm diameter filters (0.4 μm pore, Millipore) with the RGC side facing up. The filters were placed into the wells of a 24-well plate, each of which contained 800 μl of retinal explant media, consisting of neuronal growth medium (Neurobasal A) supplemented with 2% B27 (Invitrogen Ltd.), 1% N2 (Invitrogen Ltd.), l-glutamine (0.8 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml). Retinal explant cultures were maintained in humidified incubators at 37 °C and 5% CO2. Half of the media were changed on day 1 and every second day thereafter.
2.3. Cell culture and viability
Mouse Primary Retinal Microvascular Endothelial cells (MRMECs, Generon, UK) were cultured in Complete Mouse Endothelial Cell Medium (Generon, UK). Cell viability was assessed using the MTT assay. A total of 2 × 104 cells were seeded in each well of a 48-well plate and cultured for 48 h to confluency. Following serum starvation for 24 h, fresh medium supplemented with different concentrations of VEGF (25–200 ng/ml) or SP (1–80 μM) were added for a further 24 h. To assess cell viability, the MTT assay was performed according to manufacturer's instructions. In brief, 50 μl of MTT solution (Thermo Fisher Scientific, UK) was added to each culture well, and the plate was incubated for a further 2 h at 37 °C with 5% CO2. The MTT solution was replaced with HCl/isopropyl alcohol to dissolve formazan, and the optical density (OD) of the wells was measured at 562 nm on a microplate reader and was calculated using linear regression analysis.
2.4. In vitro vascular permeability assay
Permeability visualization experiments were performed using 18 × 18 mm glass coverslips coated with biotinylated gelatin and placed in 35 mm culture dishes. MRMECs were seeded (4.5 × 105 cells/dish) and cultured for 72 h to reach 100% confluency. Culture medium was changed to serum free medium supplemented with treatment (VEGF, SP or SP+VEGF) for a further 24 h. To evaluate permeability of the MRMEC monolayer, Fluorescein conjugated streptavidin (25 μg/ml final concentration) was directly added to the culture medium for 5 min, washed twice with PBS, before cell fixation with 3.7% formaldehyde in PBS 10 min, room temperature (RT). Overlay of Fluorescein and Zonula occludens-1 (ZO-1) staining demonstrates reciprocal relations between ZO-1 peripheral localization and increased local permeability of Fluorescein-streptavidin. The pattern of Fluorescein-streptavidin binding to the biotinylated gelatin underlying the cell monolayer was examined under Leica SP5-AOBS confocal laser microscope. Images were processed with ImageJ software. The percentage expression of fluorescein intensity was taken from areas with associated DAPI staining only, therefore excluding non-cellular area of fluorescence.
2.5. Immunofluorescence staining
Rat retinal explants were fixed with 4% PFA and plus 4% sucrose and snap frozen in OCT (VWR Chemicals, UK). Cryosections were prepared, washed in PBS and permeabilized with 0.1% Triton X-100. Non-specific staining was blocked with 5% BSA in PBS for 30 min at RT followed by immunostaining with goat anti-mouse GFAP (Cell Signaling Technology, UK, 1:100), rabbit anti-mouse Brn-3a (Santa Cruz Biotechnology, Inc., UK, 1:50), goat anti-mouse Rhodopsin (Abcam, UK, 1:100) antibodies. The secondary antibodies Alexa Fluor 549-labeled donkey anti-goat IgG (Invitrogen, CA) and Alexa Fluor 488-labeled donkey anti-rabbit IgG (Invitrogen, CA), both with 1:200 dilution in 2% BSA in PBS at room temperature (RT) for 1 h in the dark. Exclusion of the primary antibody acted as the negative control.
2.6. TUNEL assay
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed according to the instructions provided by the manufacturer (Roche; Indianapolis, IN). In brief, fixed retinal explant were washed in PBS, retinas were incubated in 0.1% Triton X-100 for 1 h to permeabilize the cells, rinsed three times with PBS, before incubation with the TUNEL reaction mixture for 1 h at 37 °C. Retina were mounted using anti-fading mounting medium (Vector Laboratories, Inc., Burlingame, CA), and apoptotic cells were observed using Leica SP5-AOBS confocal laser microscope. The number of TUNEL+ cells in retinal explant was counted in five random fields on each retinal explant (four per condition), and the average number of TUNEL+ cells per random field was determined for each condition tested. In vivo TUNEL staining was quantitatively analyzed by determining the intensities of TUNEL+ cells from dissected whole retina tissues (n = 6 per group) using ImageJ.
2.7. Retinal flatmount
The whole retina was carefully dissected from the eyecup, permeabilized with 0.2% Triton X-100 and non-specific binding blocked by 5% normal goat serum in PBS for 2 h RT. The retinas were then incubated with the primary antibody (purified rabbit anti-Brn-3a (1:100)) for 24 h at 4 °C. Tissues were washed before incubation with secondary Cy3 conjugated goat anti-rabbit IgG (1:250) for 2 h at RT. For TUNEL staining, retinas were incubated with the reaction mixture for 2 h at 37 °C, before being flat mounted onto microscope slides and covered with anti-fading mounting medium for confocal microscopy. The number of Brn-3a+ cell bodies was determined from four separate fields of view (the same distance from the optic nerve) per retinal flatmount and expressed as an average number of Brn-3a+ cells per field.
