Abstract
Background and Purpose
Both genetic factors and smoking are associated with ischemic stroke (IS) risk. However, little is known about the potential interaction of these factors. We aimed to assess whether smoking and a positive family history interact to increase the risk of IS.
Methods
The nationwide prospective study recruited 210,000 men and 300,000 women in 2004 to 2008 at ages 30 to 79 years. During 9.7 years of follow-up, we documented 16,923 and 20,656 incident IS cases in men and women without major chronic diseases at baseline, respectively. Multivariable Cox regression models were used to examine associations between family history and IS. Likelihood ratio tests were used to test the smoking-family history interactions on IS.
Results
About 67.8% (n=135,168) of men ever smoked regularly compared with 2.7% (n=7,775) of women. Among men, a significant interaction between family history and smoking on IS was observed (P for interaction=0.03), with more pronounced association between family history and IS among ever-regular smokers (hazard ratio [HR], 1.21; 95% confidence interval [CI], 1.16 to 1.27) than among never-smokers (HR, 1.11; 95% CI, 1.01 to 1.23). The association between family history and IS among ex-smokers after more than 10 years of cessation (HR, 1.01; 95% CI, 0.85 to 1.20) appeared similar to that among never-smokers. Among women, a similar but not significant interaction between family history and smoking on IS was observed. Ever-regular smokers who had a family history of stroke had the highest risk of IS.
Conclusions
Among Chinese men, the association of family history with IS was accentuated by smoking, and such accentuation tended to be lowered by cessation.
Keywords: Family health, Stroke, Smoking, Cohort studies, Gene-environment interaction
Introduction
Ischemic stroke (IS) is a complex disorder and the major cause of death and disability worldwide [1,2]. In China, IS accounted for more than 0.7 million deaths in 2013, bringing an enormous public health burden [3]. Notably, China is still experiencing increases in the incidence rate of IS [4]. Smoking is a major modifiable risk factor for IS [5,6]. Unfortunately, China is the world’s largest tobacco consumer. According to the 2015 Chinese Adults Tobacco Survey Report, about 52.1% of men and 2.7% of women, accounting for more than 316 million people, were current smokers [7]. In China, even though the prevalence of smoking did not change in the past 5 years, the number of smokers increased as a consequence of expanding populations [8,9]. In 2013, China was ranked highest for IS deaths attributable to tobacco consumption, which accounted for 20.3% of the population attributable risk for IS [1,10].
Recently, compelling evidence has shown that genetic susceptibility may interact with lifestyle factors on chronic diseases risk [11,12]. Previous studies found that the family history, a surrogate measure of genetic susceptibility, was associated with risk of stroke [13,14], and interacted with smoking on risk of cardiovascular outcomes such as myocardial infarction [15] and subarachnoid hemorrhage [16]. However, little is known about the potential interactions between smoking and family history of stroke on the risk of IS, especially in Chinese.
Therefore, in the present study, we examined whether tobacco smoking modified the genetic susceptibility, estimated by a family history of stroke, in men and women from the China Kadoorie Biobank (CKB) study. We also prospectively examined the joint association of family history and tobacco smoking with IS risk.
Methods
Study population
Details of the CKB study have been described previously [17,18]. Briefly, the CKB is a prospective cohort study of 512,715 adults aged 30 to 79 years recruited from 10 areas (five urban and five rural) of China. The baseline survey took place during 2004 to 2008. In each study assessment clinics, participants were interviewed by trained staff using laptop-based questionnaires, that covered demographic characteristics, socioeconomic status, lifestyle factors, general health, family history, mental health, and for women only, reproductive history. A range of physical measurements was undertaken by trained staff, including height, weight, and blood pressure. Blood samples were collected from each participant. All participants provided written informed consent. The Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (United Kingdom) approved the study.
For the present analysis, we excluded participants with previously diagnosed heart disease (n=15,472), stroke (n=8,884), or cancer (n=2,577), and those who had missing data for body mass index (n=2) or were lost to follow-up shortly after baseline (n=1). The final analysis included 487,198 participants.
Assessment of family history
The baseline questionnaire included a series of three questions regarding family history of stroke, which asked participant if their biological father, mother, or siblings ever had “stroke.” For siblings, the number with stroke was recorded. For each participant, the first-degree family members’ stroke history had been collected. In this study, a participant was classified as “having a family history of stroke” if they reported at least one first degree relatives had stroke. First-degree relatives included fathers, mothers, and siblings.
