Abstract
Background
Hypnotics and general anaesthetics impair memory by altering hippocampal synaptic plasticity. We recently reported on a neurosteroid analogue with potent hypnotic activity [(3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; 3β-OH], which does not cause developmental neurotoxicity in rat pups. Here, we investigated the effects of 3β-OH on neuronal excitability in the subiculum, the major output structure of the hippocampal formation, and synaptic plasticity at two key hippocampal synapses in juvenile rats.
Methods
Biophysical properties of isolated T-type calcium currents (T-currents) in the rat subiculum were investigated using acute slice preparations. Subicular T-type calcium channel (T-channel) subtype mRNA expression was compared using qRT–PCR. Using electrophysiological recordings, we examined the effects of 3β-OH and an endogenous neuroactive steroid, allopregnanolone (Allo), on T-currents and burst firing properties of subicular neurones, and on the long-term potentiation (LTP) in CA3-CA1 and CA1-subiculum pathways.
Results
Biophysical and molecular studies confirmed that CaV3.1 channels represent the dominant T-channel isoform in the subiculum of juvenile rats. 3β-OH and Allo inhibited rebound burst firing by decreasing the amplitude of T-currents in a voltage-dependent manner with similar potency, with 30–80% inhibition. Both neurosteroids suppressed LTP at the CA1-subiculum, but not at the CA3-CA1 Schaffer collateral synapse.
Conclusions
Neurosteroid effects on T-channels modulate hippocampal output and provide possible molecular mechanisms for the amnestic action of the novel hypnotic 3β-OH. Effects on T-channels in the subiculum provide a novel target for amnestic effects of hypnotics.
Keywords: amnesia, general anaesthetic, hippocampus, neurosteroid, T-type calcium channels, synaptic plasticity, subiculum
Editor's key points.
-
•
The role of T-type calcium channels as targets for the effects of general anaesthetics is unclear.
-
•
The novel anaesthetic neurosteroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) reduced burst firing by decreasing the amplitude of T-currents and impaired long-term potentiation in the rat hippocampus.
-
•
Modulation of hippocampal output through effects on hippocampal synaptic transmission provides a possible molecular mechanism for the amnestic action of the novel hypnotic 3β-OH.
Sedatives, hypnotics, and general anaesthetics cause a temporary loss of consciousness and memory loss (amnesia). While loss of consciousness may be because of effects on various subcortical and cortical targets, it is believed that temporary memory impairment originates within the hippocampal formation. These agents produce strong suppression of long-term potentiation (LTP) at the CA3-CA1 synapse,1, 2, 3 a leading cellular model of synaptic plasticity and memory processing.4 Both inhalation and injectable anaesthetics impair hippocampal dependent learning and memory at concentrations that are often below those needed for their hypnotic effect.5, 6, 7
The flow of information between hippocampus and numerous subcortical and cortical structures is strongly modulated by the subiculum, the major output structure of the hippocampal formation. Most subicular neurones are capable of burst firing,8, 9 a high-frequency (>100 Hz) barrage of action potentials, which appears to be an important means of distributing information across brain networks.10 The CaV3.1 isoform of T-type calcium channels (T-channels) contributes significantly to burst firing of subicular neurones, and T-channels are important regulators of neuronal excitability and synaptic plasticity in the subiculum.11
At a molecular level, most general anaesthetics act on N-methyl-D-aspartate (NMDA)-type glutamate, gamma-aminobutyric acid type A (GABAA), or both receptors,12 but also have underappreciated effects on other targets, such as voltage-gated calcium channels.13 For example, isoflurane blocks T-channels in thalamocortical neurones at clinically relevant concentrations.14 We recently described a neurosteroid analogue with potent hypnotic activity in rat pups ([3β,5β,17β]-3-hydroxyandrostane-17-carbonitrile; 3β-OH) that inhibits neuronal T-currents and inhibits glutamate-mediated synaptic transmission presynaptically, but unlike most other neurosteroids, it lacks effects on postsynaptic GABAA currents.15 In the present study, we investigated the effects of 3β-OH and an endogenous neuroactive steroid, allopregnanolone (Allo), on burst firing properties of subicular neurones and synaptic plasticity at two important hippocampal synapses in juvenile rats. We found that both neurosteroids strongly affected burst firing by decreasing T-currents and suppressed LTP at the CA1-subiculum, but not at CA3-CA1 synapses. Thus, T-channels in the subiculum are a potentially important target for the amnestic effects of anaesthetics.
Methods
Drugs
The neurosteroid analogue 3β-OH was synthesised as described,15 whereas Allo was obtained from Sigma Aldrich Chemical Co. (#P-8887, Saint Louis, MO, USA). Purification of 3β-OH was by flash column chromatography on silica gel; preparations showed a single component on thin layer chromatography on silica gel plates. Both compounds were dissolved in 100% dimethyl sulfoxide (Sigma Aldrich Chemical Co.) as 3 or 10 mM stock solutions, and freshly diluted to the final concentrations in the external solution at the time of electrophysiology experiments. For the loss of righting reflex (LORR) study, 3β-OH was solubilised in 2-hydroxypropyl-β-cyclodextrin 15% (Santa-Cruz Biotechnology, Santa Cruz, CA, USA).