2.8. SDS-PAGE analysis
Cultured MRMEC cells were washed twice with ice-cold PBS before lysis with ice-cold CelLytic™ MT Cell Lysis Reagent (Sigma-Aldrich, UK). Cell lysates were centrifuged at 14,000 g for 10 min at 4 °C, the supernatant was transferred to fresh tubes and protein concentration of samples determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, UK). Equal amounts of protein were mixed with 4x SDS sample buffer, boiled for 10 min at 90 °C, and resolved using 4%–20% NuPAGE gels (Invitrogen, UK). Proteins were transferred to PVDF membranes (Invitrogen, UK), blocked with 5% skimmed milk for 2 h, and incubated overnight at 4 °C with primary antibodies: Anti-ZO-1 (Merck, UK; 1: 1000), anti-p38 MAPK (Cell Signaling Technology, UK; 1: 1000), anti-p44/42 MAPK (Cell Signaling Technology, UK; 1: 1000) and β-actin (Cell Signaling Technology, UK; 1:1000). Excess antibody was removed by washing the membrane in PBS/0.1% Tween 20, and the membranes were incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies (Abcam, UK; 1:2000). Following further washes in PBS/0.1% Tween 20, the signals were developed with ECL reagent (Sigma, UK) and captured by an electronic imaging system (Konica Minolta). Restore™ PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific, UK) was used to re-blot the membranes. The density of protein was quantified using the ImageJ.
2.9. Statistics
All experiments were repeated at least three times. Results are therefore presented as means ± standard deviation (S.D.). Comparisons of two individual experimental groups were performed by unpaired Student's t-test and Mann-Whitney test. For multiple comparisons, nonparametric analysis was performed using one-way ANOVA test with Dunn's test. All the analysis was performed using GraphPad Prism 6 (GraphPad Software, version 6.01, La Jolla, USA). Two-tailed tests were used throughout. The significant differences were considered at P ≤ 0.05.
3. Results
3.1. SP regulates ZO-1 expression in cultured MRMECs, through MAP-kinase inactivation.
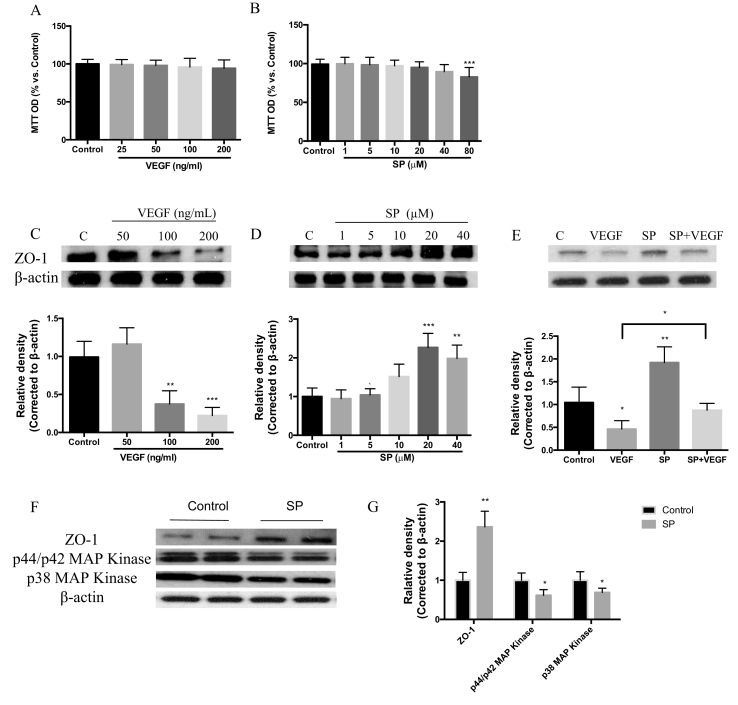
Toward initial understanding of role of SP in regulation of vascular barriers we examined the effects of SP on the tight junction proteins in primary retinal microvascular endothelial cells (MRMECs). We assessed the response of MRMECs following exposure to VEGF, SP or a combination of VEGF and SP. At all concentrations tested, VEGF had no effect on cell viability, while we established that SP demonstrated toxicity at the high dose of 80 (μM) in vitro (Fig. 1A–B), which we therefore omitted from further experiments. VEGF resulted in a dose-dependent reduction of ZO-1 in MRMECs as shown by Western blot, whereas SP at non-toxic doses increased significantly ZO-1 expression in a dose-dependent manner (Fig. 1C–D). Treatment of cultured MRMECs with VEGF led to a significant (52%) reduction of ZO-1 expression as compared to untreated control. Incubation of the cells with SP (20 μM), applied 6 h before VEGF treatment, showed an increase in ZO-1 expression by 43% compared to VEGF alone. (Fig. 1E).
Fig. 1.
Effects of Substance P on the expression of tight junction protein ZO-1 in MRMEC cells. (A, B) Cells were deprived of serum for 24 h and then incubated in the presence of SP or VEGF. MRMEC viability was determined in each group using the MTT assay. Data were normalized by control. (C, D) ZO-1 expression in MRMECs deprived of serum for 24 h, upon treatment with SP (0–40 μM) or VEGF (0–200 ng/ml). (E) ZO-1 expression in MRMECs deprived of serum for 24 h and then incubated in the presence of SP (20 μM), VEGF (100 ng/ml) or SP with VEGF (pre-incubated with SP for 6 h before addition of VEGF). (F, G) p44/p42 MAPK, p38 MAPK and ZO-1 expression in MRMECs treated with SP (20 μM) or VEGF (100 ng/ml). Data represents means ± SD of relative values vs control from 3 independent experiments. *P < 0.05; ***P < 0.001, statistical analysis was performed with one-way ANOVA with Dunn's test for multiple comparisons.