Assessment of smoking and other covariates
Our baseline questionnaire collected smoking information including frequency, amount, and types of tobacco smoked (factory cigarettes, hand-rolled cigarettes, pipe or water pipe, cigars), depth of inhalation, and the ages at which participants started smoking regularly and stopped smoking, with additional information on the main reason for cessation (ill or stopped by choice) among those who had stopped smoking. Among current and former (or ex) regular smokers, the amount of tobacco smoked (g/day) was calculated, assuming 1 g of tobacco per factory cigarette, and 2 g per cigar, with quantities smoked in pipes and hand-rolled cigarettes given as liang/month (1 liang equivalent to 50 g). Smoking duration in years was derived from the age at starting smoking regularly to age at baseline (current smokers) or duration since quitting at baseline (ex-smokers).
For the present study, participants were categorized into four groups according to the smoking status in line with previous CKB studies [19]. Never-smokers were defined as those who had not smoking at recruitment and who had not smoked more than 100 cigarettes during their lifetime. Ever-regular smokers were defined as those who had ever smoked at least one cigarette or equivalent per day for at least 6 months, or who had stopped smoking at least 6 months before recruitment because of ill. Regular smokers who had stopped smoking by choice for at least 6 months at baseline were classified as ex-smokers. Participants who did not meet the criteria for never-smoker, ever-regular smoker, or ex-smoker were classified as occasional smokers.
The baseline questionnaire also included other covariates, such as sociodemographic characteristics (age, sex, education, marital status, and household income), lifestyle behaviors (physical activity, alcohol consumption, intakes of red meat, fresh fruits, and vegetables), and menopausal status (for women). Trained staff measured weight and height using calibrated instruments. Prevalent diabetes was defined as measured fasting blood glucose ≥7.0 mmol/L, measured random blood glucose ≥11.1 mmol/L, or self-reported diagnosis of diabetes. Prevalent hypertension was defined as measured systolic blood pressure ≥140 mm Hg, measured diastolic blood pressure ≥90 mm Hg, self-reported diagnosis of hypertension, or self-reported use of antihypertensive agents at baseline.
Follow-up for incident IS
All the participants were followed up from the time they enrolled in the study. We ascertained the vital status of each participant through the linkage with established chronic disease registries, and the disease surveillance points system in the study areas, as well as the national health insurance system. Almost all the participants (>98%) across the 10 study areas had been successfully linked to the health insurance databases. Annual active follow-up was performed by checking against local residential and health insurance records and was confirmed by street committees or village administrators. Fatal and non-fatal events were coded by the trained staff, blinded to baseline information, using the International Classification of Diseases, 10th Revision. All reported fatal or non-fatal major diseases events (including stroke) from different sources were reviewed and integrated centrally by trained clinical staff, who were blinded to baseline information. By December 2018, we have retrieved medical records for 31,069 newly reported IS cases during follow-up, among which 21,426 (69%) were confirmed as IS cases.
Statistical analysis
As the prevalence of smoking and the number of cigarettes smoked per day are much higher in men compared with women, all analyses were done separately in men and women. Percentages and mean±standard deviation of baseline characteristics were calculated across family history of stroke standardized by age and study area, when appropriate. We calculated person-years at risk from the baseline date to diagnosis of IS, death, loss to follow-up, or the censoring date (December 31, 2016), whichever occurred first. Cox regression was used to estimate the hazard ratios (HRs) of family history, with age as the underlying time scale and stratified jointly by 10 study areas and age at baseline in 5-year intervals. Multivariable models were adjusted for education (no formal school, primary school, middle school, high school, or college or university or higher), marital status (married, widowed, divorced/separated, or never married), household income (<10,000, 10,000 to 19,999, ≥20,000 Chinese renminbi/yr), alcohol consumption (less than weekly, ex-regular drinkers, weekly; or <15, 15 to 29, 30 to 59, or ≥60 g/day of pure alcohol), smoking status (never-smokers or occasional smokers, or ex-smokers, or current smokers of 1 to 14, 15 to 24, or ≥25 cigarettes or equivalents per day), physical activity as metabolic equivalent of task hours per day, intake of red meat, fresh fruits, and vegetables (daily, 4 to 6 days/week, 1 to 3 days/week, monthly, or rarely or never), menopause status (for women only, premenopausal, perimenopause, postmenopausal, or missing), body mass index (in kg/m2), and prevalent diabetes and hypertension at baseline (presence or absence).