Animals
Juvenile male and female Sprague-Dawley rats (P15–P32) were used. For patch-clamp experiments, we used P15–P28 rats, and for LTP experiments we used P28–P32 rats, in order to make a direct comparison with our previous studies of a T-channel antagonist that selectively inhibits all three subtypes without affecting other classes of voltage-gated Ca2+ channels (TTA-P2; 3,5-dichloro-N-[1-{2,2-dimethyl-tetrahydro-pyran-4-ylmethyl}-4-fluoro-piperidin-4-ylmethyl]-benzamide).11 Analysis of T-type calcium channel isoform mRNA expression was performed on subicular tissue of P21–P22 rats. We used P20–P29 rats to assess the LORR after i.p. administration of 3β-OH. Animals were housed within accredited animal facilities according to protocols approved by the University of Colorado Anschutz Medical Campus, the University of Virginia, or the Washington University Animal Studies Committee. Treatments of rats adhered to guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimise animal suffering and the number of animals necessary to produce reliable scientific data. Details of specific experimental procedures are provided in the online Supplementary material. Data are shown as mean (standard error of mean).
Results
Biophysical properties of T-currents in the subiculum
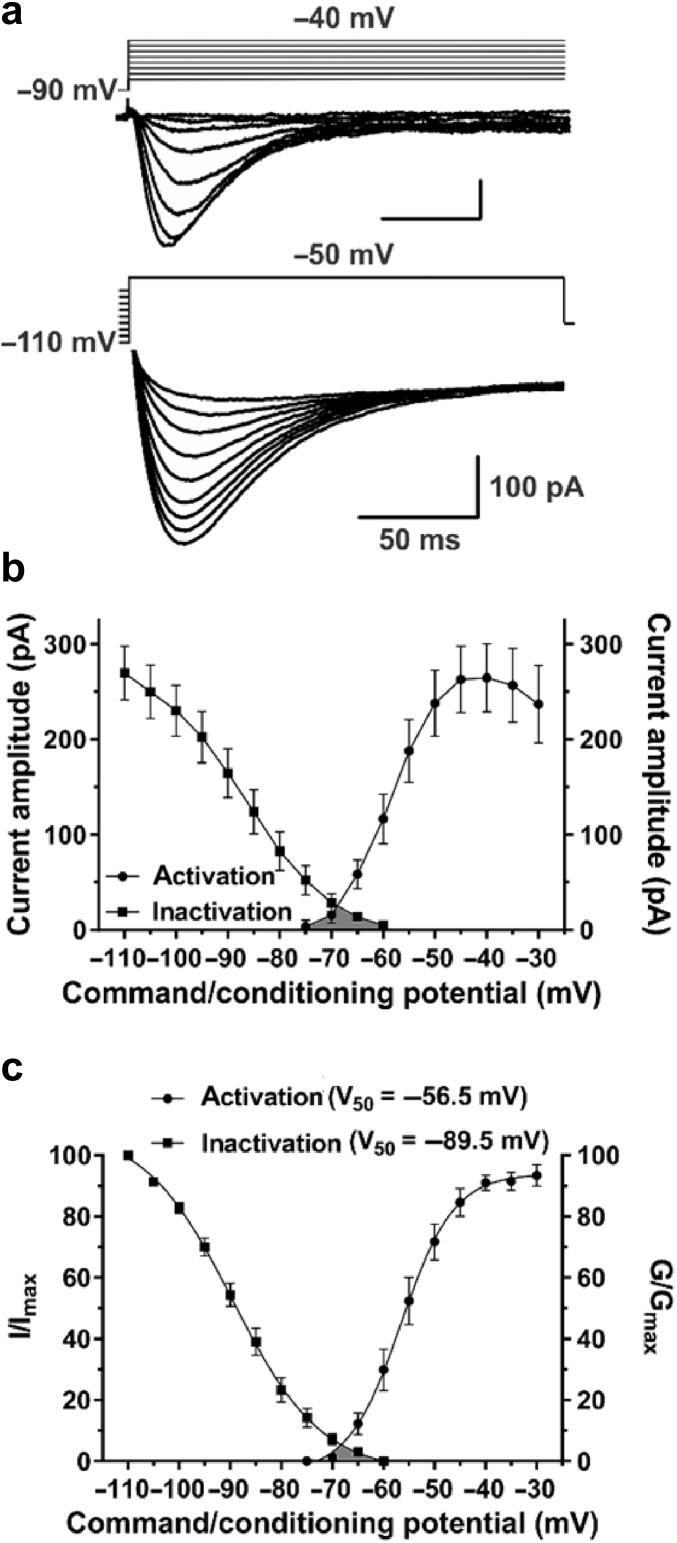
We previously described isolated T-currents in the subiculum of rat pups.15 Here, we investigated the biophysical properties of T-currents in juvenile rats when dendritic arborisation is well underway.17 The voltage dependence of T-current activation was measured using typical I-V relationships (Vh=−90 mV, Vt=−75 to −30 mV in 5 mV increments), whereas an independent steady-state protocol was used to measure the voltage dependence of T-current inactivation (Fig. 1a). As shown in Fig. 1b, T-current amplitudes were: 270 (28) pA after the long prepulse at −110 mV (n=19 neurones, 10 rats), and 264 (36) pA (n=15 neurones, nine rats) at the peak of activation (Vt=−40 mV). I/Imax and G/Gmax curves are shown on the same graph (Fig. 1c), along with corresponding V50 values (n=20 and 14 neurones, respectively). The average slope of activation curves in P15–P23 rats [4.5 (0.5)] was almost identical to that reported in rat pups11; however, the average V50 value was significantly hyperpolarised by ∼4 mV (P=0.043, two-tailed t-test). Overlap of the activation and inactivation curves revealed a ‘window’ current (ITwindow), shown in grey shaded areas in Fig. 1b and c. These currents result from a small fraction of T-channels that are persistently available near the resting membrane potential, which in turn allows a basal inward flux of calcium ions.18
Fig 1.