To understand the potential mechanism for the observed SP protective effect, we examined expression of MAP-kinase which is recognised to modulate the cellular expression of tight junction proteins in response to various stimuli [24]. In MRMECs, we showed that SP treatment significantly reduced the phosphorylated forms, p38-MAPK and p44/p42 MAPK. The data supported that treatment of MRMECs with SP led to down-regulation of activated MAPK isoforms, accompanied by increased expression of ZO-1, compared to VEGF alone (Fig. 1F–G).
3.2. SP inhibits VEGF-induced vascular permeability by preventing dissociation of endothelial ZO-1.
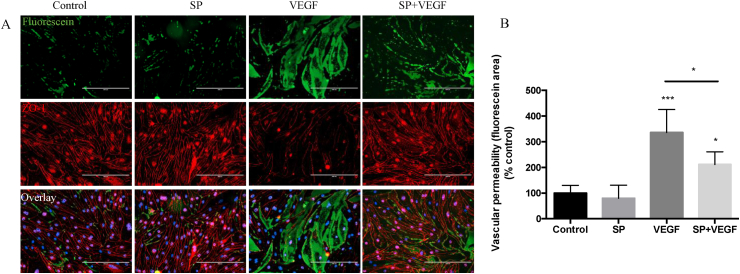
To determine how the SP-mediated increase in ZO-1 expression may influence the barrier function of MRMEC cells, we exploited an in vitro vascular permeability assay. This approach permitted dual assessment of endothelial cell integrity (tight junction expression of ZO-1) and extent of vascular leakage (fluorescence intensity of fluorescent-positive intercellular signal) in response to SP and VEGF treatments.
When MRMEC cells monolayer were treated with VEGF (100 ng/ml, 24 h), a 6-fold increase in fluorescence intensity compared to control (untreated cells) was observed indicating increased permeability. SP partially protected against the increased permeability through preservation of the cell junction integrity demonstrated by attenuation of fluorescence intensity by 36% compared to VEGF alone (p < 0.001) (Fig. 2A–B). Furthermore, ZO-1 immunostaining confirmed the loss of tight junction (TJ) integrity following VEGF treatment, which was again attenuated by SP pre-treatment (Fig. 2A).
Fig. 2.
Substance P inhibits VEGF-induced retinal vascular permeability in vitro. (A) Immunostaining of MRMECs for Fluorescein-streptavidin to detect permeable areas and for ZO-1 to show cell-cell contact; cells deprived of serum for 24 h and incubated in the presence of SP (20 μM), VEGF (100 ng/ml) or SP with VEGF (pre-incubated with SP for 6 h before addition of VEGF) for 24 h (n = 4 per group). (B) Fluorescein intensity associated with DAPI expression was calculated by Image J in all treatment groups. Scale bar, 200 μm. Data represents means ± SD of relative values vs control from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, statistical analysis was performed with one-way ANOVA with Dunn's test for multiple comparisons.
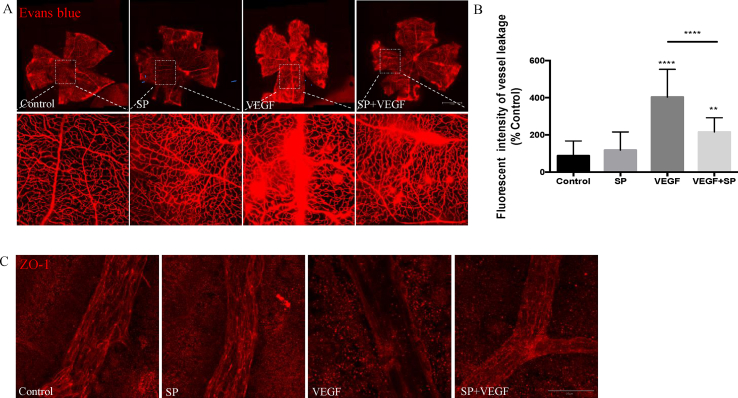
To support the in vitro data, we investigated the protective effect of SP on VEGF-induced vessel leakage in vivo. Groups of C57BL/6J mice were treated with a single intravitreal injection of VEGF, SP, SP+VEGF or vehicle control. Vascular leakage was measured at 48 h post-injection by extravasation of intravascular Evans Blue dye. Flat-mount retinal preparations showed that intravitreal injection of VEGF induced a 70% increase in Evans blue extravasation as determined via fluorescent intensity compared to control. Intravitreal injection of SP together with VEGF suppressed the VEGF-induced leakage in retinal vessels by 47%. By contrast, only minimal vascular leakage was observed in animals receiving normal saline or SP alone (Fig. 3A–B).
Fig. 3.