Interactions between the family history of stroke and tobacco smoking on IS were tested by using likelihood ratio tests to compare models with and without cross-product terms (e.g., interaction term: family history of stroke × smoking categories). The HRs for IS were also calculated by strata of smoking characteristic subgroups, using individuals who reported no family history of stroke as the reference group. We further examined the risk of incident IS according to the joint classification of family history and smoking, in which smoking variables were included two categories (never-smokers and ever-regular smokers). We performed all statistical analyses using Stata version 14.2 (StataCorp., College Station, TX, USA).
Results
Population characteristics according to family history of stroke
Overall, the mean baseline age was 51.5±10.5 years, 67.8% (n=135,168) of men ever smoked regularly compared with 2.7% (n=7,775) of women. Of the 199,240 men and 287,958 women included, 17.8% of men and 17.5% of women had a family history of stroke. Both men and women who had a family history of stroke were, on average, more likely to reside in the urban area, have a higher level of education, and have hypertension at baseline (Table 1).
Table 1.
Baseline characteristics of 487,198 participants according to family history of stroke
| Characteristic | Family history in men (n=199,240) |
Family history in women (n=287,958) |
||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| No. of participants | 163,791 (82.2) | 35,449 (17.8) | 237,436 (82.5) | 50,522 (17.5) |
| Age (yr) | 52.4±11.0 | 52.1±9.8 | 50.9±10.5 | 51.3±9.4 |
| Urban area (%) | 41.9 | 45.9 | 42.4 | 48.5 |
| Middle school and above (%) | 57.1 | 60.9 | 42.5 | 46.8 |
| Married (%) | 92.8 | 93.7 | 89.3 | 89.9 |
| Household income ≥20,000 yuan/y (%) | 45.5 | 47.0 | 40.3 | 42.5 |
| Postmenopausal (%) | - | - | 50.8 | 50.6 |
| Smoking category (%) | ||||
| Never-smokers | 14.4 | 13.3 | 95.1 | 94.9 |
| Ex-smokers | 6.6 | 6.8 | 0.4 | 0.4 |
| Occasional smokers | 11.4 | 11.2 | 1.8 | 2.0 |
| Ever-regular smokers | 67.7 | 68.7 | 2.7 | 2.8 |
| Weekly alcohol drinker (%) | 33.7 | 35.1 | 2.1 | 2.1 |
| Average weekly consumption (day)* | ||||
| Red meat | 4.0 | 4.0 | 3.5 | 3.6 |
| Fresh vegetable | 6.8 | 6.8 | 6.8 | 6.8 |
| Fresh fruit | 2.2 | 2.3 | 2.7 | 2.9 |
| Total physical activity (MET hr/day) | 22.5 | 23.0 | 20.8 | 20.9 |
| Body mass index (kg/m2) | 23.3 | 23.6 | 23.7 | 24.0 |
| Prevalent diabetes (%) | 5.0 | 5.5 | 5.4 | 6.3 |
| Prevalent hypertension (%) | 34.5 | 42.2 | 30.9 | 38.3 |
Values are presented as number (%) or mean±standard deviation.
MET, metabolic equivalent.
Average weekly consumptions of red meat, fresh vegetables, and fruits were calculated by assigning participants to the midpoint of their consumption category.
Family history of stroke and IS
During 10 years of follow-up (4.7 million person years), we documented 16,923 incident IS cases in men and 20,656 in women. Multivariate-adjusted analyses showed that family history of stroke was associated with an increased IS risk (Supplementary Table 1). In the whole cohort, compared with participants who reported no family history of stroke, the adjusted HR for IS was 1.19 (95% confidence interval [CI], 1.16 to 1.22) for those who reported a family history of stroke. The respective HRs were 1.19 (95% CI, 1.15 to 1.23) among men and 1.19 (95% CI, 1.15 to 1.23) among women. There was no heterogeneity between men and women (P for interaction=0.92).
Interactions between family history of stroke and smoking on IS risk
Among men, we observed a significant interaction between family history of stroke and IS risk across the stratum for tobacco smoking (P for interaction=0.03), with a stronger association among ever-regular smokers than among never-smokers (Table 2). Compared with men who reported no family history of stroke, the adjusted HRs for those reported a family history of stroke were 1.11 (95% CI, 1.01 to 1.23) among never-smokers and 1.21 (95% CI, 1.16 to 1.27) among ever-regular smokers. Among women, differences in the associations between family history and IS risk across the smoking status were observed, but the interaction was not significant (P for interaction=0.49). The respective HRs for those who reported a family history of stroke were 1.19 (95% CI, 1.15 to 1.23) among never-smokers, and 1.32 (95% CI, 1.14 to 1.53) among ever-regular smokers. Likewise, compared with never-smokers, the adjusted HRs for ever-regular smokers were greater in those who reported a family history of stroke than those who did not (Table 3).