Biophysical properties of T-type calcium currents (T-currents) in the juvenile rat subiculum. (a) Average T-current I-V traces from representative subicular neurones for Vt from −75 to −40 mV (the peak of activation) from Vh of −90 mV in 5 mV increments (top), and traces generated using a double-pulse protocol with 3.6-s prepulses to variable voltages (from −110 to −70 mV in 5 mV increments) and test potential of −50 mV (bottom); not all data points are displayed. (b) Average current amplitudes of the voltage dependence for activation and steady-state inactivation. (c) The voltage dependence of activation (G/Gmax) and steady-state inactivation (I/Imax) is shown with V50 values noted in the parentheses. Note the ‘window’ current depicted by the grey area below the intersection of the curves. Results are expressed as mean (standard error of mean).
mRNA expression of CACNA1G, CACNA1H, and CACNA1I in the rat subiculum
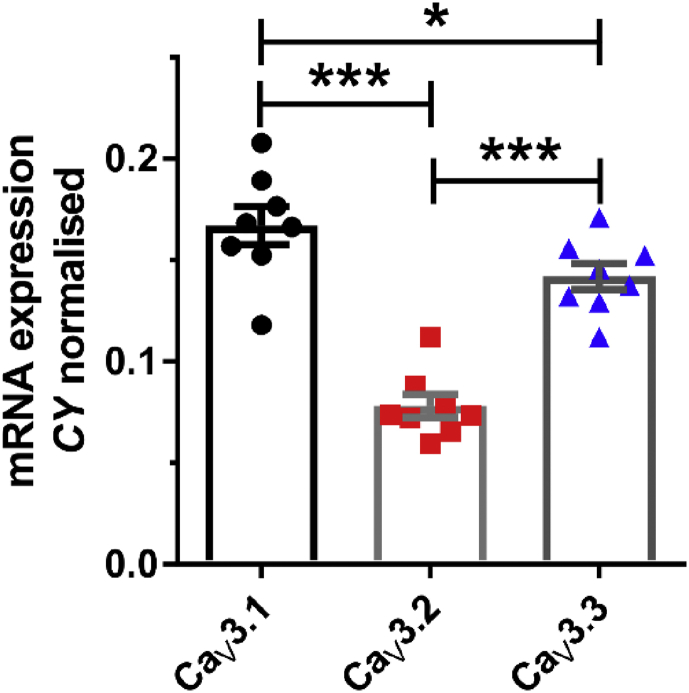
The three known isoforms of T-type calcium channels (CaV3.1, CaV3.2, and CaV3.3) are encoded by CACNA1G, CACNA1H, and CACNA1I genes, respectively.19 Based on our previous findings of mRNA expression levels in rat pups and insensitivity of burst firing to low concentrations of Ni2+,11 and available in situ hybridisation data,20 it appears that the CaV3.1 isoform constitutes the largest portion of T-channels in the subiculum. To investigate whether this is also the case in juvenile rats, we used qRT–PCR to assess mRNA expression of all three genes encoding different T-channel isoforms in the subiculum of P21–P22 rats. Indeed, CaV3.1 was the dominant T-channel isoform at this age as well (Fig. 2; P<0.001 vs CaV3.2 and P=0.025 vs CaV3.3; one-way analysis of variance (ANOVA) followed by Holm-Sidak's test; n=8 rats). In contrast to our findings in rat pups, relatively high CaV3.3 isoform mRNA expression was observed in juvenile rats (P<0.001 vs CaV3.2), which is likely related to the presence of more extensive dendritic branching in neurones from juvenile animals.
Fig 2.
mRNA expression of all three T-type calcium channel isoforms in the juvenile rat subiculum. qRT–PCR analysis on excised subicular tissue revealed the dominant expression of CaV3.1, followed by CaV3.3 isoform. Results are expressed as mean (standard error of mean). *P<0.05 and †P<0.001, one-way analysis of variance followed by Holm-Sidak's post hoc test.
Hypnotic effect of 3β-OH in juvenile rats
The neurosteroid analogue 3β-OH inhibits T-currents and produces potent hypnotic effects in rat pups.15 This finding was corroborated in the present study using juvenile rats. The ED50 for the LORR obtained when the compound was injected i.p. was 16.0 (0.4) mg kg−1, with a slope of 3.4 (0.4). At a dose of 50 mg kg−1 i.p., 3β-OH had an average onset of action (time to LORR) of 14.9 (1.8) min, whereas the average duration of LORR was 108 (19) min (data not shown). In juvenile rats, this ED50 was five-fold higher than in rat pups,15 which may reflect differences in pharmacokinetics, in the maturation of thalamocortical circuitry, or both.21 All animals remained euthermic during LORR and resumed normal locomotor activity after effects of 3β-OH dissipated (data not shown). These results clearly demonstrate that 3β-OH is an efficacious and apparently safe hypnotic in juvenile rats.