Substance P prevents vascular leakage in the mouse retina. Groups of C57BL/6J mice received intravitreal injections of SP (20 nmol), VEGF (100 ng), SP in combination with VEGF or vehicle control (n = 6 per group). (A) Representative images 48 h following treatment demonstrate differences in the vasculature (leakage of Evans blue dye, which appears as red). Scale bar = 50 μm. (B) The fluorescent intensity of Evans blue was quantified by Image J. (C) Representative confocal images of retinal vessels immunostained with ZO-1. Scale bar = 20 μm. Data represents means ± SD of relative values vs control from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001, statistical analysis was performed with one-way ANOVA with Dunn's test for multiple comparisons. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Deploying ZO-1 immunofluorescence staining, we assessed the spatial relationship between TJ protein expression and changes in paracellular vascular permeability. Classical staining of intact vasculature exposes the demarcated lateral membranes of retinal microvascular endothelial cells. Localization and integrity of junctional complexes were not altered following exposure to SP or saline alone. However, intravitreal injection of VEGF resulted in a dramatic loss of staining of the ZO-1 junctional protein network. Combined administration of SP with VEGF led to a significant increase in the expression of ZO-1 protein (Fig. 3C). Collectively this data supports a protective effect of SP on retinal vascular integrity.
3.3. SP protects retinal ganglion cell against apoptotic cell death induced by NMDA ex vivo retinal explants
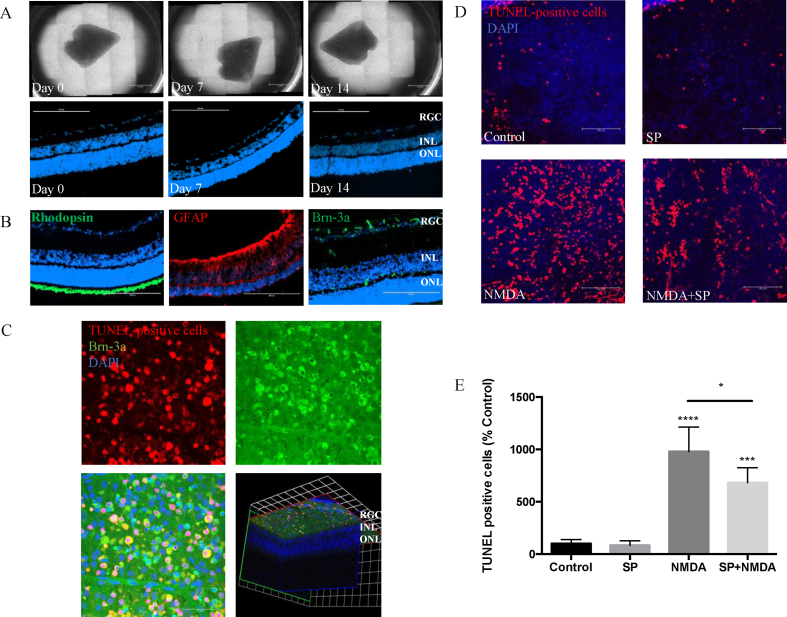
Given the distribution of neurotransmitter SP expression in the inner retina, next we wished to determine whether SP conferred a homeostatic role and delivered a protective effect leading to preservation of retinal microarchitecture and other cell types, specifically retinal ganglion cells. Ex vivo retinal explants cultured for 2 weeks maintained the laminar structure of the retina, with no loss of cells from the outer and inner retinal layers (Fig. 4A). Furthermore, immunostaining demonstrated major cell types were preserved after 2 weeks in culture. Brn-3a+ retinal ganglion cell (RGC) axon bundles were observed in the retinal nerve fiber layer, but also GFAP+ astrocytes and Müller cells and Rhodopsin+ photoreceptors were retained (Fig. 4B).
Fig. 4.
Substance P protects against apoptotic cell death in the retinal explants induced by NMDA. Gross morphology and tissue viability of rat retinal explants, cultured in B27/N2 and maintained to Day 14. (A) Wholemount photographs. Scale bar, 2 mm. Sectioned explants, stained with DAPI to visualize nuclei, demonstrated good survival of cells in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL). Scale bar, 200 μm. (B) Immuno-staining of retinal sections from day 14 explant for different cell markers (Rhodopsin, Brn-3a, GFAP), counterstained with DAPI (blue). Scale bar, 200 μm. (D, E) Representative images showing TUNEL-positive cells in rat explants exposed to NMDA (10 μM), SP (20 μM) or pretreated with SP 6 h before NMDA exposure (n = 6 per group). Scale bar, 100 μm. Quantification of TUNEL positive cells expressed as percentage of control. (C) Representative images of retinal wholemount obtained from eyes exposed to NMDA for 24 h (10 nmol, intravitreal injection), showing TUNEL+ (red), Brn-3a+ (green), and cell nuclei stained with DAPI (blue). 3D image indicates NMDA specifically induced TUNEL+ in GCL. Scale bar, 50 μm. Data represents means ± SD of relative values vs control from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, statistical analysis was performed with one-way ANOVA with Dunn's test for multiple comparisons. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To evaluate the role of SP on RGC health, we employed the well-established NMDA-induced model of excitotoxic damage in retinal explants [25]. Quantification of TUNEL+ cells shows NMDA treatment results in a 10-fold increase in the number of apoptotic cells. Pre-treatment of the explant with SP for 6 h prior to NMDA exposure significantly reduced the cytotoxic effects, as shown by a reduction in the number of TUNEL+ cells by 27% compared to NMDA alone, SP treatment alone did not lead to any apoptosis or retinal toxicity, with comparable number of TUNEL+ cells to control explants (Fig. 4D and E). To confirm with an in vivo correlate we analyzed wholemounts obtained from eyes exposed to NMDA for 24 h (10 nmol, intravitreal injection) where upon following incubation with NMDA for 24 h RGC NMDA-induced excitotoxic damage of RGC cells undergoing apoptosis was noted. (Fig. 4C).
3.4. SP protects ganglion cells from NMDA excitotoxicity in vivo
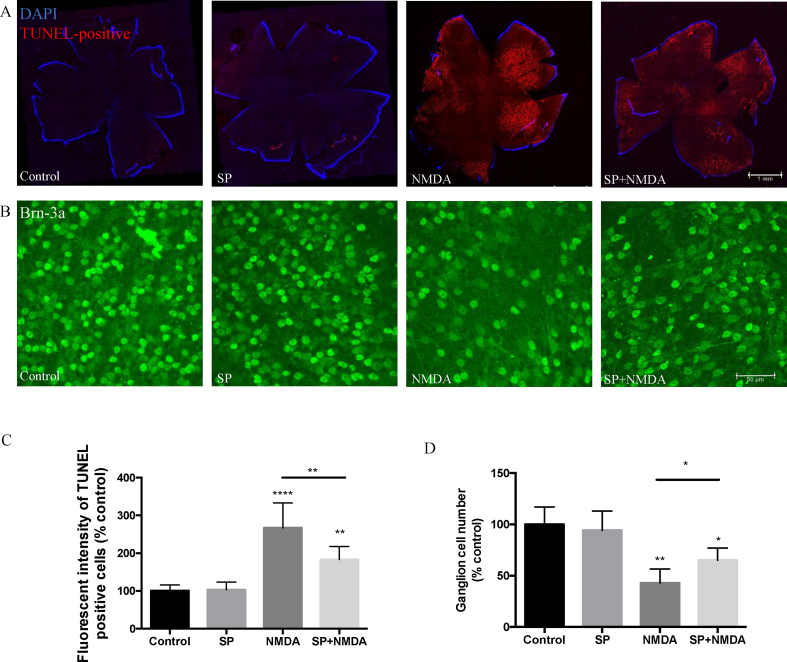
To validate the protective effect of SP in suppressing NMDA-induced RGC apoptosis, we assessed in vivo efficacy. In these experiments, intravitreal administration of NMDA (10 nmol) was used to induce neuronal excitotoxic damage in the eyes of mice. A dose-response was undertaken previously to ascertain the optimal dosing of SP (10 nmol–40 nmol). We found that 20 nmol of SP demonstrated the optimal protective effect in NMDA-induced ganglion cell apoptosis model (Supplemental Figs. 1A–B). The extent of NMDA-induced RGC apoptosis was quantified by immuno-staining (TUNEL and Brn-3a positivity) of ex vivo retinal whole-mounts (Fig. 5A–D). We observed a significantly higher fluorescence in the NMDA-injected eyes than PBS-injected contralateral eyes. Combined administration of SP and NMDA, resulted in a significant reduction (34%) in the fluorescence intensity of TUNEL+ cells in the retina as compared to NMDA alone. Of note, no significant differences were detected between the SP-injected experimental eyes and the respective control (Fig. 5A, C).
Fig. 5.
Substance P protects neuronal cell death induced by NMDA in murine retina. Representative images of retinal whole-mounts prepared for TUNEL assay (A) and Brn-3a immunostaining (n = 6 per group). (B) 24 h following a single intravitreal injection of SP (20 nmol), NMDA (10 nmol), SP combined NMDA or vehicle control (n = 6 per group). (C) The fluorescence intensity of TUNEL+ was quantified by Image J, showing that SP decreases the intensity of apoptotic retinal ganglion cells induced by NMDA. (D) Brn-3a+ were counted by Image J, showing that SP protects against the loss of retinal ganglion cells. Data represents means ± SD of relative values vs control from 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, statistical analysis was performed with one-way ANOVA with Dunn's test for multiple comparisons.
The number of Brn-3a+ cells was also evaluated from the retinal flat mount, and showed that NMDA reduced decreased the number of Brn-3a+ cells to 45% of control. Conversely, after SP was injected along with NMDA injection in the mouse vitreous, there was a statistically significant protective effect as larger number of Brn-3a+ cells remained (from 45% to 68% of control) (Fig. 5B, D).
4. Discussion
In this study, we demonstrated a protective role of SP against NMDA-induced retinal ganglion cell death in vitro and in vivo. Furthermore, our results revealed that SP suppresses VEGF-induced increase in vascular permeability. These findings suggest that modulating SP levels can be a potential new avenue of treatments to preserve the neurovascular unit in retinal neurodegenerative and vascular diseases.