Effects of 3β-OH on T-currents in subicular neurones
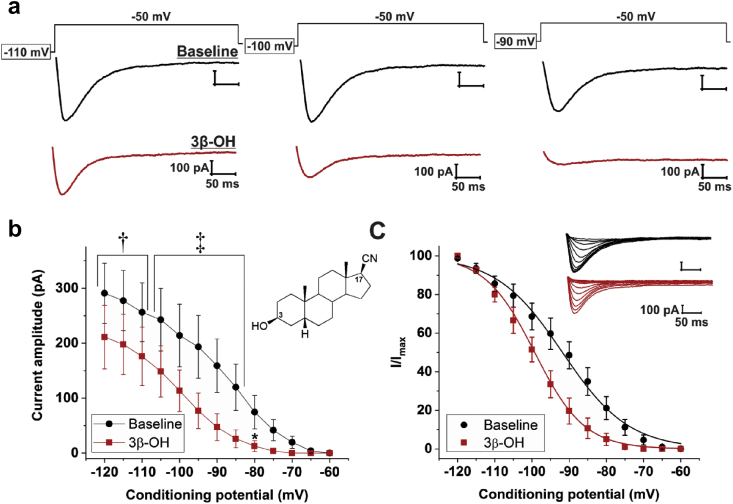
The effects of 3β-OH on T-currents in juvenile rats were tested using a standard steady-state inactivation protocol. Original traces from a representative subicular neurone are presented in Fig. 3a. 3β-OH significantly decreased T-current amplitude across the range of conditioning potentials, as assessed by two-way repeated measures ANOVA followed by Holm-Sidak's multiple comparisons test (Fig. 3b; interaction: F[12,60]=3.83, P<0.001; post hoc: P=0.002 for potentials from −120 to −110 mV, P<0.001 for potentials from −105 to −85 mV, and P=0.017 for −80 mV; n=6 neurones, three rats). This effect was strongly voltage-dependent, ranging from ∼30% inhibition at −120 mV to ∼80% inhibition at the −80 mV prepulse. Addition of 3β-OH 1 μM produced a significant hyperpolarising shift in V50 of ∼9 mV (Fig. 3c; −99.5 [2.0] mV vs −90.5 [3.0] mV; P=0.013; paired t-test). Thus, 3β-OH is a potent and effective T-channel antagonist, confirming our previous results in dorsal root ganglion neurones,22 nucleus reticularis thalami,23 and the subiculum from rat pups.15
Fig 3.
(3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile blocks T-type calcium currents (T-currents) in subicular neurones. (a) Original T-current traces from a representative subicular neurone at baseline (black trace) and after application of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) 1 μM (red trace), generated using a double-pulse protocol with three different 3.6-s prepulses: −110 mV (left), −100 mV (middle), and −90 mV (right). (b) Average current amplitudes exhibit a significant decrease after the application of 3β-OH 1 μM across different conditioning potentials. The inset shows the chemical structure of 3β-OH, a neuroactive steroid analogue based on a progesterone scaffold with OH and CN groups in a beta-conformation at positions 3 and 17, respectively. (c) Steady-state inactivation curves (I/Imax) display a leftward shift upon the application of 3β-OH. Inset shows original traces from a representative subicular neurone at baseline (black trace) and after 3β-OH (red trace), generated using our standard steady-state inactivation protocol. Results are expressed as mean (standard error of mean). *P<0.05, †P<0.01, and ‡P<0.001, two-way repeated measures analysis of variance followed by Holm-Sidak's post hoc test.
Effects of 3β-OH on rebound burst firing in subicular neurones
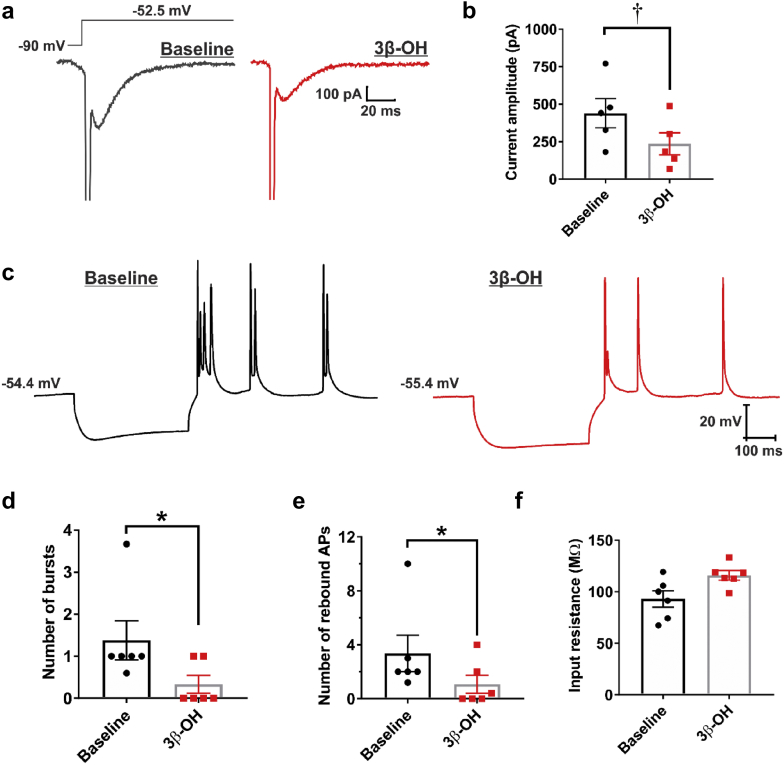
Hyperpolarising stimuli, such as inhibitory postsynaptic potentials, can deinactivate T-channels and cause ‘rebound’ burst firing of thalamocortical neurones in an oscillatory manner.24 This phenomenon is also observed in a large population of pyramidal neurones of the subiculum.8, 11 We tested whether 3β-OH 3 μM affects rebound burst firing by blocking T-channels in the subiculum of juvenile rats. Using voltage-clamp recordings, we investigated the effects of 3β-OH on calcium currents (mostly consisting of T-currents) evoked from −90 mV (Fig. 4a. 3β-OH reduced the maximal calcium current by ∼50% (Fig. 4b; P=0.003, paired t-test, n=5 neurones). In current-clamp mode, we investigated burst firing properties in response to a brief hyperpolarising stimulus (Fig. 4c). 3β-OH significantly decreased the number of bursts (Fig. 4d; P=0.034, paired t-test, n=6 neurones), and the number of rebound action potentials (Fig. 4e; P=0.031). The membrane potential was held at approximately the same value (baseline: −54.7 [1.7] mV vs 3β-OH: −54.6 [2.0] mV), whereas the average input resistance was not significantly affected upon the addition of 3β-OH (Fig. 4f; P=0.054). Our data suggest that the effects of 3β-OH on burst firing are preserved across different age groups in rats.