Regulation of vascular permeability plays a major role in the pathophysiology of neovascular diseases, with numerous VEGF-inhibitory strategies to reduce retinal vascular permeability [26]. The integrity of endothelial cell–cell junctions and vascular barrier function is regulated by a series of adhesion molecules that form three types of junctions, tight, gap and adherens [27,28,29]. Among the various protein components of tight junctions, ZO-1 is a phosphoprotein that participates in multiple protein–protein interactions and has been implicated in the regulation of tight junction integrity [30,31,32]. Furthermore, VEGF disrupts tight junctions by altering phosphorylation of ZO-1 and occludin through a Src-dependent pathway [33,34]. In our experiments, and in agreement with previous studies, we were also able to demonstrate the inhibitory effect of VEGF on the expression of tight junction protein ZO-1 in endothelial cells. However here, we demonstrated that VEGF and SP have opposite effects on the expression of ZO-1 in the MRMEC monolayers. SP increased ZO-1 expression, which supported our initial hypothesis that SP maintains the vascular integrity.
To further assess the mechanism of action of SP, we assessed vascular permeability both in vitro and in vivo. We observed that SP can attenuate VEGF-induced vascular leakage of microvascular retinal endothelial cell monolayer. These results suggest that SP improves barrier function to maintain homeostasis of retinal endothelial cells. Our results support other data demonstrating that SP up-regulates the tight-junction protein ZO-1 in various cell types, including epithelial cells [35], and also promotes wound healing and tissue integrity [36,37].
During pathological conditions, many components may interplay to determine either death or survival of RGCs [11], but there are many mechanisms for RGC death that are shared among different diseases [38]. Changes in electrical activity [39] and growth factor deprivation [40] likely contribute to RGC death after optic nerve injury, while other mechanisms, such as excitotoxicity [41], ischemia and hypoxia [7], oxidative stress [42] and abnormal protein trafficking, induce RGC death. Xie's [14] results from studies in diabetic rats showed that the levels of SP in the retina and serum were significantly reduced, with an associated increase in apoptosis and caspase-3 activity in the retina. More importantly, restoration of endogenous SP paralleled the inhibition of the apoptosis of the RGC and the caspase-3 activity in the diabetic animals. This indicates its potential use in the development of therapeutic strategies to fight retinal disease.
Here we extend the known beneficial effect to show that SP exerts neuroprotective properties on RGC in NMDA-induced neuro-excitotoxicity apoptotic cell death both in vitro and in an animal model. For SP neuroprotective property we speculated that SP exerts its protective function mainly through MAPK signaling, as MAPK has been shown to regulate tight junction function. In other tissues, p38 MAPK is activated by tumor necrosis factor α and interferon γ, leading to down-regulation of occludin, ZO-1 and claudin-2, which contributes to the increase in paracellular permeability [43]. In our experiments, we similarly found that both phosphorylated forms of MAPK (p38 and p44/42) were reduced upon SP treatment, alongside ZO-1 upregulation, supporting that SP inactivated MAPK to maintain vascular integrity. Furthermore, several cytokines such as IL-10 and IL-12 have been observed to mediate partially the effect of SP in several organs/tissues [44,45]. There appear to be opposing data on the role of SP on endothelial cells. For example, SP has been reported to regulate angiogenesis directly by inducing endothelial cells to produce nitric oxide [46], or indirectly via its interactions with mast cells and granulocytes [47], while VEGF blockade does not abolish the proangiogenic property of SP [48]. On the other side, in support of our findings, SP can reportedly prevent laser-induced retinal degeneration in vivo, by suppressing inflammation and reducing neovascularisation [49]. These differences are likely attributable to different animal models studied, the type and kinetics of retinal insult, selective mechanisms potentially affecting only some subtypes of RGC or experimental methods that select some types of RGC for observation over others (e.g. central retina versus peripheral) [11]. Nevertheless, as we continue to decode the molecular mechanisms of SP, other important potential therapies may be incorporated into a multifaceted approach. It will be important moving forward to transition as many of these are possible into well-structured clinical trials in humans.
In conclusion, we have shown that SP is neuroprotective, suppressing apoptosis of retinal ganglion cells induced by NMDA, and SP also protects against VEGF-induced microvascular leakage. Both effects demonstrate the significant role that the neuropeptide transmitter plays in maintaining neurovascular unit homeostasis.