Fig 4.
(3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile attenuates rebound burst firing in subicular neurones. (a) Original traces from a representative subicular neurone showing that (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) reduced the amplitude of inward calcium currents (evoked using Vh of −90 mV and Vt of −52.5 mV). (b) Bar graph shows a significant decrease in the average calcium current amplitude after application of 3 μM 3β-OH. (c) Original traces from a representative subicular neurone depicting active membrane responses to a 200 pA hyperpolarising stimulus in the absence (black trace) or presence of 3β-OH 3 μM (red trace). (d) The average number of rebound bursts and the average number of rebound action potentials (e) was significantly decreased upon the addition of 3β-OH, whereas the average input resistance was not significantly affected (f). Results are expressed as mean (standard error of mean). *P<0.05 and †P<0.01, paired t-test.
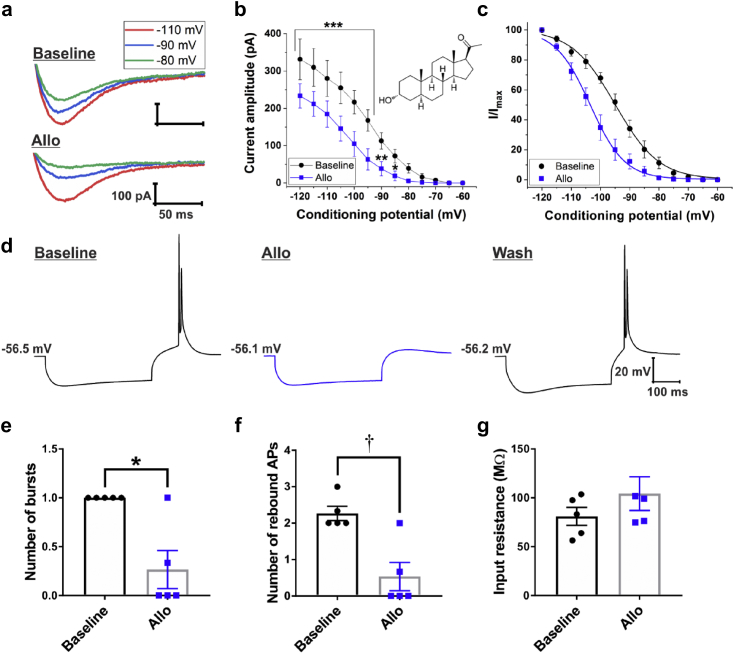
Effects of Allo on T-current properties and rebound burst firing in subicular neurones
Allo partially blocks T-currents in rat sensory neurones with an IC50 of 0.9 μM.25 We assessed whether Allo exhibits similar effects on T-currents in subicular neurones, and compared these effects with those of 3β-OH using the same voltage- and current-clamp protocols (Fig. 5a). Allo (1 μM) showed a strikingly similar T-current blocking effect as the same concentration of 3β-OH (Fig. 5a–c). Allo significantly decreased T-current amplitudes starting from the prepulse of −120 mV (30% inhibition) to a prepulse of −85 mV (75% inhibition; Fig. 5b; interaction: F[12,72]=5.34, P<0.001; post hoc: P<0.001 for potentials from −120 to −95 mV, P=0.002 for −90 mV, and P=0.046 for −85 mV; n=7 neurones, three rats), which was accompanied by a hyperpolarising shift in V50 of 10 mV (Fig. 5c).
Fig 5.
Allopregnanolone blocks T-type calcium channels (T-channels) in a voltage-dependent manner and attenuates rebound burst firing in subicular neurones. (a) Original traces from a representative subicular neurone displaying that allopregnanolone (Allo) reduced the amplitude of inward calcium currents evoked using three different 3.6-s prepulses: −110 mV (red), −90 mV (blue), and −80 mV (green), and Vt of −50 mV. (b) Average current amplitudes exhibit a significant decrease after application of 1 μM Allo across different conditioning potentials. The inset shows the chemical structure of Allo (*P<0.05, †P<0.01, and ‡P<0.001, two-way repeated measures analysis of variance followed by Holm-Sidak's post hoc test). (c) Steady-state inactivation curves (I/Imax) display a leftward shift upon application of Allo. (d) Original traces from a representative subicular neurone depicting active membrane responses to a 200 pA hyperpolarising stimulus in the absence (black trace), presence of Allo 3 μM (blue trace), and after drug wash-out (black trace). (d) The average number of rebound bursts and average number of rebound action potentials (e) was significantly decreased upon addition of Allo, whereas the average input resistance was not significantly affected (f). Results are expressed as mean (standard error of mean). *P<0.05 and †P<0.01, paired t-test.
The effects of Allo on T-current-dependent rebound burst firing was investigated in subicular neurones (Fig. 5d). The effects of Allo mirrored those of 3β-OH 3 μM: the number of bursts (Fig. 5e; P=0.020, paired t-test, n=5 neurones, three rats), and the number of rebound action potentials (Fig. 5f; P=0.002), were significantly decreased, whereas the average input resistance was slightly higher upon the addition of Allo (Fig. 5g; P=0.103). We conclude that both the endogenous (Allo) and synthetic (3β-OH) neuroactive steroid analogues block isolated T-type calcium currents and rebound bursting in the subiculum, with similar potency.
Effects of 3β-OH and Allo on synaptic plasticity at the CA3-CA1 and CA1-subiculum synapses
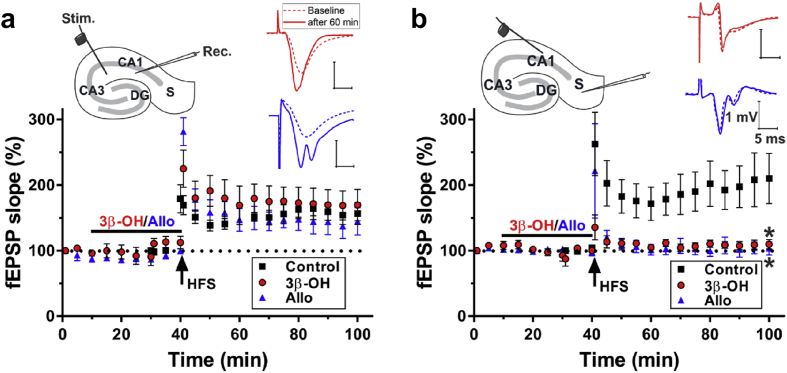
Most hypnotics and general anaesthetics suppress LTP at the CA3-CA1 synapse.26, 27, 28 TTA-P2, a structurally unrelated T-channel antagonist, alters LTP at the CA1-subiculum, but not at the CA3-CA1 synapse.11 As our results revealed T-channel-blocking properties of 3β-OH and Allo on subicular T-currents, we speculated that these neuroactive steroids also have significant effects on synaptic plasticity in hippocampal slices. 3β-OH or Allo at 1 μM did not significantly alter basal transmission (control: 100 [2]% of baseline; 3β-OH: 112 [10]%; Allo: 99 [2]%, n=5 in each group; F(2,12)=1.54, P=0.255, one-way ANOVA) or LTP at the CA3-CA1 synapse (Fig. 6a; control: 156 [16]% of baseline 60 min after high frequency stimulation [HFS]; 3β-OH: 169 [24]%, red trace; Allo: 144 [21]%, blue trace). This finding was confirmed using 3β-OH 10 μM (138 [9]%, n=5; data not shown). These data are consistent with our previous study that showed the lack of effect of Allo on LTP induction at this synapse.29 Conversely, 3β-OH and Allo, both at 1 μM, significantly suppressed LTP at the CA1-subiculum synapse (Fig. 6b; control: 210 [38]% of baseline 60 min after HFS; 3β-OH: 110 [7]%; Allo: 103 [10]%; n=5, 7, and 5, respectively), as assessed by one-way ANOVA followed by Holm-Sidak's post hoc test (F[2,14]=7.86, P=0.005; 3β-OH: P=0.010 and Allo: P=0.010, both vs control). Taken together, these results confirm our findings that modulating T-channels can have a crucial influence on synaptic plasticity in the CA1-subiculum, but not in CA3-CA1 pathway.
Fig 6.
Long-term potentiation is impaired by (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile or allopregnanolone at the CA1-subiculum, but not at the CA3-CA1 synapse. (a) Schematic diagram of hippocampal formation with stimulation probe for CA1 recordings placed along the Schaffer collateral pathway. Extracellular recordings were obtained in the apical dendritic layer of the CA1 region. Inset shows original excitatory postsynaptic potential (EPSP) traces in baseline (dashed line) conditions and 60 min after high frequency stimulation (HFS) in the presence of (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) 1 μM or Allo 1 μM (solid line). Constant current pulses (0.1 ms, 100 Hz×1 s) HFS successfully induced long-term potentiation (LTP) in CA1 in the presence of either 3β-OH or Allo measured 60 min after HFS. (B) Schematic diagram of hippocampal formation with stimulation probe for LTP induction in the subiculum located towards the end of CA1 in the stratum oriens/alveus region, and with the recording pipette in the molecular layer of the subiculum. Original EPSP traces shown in inset depict the absence of LTP at the CA1-subiculum synapse after the addition of 3β-OH 1 μM or Allo 1 μM (solid line), as compared with baseline conditions (dashed line). The normalised EPSP slopes show that HFS (200 Hz×1 s×2) did not induce subicular LTP in the presence of either 3β-OH or Allo. Results are expressed as mean (standard error of mean). *P<0.05 vs control, one-way analysis of variance followed by Holm-Sidak's post hoc test.
Discussion
We have shown that burst firing of subicular neurones relies on T-channel activity,11 and that 3β-OH, a novel neuroactive steroid with hypnotic effects, is a potent blocker of subicular and thalamic T-currents in rat pups.15 Here, we confirm the hypnotic and T-channel-blocking properties of 3β-OH in juvenile rats and, furthermore, report on its differential effects at two hippocampal synapses important for memory processing: CA3-CA14 and CA1-subiculum.30
First, we detected isolated T-currents in subicular neurones with minimal differences in biophysical properties compared with rat pups,11 which clearly showed a dominant expression of the CaV3.1 T-channel isoform. However, here we report an increase in mRNA expression of CaV3.3 isoform compared with rat pups. This increase of CaV3.3 expression coincides with distal dendritic localisation of this T-channel isoform in the subiculum,31 which may explain why we did not detect substantial T-currents with CaV3.3-like properties (e.g. slow activation and inactivation kinetics) using somatic recordings in rat pups. The development of the dendritic arbor and change in the expression of CaV3.3 may also account for the slight but significant hyperpolarising shift in V50 for T-current activation, which yielded a ‘window current’.