Disclosure statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
This work was supported by The Dunhill Medical Trust [grant number: R474/0216] and China Sponsorship Council.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yexcr.2019.04.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ames A. Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can. J. Physiol. Pharmacol. 1992;70(Suppl):S158–S164. doi: 10.1139/y92-257. http://www.ncbi.nlm.nih.gov/pubmed/1295666 [DOI] [PubMed] [Google Scholar]
- 2.Anderson B., Saltzman H.A. Retinal oxygen utilization measured by hyperbaric blackout. Arch. Ophthalmol. 1964;72 doi: 10.1001/archopht.1964.00970020794009. http://www.ncbi.nlm.nih.gov/pubmed/14205438 (Chicago, Ill. 1960) 792–5. [DOI] [PubMed] [Google Scholar]
- 3.Kaur C., Rathnasamy G., Foulds W.S., Ling E.-A. Cellular and molecular mechanisms of retinal ganglion cell death in hypoxic-ischemic injuries. J. Neurol. Exp. Neurosci. 2015 [Google Scholar]
- 4.Petrella R.J., Blouin J., Davies B., Barbeau M. Prevalence, demographics, and treatment characteristics of visual impairment due to diabetic macular edema in a representative Canadian cohort. J. Ophthalmol. 2012;2012:159167. doi: 10.1155/2012/159167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson R. University of Utah Health Sciences Center; 1995. Visual Responses of Ganglion Cells.http://www.ncbi.nlm.nih.gov/pubmed/21413404 [PubMed] [Google Scholar]
- 6.Laha B., Stafford B.K., Huberman A.D. Regenerating optic pathways from the eye to the brain. Science. 2017;356:1031–1034. doi: 10.1126/science.aal5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur C., Foulds W.S., Ling E.-A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008;2:879–889. doi: 10.2147/opth.s3361. http://www.ncbi.nlm.nih.gov/pubmed/19668442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupp-Montpetit K., Moody M.L. Visual loss as a complication of nonophthalmologic surgery: a review of the literature. AANA J. (Am. Assoc. Nurse Anesth.) 2004;72:285–292. http://www.ncbi.nlm.nih.gov/pubmed/15354918 [PubMed] [Google Scholar]
- 9.Costa V.P., Harris A., Stefánsson E., Flammer J., Krieglstein G.K., Orzalesi N., Heijl A., Renard J.-P., Serra L.M. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog. Retin. Eye Res. 2003;22:769–805. doi: 10.1016/s1350-9462(03)00064-8. http://www.ncbi.nlm.nih.gov/pubmed/14575724 [DOI] [PubMed] [Google Scholar]
- 10.Tezel G., Wax M.B. Hypoxia-inducible factor 1α in the glaucomatous retina and OpticNerve head. Arch. Ophthalmol. 2004;122:1348. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- 11.Corredor R.G., Goldberg J.L. Retinal ganglion cell life and death – mechanisms and implications for Ophthalmology. Eur. Ophthalmic Rev. 2009;03:109. [Google Scholar]
- 12.Duh E.J., Sun J.K., Stitt A.W. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashaghi A., Marmalidou A., Tehrani M., Grace P.M., Pothoulakis C., Dana R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016;73:4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J.-H., Guo Z., Zhang T., Meng X.X., Xie L.-S. Restoration of endogenous substance P is associated with inhibition of apoptosis of retinal cells in diabetic rats. Regul. Pept. 2013;187:12–16. doi: 10.1016/j.regpep.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Bagnoli P., Dal Monte M., Casini G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol. 2003;18:1219–1242. doi: 10.14670/HH-18.1219. [DOI] [PubMed] [Google Scholar]
- 16.Denis P., Nordmann J.P., Elena P.P., Dussaillant M., Saraux H., Lapalus P. Physiological roles of dopamine and neuropeptides in the retina. Fundam. Clin. Pharmacol. 1993;7:293–304. doi: 10.1111/j.1472-8206.1993.tb00243.x. http://www.ncbi.nlm.nih.gov/pubmed/8406293 [DOI] [PubMed] [Google Scholar]
- 17.D'Alessandro A., Cervia D., Catalani E., Gevi F., Zolla L., Casini G. Protective effects of the neuropeptides PACAP, substance P and the somatostatin analogue octreotide in retinal ischemia: a metabolomic analysis. Mol. Biosyst. 2014;10:1290. doi: 10.1039/c3mb70362b. [DOI] [PubMed] [Google Scholar]
- 18.Ziche M., Morbidelli L., Pacini M., Geppetti P., Alessandri G., Maggi C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990;40:264–278. doi: 10.1016/0026-2862(90)90024-l. http://www.ncbi.nlm.nih.gov/pubmed/1701206 [DOI] [PubMed] [Google Scholar]
- 19.Yang J., Meng X., Xie L., Guo Z. Acute myocardial ischemia up-regulates substance P in the retina of rats. Neurosci. Lett. 2008;443:218–222. doi: 10.1016/j.neulet.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 20.Troger J., Neyer S., Heufler C., Huemer H., Schmid E., Griesser U., Kralinger M., Kremser B., Baldissera I., Kieselbach G. Substance P and vasoactive intestinal polypeptide in the streptozotocin-induced diabetic rat retina. Investig. Ophthalmol. Vis. Sci. 2001;42:1045–1050. http://www.ncbi.nlm.nih.gov/pubmed/11274084 [PubMed] [Google Scholar]
- 21.Kunt T., Forst T., Schmidt S., Pfützner A., Schneider S., Harzer O., Löbig M., Engelbach M., Goitom K., Pohlmann T., Beyer J. Serum levels of substance P are decreased in patients with type 1 diabetes. Exp. Clin. Endocrinol. Diabetes. 2000;108:164–167. doi: 10.1055/s-2000-7738. [DOI] [PubMed] [Google Scholar]
- 22.Cai J., Wu L., Qi X., Shaw L., Li Calzi S., Caballero S., Jiang W.G., Vinores S.A., Antonetti D., Ahmed A., Grant M.B., Boulton M.E. Placenta growth factor-1 exerts time-dependent stabilization of adherens junctions following VEGF-induced vascular permeability. PLoS One. 2011;6:e18076. doi: 10.1371/journal.pone.0018076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Johnson T.V., Martin K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Investig. Opthalmology Vis. Sci. 2008;49:3503. doi: 10.1167/iovs.07-1601. [DOI] [PubMed] [Google Scholar]