The neurosteroid analogue 3β-OH and the endogenous neurosteroid Allo blocked T-currents in subicular neurones with similar potency as described.15, 25 Inhibition of T-currents was accompanied by the attenuation of rebound burst firing without significant changes in passive membrane properties of subicular neurones. These effects largely mirror our findings with the structurally unrelated selective T-channel antagonist TTA-P2,11 thus indirectly confirming the crucial role of T-channels in mediating burst firing in the subiculum. 3β-OH also blocks CaV3.2 T-currents in dissociated neurones of the dorsal root ganglion25 and in the nucleus reticularis thalami,23 an inhibitory thalamic nucleus rich in CaV3.2 and CaV3.3 T-channel isoforms.20 Taken together, our results show that 3β-OH modulates neuronal excitability acting through T-channels, regardless of which T-channel isoform is dominant in neuronal tissue.
LTP represents a persistent activity-dependent change in synaptic efficacy usually studied by stimulating the Schaffer collateral pathway (CA3-CA1 synapses). Amnestic actions of hypnotics and anaesthetics are usually studied using this particular synapse as a cellular model of learning and memory, with limited data on their effects on other hippocampal synapses. Most studies have focused on either GABAA or NMDA receptors as the major molecular targets for hypnotic/anaesthetic-induced impairment of synaptic plasticity,3, 32, 33 with very little, if any, data on the contribution of voltage-gated calcium channels to these effects. The results presented here show that 3β-OH and Allo are devoid of effects on LTP induction at the CA3-CA1 synapse, thus corroborating our previous findings that T-channels do not play an important role in regulating synaptic plasticity in this hippocampal subregion.11 This is also consistent with a lack of effect of 3β-OH on NMDA receptors,15 as LTP in the CA3-CA1 pathway relies heavily on activation of these glutamate receptors.34 In contrast, both 3β-OH and Allo strongly suppressed the induction and maintenance of LTP at the CA1-subiculum synapse, very similar to TTA-P2.11 This effect should likely be accompanied with specific alterations in memory processing, and this hypothesis needs to be tested in future behavioural experiments aimed at assessing learning and memory processes that require intact function of the hippocampal formation. Towards this end, we recently reported that CaV3.1−/− mice exhibit specific deficits in spatial navigation and recognition memory.35
3β-OH is a potent hypnotic in both rats and mice15 (unpublished data). This hypnotic effect is apparently preserved across different age groups. The concentration range used in this study (1–3 μM) probably reflects sub-hypnotic doses in juvenile rats, but is nonetheless high enough to modulate both neuronal excitability and synaptic plasticity in the subiculum. We propose that the predominant action of 3β-OH on subicular T-channels and LTP in the CA1-subiculum synapse may result in amnestic effects different from those seen with other commonly used injectable anaesthetics, such as propofol or ketamine, that have very little effect on neuronal T-type currents at clinically relevant concentrations.16, 36, 37
Authors' contributions
Performed experiments and analysed the data: SMJ, YI, SLjJ, VT, KK, BA.
Designed the studies, supervised the overall project, and performed final manuscript preparation: SMJ, VJT, DFC, CFZ, SMT.
Declaration of interest
CFZ serves on the Scientific Advisory Board of Sage Therapeutics and holds stock in Sage Therapeutics. Sage Therapeutics was not involved in the funding or design of the present study.
Other authors have no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Funding
Department of Anesthesiology at the University of Colorado Anschutz Medical campus, National Institutes of Health grants R01 GM102525 (to SMT); R01 GM118197, R01 HD144517, R21 HD080281, R0144517-S, GM118197-S and March of Dimes National Award, USA (to VJT), CU Medicine Endowment (to VJT); MH114866 (CFZ) and funds from the Taylor Family Institute for Innovative Psychiatric Research (to DFC).
Editorial decision: 21 December 2018
Handling editor: H.C. Hemmings Jr
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2019.01.029.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Maciver M.B., Tauck D.L., Kendig J.J. General anesthetic modification of synaptic facilitation and long-term potentiation in hippocampus. Br J Anaesth. 1989;62:301–310. doi: 10.1093/bja/62.3.301. [DOI] [PubMed] [Google Scholar]
- 2.Simon W., Hapfelmeier G., Kochs E., Zieglgänsberger W., Rammes G. Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology. 2001;94:1058–1065. doi: 10.1097/00000542-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Nagashima K., Zorumski C.F., Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005;103:318–326. doi: 10.1097/00000542-200508000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Newcomer J.W., Farber N.B., Jevtovic-Todorovic V. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 6.Ren Y., Zhang F.J., Xue Q.S., Zhao X., Yu B.W. Bilateral inhibition of γ-aminobutyric acid type a receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. Anesthesiology. 2008;109:775–781. doi: 10.1097/ALN.0b013e31818a37c4. [DOI] [PubMed] [Google Scholar]
- 7.Perouansky M., Rau V., Ford T. Slowing of the hippocampal θ rhythm correlates with anesthetic-induced amnesia. Anesthesiology. 2010;113:1299–1309. doi: 10.1097/ALN.0b013e3181f90ccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staff N.P., Jung H.Y., Thiagarajan T., Yao M., Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- 9.Jarsky T., Mady R., Kennedy B., Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol. 2008;547:535–547. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- 10.O’Mara S.M., Sanchez-vives M.V., Brotons-mas J.R., Hare E.O. Progress in neuro-psychopharmacology & biological psychiatry roles for the subiculum in spatial information processing , memory , motivation and the temporal control of behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:782–790. doi: 10.1016/j.pnpbp.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Joksimovic S.M., Eggan P., Izumi Y. The role of T-type calcium channels in the subiculum: to burst or not to burst? J Physiol. 2017;595:6327–6348. doi: 10.1113/JP274565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph U., Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 13.Orestes P., Todorovic S.M. Are neuronal voltage-gated calcium channels valid cellular targets for general anesthetics? Channels. 2010;4:518–522. doi: 10.4161/chan.4.6.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckle V.S., DiGruccio M.R., Uebele V.N., Renger J.J., Todorovic S.M. Inhibition of T-type calcium current in rat thalamocortical neurons by isoflurane. Neuropharmacology. 2012;63:266–273. doi: 10.1016/j.neuropharm.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atluri N., Joksimovic S.M., Oklopcic A. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br J Anaesth. 2018;120:768–778. doi: 10.1016/j.bja.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todorovic S.M., Lingle C.J. Pharmacological properties of T-type Ca2+ current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol. 1998;79:240–252. doi: 10.1152/jn.1998.79.1.240. [DOI] [PubMed] [Google Scholar]
- 17.Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cain S.M., Snutch T.P. Contributions of T-type calcium channel isoforms to neuronal firing. Channels. 2010;4:475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 20.Talley E.M., Cribbs L.L., Lee J.H., Daud A., Perez-Reyes E., Bayliss D.A. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren R.A., Jones E.G. Maturation of neuronal form and function in a mouse thalamo-cortical circuit. J Neurosci. 1997;17:277–295. doi: 10.1523/JNEUROSCI.17-01-00277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todorovic S.M., Pathirathna S., Brimelow B.C. 5beta-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 23.Joksovic P.M., Covey D.F., Todorovic S.M. Inhibition of T-type calcium current in the reticular thalamic nucleus by a novel neuroactive steroid. Ann N Y Acad Sci. 2007;1122:83–94. doi: 10.1196/annals.1403.006. [DOI] [PubMed] [Google Scholar]
- 24.Llinás R.R., Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 25.Pathirathna S., Brimelow B.C., Jagodic M.M. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Wei H., Xiong W., Yang S. Propofol facilitates the development of long-term depression (LTD) and impairs the maintenance of long-term potentiation (LTP) in the CA1 region of the hippocampus of anesthetized rats. Neurosci Lett. 2002;324:181–184. doi: 10.1016/s0304-3940(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 27.Ishizeki J., Nishikawa K., Kubo K., Saito S., Goto F. Amnestic concentrations of sevoflurane inhibit synaptic plasticity of hippocampal CA1 neurons through gamma-aminobutyric acid-mediated mechanisms. Anesthesiology. 2008;108:447–456. doi: 10.1097/ALN.0b013e318164cfba. [DOI] [PubMed] [Google Scholar]
- 28.Piao M.H., Liu Y., Wang Y.S., Qiu J.P., Feng C.S. Volatile anesthetic isoflurane inhibits LTP induction of hippocampal CA1 neurons through α4β2nAChR subtype-mediated mechanisms. Ann Fr Anesth Reanim. 2013;32:e135–e141. doi: 10.1016/j.annfar.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Izumi Y., Murayama K., Tokuda K., Krishnan K., Covey D.F., Zorumski C.F. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur J Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Mara S.M., Commins S., Anderson M. Synaptic plasticity in the hippocampal area ca1- subiculum projection : implications for theories of memory. Hippocampus. 2000;10:447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Mckay B.E., Mcrory J.E., Molineux M.L. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers F.C., Zarnowska E.D., Laha K.T. Etomidate impairs long-term potentiation in vitro by targeting α5-subunit containing GABAA receptors on nonpyramidal cells. J Neurosci. 2015;35:9707–9716. doi: 10.1523/JNEUROSCI.0315-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang D.X., Levy W.B. Ketamine blocks the induction of LTP at the lateral entorhinal cortex-dentate gyrus synapses. Brain Res. 1992;593:124–127. doi: 10.1016/0006-8993(92)91273-h. [DOI] [PubMed] [Google Scholar]
- 34.Collingridge G.L., Bliss T.V.P. NMDA receptors - their role in long-term potentiation. Trends Neurosci. 1987;10:288–293. [Google Scholar]
- 35.Joksimovic S.M., Busquet N., Valdez R., Todorovic S.M. 2018 Neuroscience Meeting Planner, Society for Neuroscience, San Diego, CA. 2018. Cognitive and affective behavioral phenotype of CaV3.1 knock-out mice: The importance of subicular circuitry. Program No. 461.05.https://abstractsonline.com/pp8/#!/4649/presentation/5057 [Google Scholar]
- 36.Todorovic S.M., Perez-Reyes E., Lingle C.J. Anticonvulsants but not general anesthetics have differential blocking effects on different T-type current variants. Mol Pharmacol. 2000;58:98–108. doi: 10.1124/mol.58.1.98. [DOI] [PubMed] [Google Scholar]
- 37.Joksovic P.M., Brimelow B.C., Murbartián J., Perez-Reyes E., Todorovic S.M. Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant CaV3.3 channels. Br J Pharmacol. 2009;144:59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.