- 24.Dörfel M.J., Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kugler M., Schlecht A., Fuchshofer R., Kleiter I., Aigner L., Tamm E.R., Braunger B.M. Heterozygous modulation of TGF-beta signaling does not influence Müller glia cell reactivity or proliferation following NMDA-induced damage. Histochem. Cell Biol. 2015;144:443–455. doi: 10.1007/s00418-015-1354-y. [DOI] [PubMed] [Google Scholar]
- 26.Keane P.A., Sadda S.R. Development of anti-VEGF therapies for intraocular use: a guide for clinicians. J. Ophthalmol. 2012;2012:483034. doi: 10.1155/2012/483034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimi N. Defenders and challengers of endothelial barrier function. Front. Immunol. 2017;8:1847. doi: 10.3389/fimmu.2017.01847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. http://www.ncbi.nlm.nih.gov/pubmed/8276896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. http://www.ncbi.nlm.nih.gov/pubmed/3048970 [DOI] [PubMed] [Google Scholar]
- 30.Itoh M., Nagafuchi A., Yonemura S., Kitani-Yasuda T., Tsukita S., Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J. Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. http://www.ncbi.nlm.nih.gov/pubmed/8486731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willott E., Balda M.S., Fanning A.S., Jameson B., Van Itallie C., Anderson J.M. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc. Natl. Acad. Sci. U.S.A. 1993;90 doi: 10.1073/pnas.90.16.7834. http://www.ncbi.nlm.nih.gov/pubmed/8395056 7834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. http://www.ncbi.nlm.nih.gov/pubmed/10601346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonetti D.A., Barber A.J., Hollinger L.A., Wolpert E.B., Gardner T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 34.Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta Biomembr. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Ko J.-A., Yanai R., Nishida T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett. 2009;583:2148–2153. doi: 10.1016/j.febslet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Leal E.C., Carvalho E., Tellechea A., Kafanas A., Tecilazich F., Kearney C., Kuchibhotla S., Auster M.E., Kokkotou E., Mooney D.J., LoGerfo F.W., Pradhan-Nabzdyk L., Veves A. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015;185:1638–1648. doi: 10.1016/j.ajpath.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.V Delgado A., McManus A.T., Chambers J.P. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp. Biol. Med. 2005;230:271–280. doi: 10.1177/153537020523000407. http://www.ncbi.nlm.nih.gov/pubmed/15792949 [DOI] [PubMed] [Google Scholar]
- 38.Tezel G., Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Investig. Opthalmology Vis. Sci. 2004;45:4049. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heck N., Golbs A., Riedemann T., Sun J.-J., Lessmann V., Luhmann H.J. Activity-dependent regulation of neuronal apoptosis in neonatal mouse cerebral cortex. Cerebr. Cortex. 2008;18:1335–1349. doi: 10.1093/cercor/bhm165. [DOI] [PubMed] [Google Scholar]
- 40.Chau R.M., Ren F., Huang W.Q. Programmed cell death of neonatal rat retinal ganglion cells due to turn-off expression of a novel 30-kD trophic factor and/or the lack of this factor supplied from the superior colliculus. Ann. N. Y. Acad. Sci. 1992;663:466–470. doi: 10.1111/j.1749-6632.1992.tb38704.x. http://www.ncbi.nlm.nih.gov/pubmed/1336334 [DOI] [PubMed] [Google Scholar]
- 41.Massoll C., Mando W., Chintala S.K. Excitotoxicity upregulates SARM1 protein expression and promotes Wallerian-like degeneration of retinal ganglion cells and their axons. Investig. Ophthalmol. Vis. Sci. 2013;54:2771–2780. doi: 10.1167/iovs.12-10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog. Retin. Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrick D., Leone A., Shellenberger J., Dudowicz K., King J. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol. 2006;6:2. doi: 10.1186/1472-6793-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho W.Z., Kaufman D., Uvaydova M., Douglas S.D. Substance P augments interleukin-10 and tumor necrosis factor-alpha release by human cord blood monocytes and macrophages. J. Neuroimmunol. 1996;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. http://www.ncbi.nlm.nih.gov/pubmed/8982105 [DOI] [PubMed] [Google Scholar]
- 45.Kincy-Cain T., Bost K.L. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 1997;158:2334–2339. http://www.ncbi.nlm.nih.gov/pubmed/9036982 [PubMed] [Google Scholar]
- 46.Ziche M., Morbidelli L., Masini E., Amerini S., Granger H.J., Maggi C.A., Geppetti P., Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaik-Dasthagirisaheb Y.B., Varvara G., Murmura G., Saggini A., Potalivo G., Caraffa A., Antinolfi P., Tetè S., Tripodi D., Conti F., Cianchetti E., Toniato E., Rosati M., Conti P., Speranza L., Pantalone A., Saggini R., Theoharides T.C., Pandolfi F. Vascular endothelial growth factor (VEGF), mast cells and inflammation. Int. J. Immunopathol. Pharmacol. 2013;26:327–335. doi: 10.1177/039463201302600206. [DOI] [PubMed] [Google Scholar]
- 48.Katsanos G.S., Anogeianaki A., Orso C., Tete S., Salini V., Antinolfi P.L., Sabatino G. Impact of substance P on cellular immunity. J. Biol. Regul. Homeost. Agents. 2008;22 http://www.ncbi.nlm.nih.gov/pubmed/18597700 (n.d.) 93–8. [PubMed] [Google Scholar]
- 49.Hong H.S., Kim S., Nam S., Um J., Kim Y.H., Son Y. Effect of substance P on recovery from laser-induced retinal degeneration. Wound Repair Regen. 2015;23:268–277. doi: 10.1111/wrr.12264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.