Table 2.
Risk of incident ischemic stroke according to family history of stroke, stratified by smoking category
| Variable | Family history of stroke |
Pinteraction | |||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| Total no. | Cases | HR (95% CI) | Total no. | Cases | HR (95% CI) | ||
| Men | 0.03 | ||||||
| Never-smokers | 23,611 | 2,222 | Ref | 4,666 | 578 | 1.11 (1.01–1.23) | |
| Ex-smokers | 10,650 | 1,114 | Ref | 2,491 | 332 | 1.13 (0.99–1.28) | |
| Occasional smokers | 18,413 | 1,286 | Ref | 4,207 | 420 | 1.18 (1.05–1.32) | |
| Ever-regular smokers | 111,117 | 8,311 | Ref | 24,085 | 2,660 | 1.21 (1.16–1.27) | |
| Women | 0.49 | ||||||
| Never-smokers | 225,929 | 14,453 | Ref | 47,891 | 4,615 | 1.19 (1.15–1.23) | |
| Ex-smokers | 848 | 133 | Ref | 196 | 37 | 1.04 (0.70–1.54) | |
| Occasional smokers | 4,228 | 304 | Ref | 1,049 | 125 | 1.20 (0.95–1.50) | |
| Ever-regular smokers | 6,431 | 733 | Ref | 1,386 | 256 | 1.32 (1.14–1.53) | |
Stratified by age and study area and adjusted for level of education; marital status; household income; alcohol consumption; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; menopause status (only for women); body mass index; prevalent hypertension; prevalent diabetes.
HR, hazard ratio; CI, confidence interval.
Table 3.
Risk of incident ischemic stroke according to smoking category, stratified by family history of stroke
| Variable | Family history of CVD |
|
|---|---|---|
| No | Yes | |
| Men | ||
| Never-smokers | 1.00 | 1.00 |
| Ex-smokers | 0.99 (0.92–1.07) | 1.05 (0.92–1.19) |
| Occasional smokers | 0.99 (0.92–1.06) | 1.03 (0.91–1.17) |
| Ever-regular smokers | 1.17 (1.11–1.23) | 1.30 (1.19–1.43) |
| Women | ||
| Never-smokers | 1.00 | 1.00 |
| Ex-smokers | 0.98 (0.82–1.17) | 0.90 (0.65–1.23) |
| Occasional smokers | 0.98 (0.87–1.11) | 1.07 (0.90–1.28) |
| Ever-regular smokers | 1.09 (1.00–1.18) | 1.29 (1.14–1.47) |
Values are presented as hazard ratio (95% confidence interval). Stratified by age and study area and adjusted for level of education; marital status; household income; alcohol consumption; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; menopause status (only for women); body mass index; prevalent hypertension; prevalent diabetes.
We further examined the interaction between each type of family history and the smoking history (Supplementary Table 2). We only found a significant interaction between parental history and smoking status among men. Nevertheless, the associations between each type of family history and IS risk seemed to be less evident in never-smokers compared with ever-regular smokers.
Stratified analyses among smokers
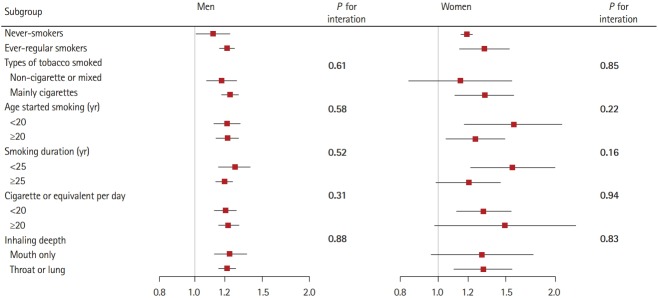
Among ever-regular smokers, we further performed stratified analyses according to baseline smoking characteristics: types of tobacco smoked, age started smoking, smoking duration, smoking amount, and depth of inhalation. Among men, the HRs for family history on IS were markedly higher among all subgroups compared with never-smokers (Figure 1), but did not significantly differ across different smoking characteristics subgroups (all P for interaction >0.05). Similar but more evident associations were observed among women.
Figure 1.
Subgroup analysis of associations between family history and ischemic stroke according to baseline smoking characteristics. Hazard ratios for ischemic stroke are for comparison of men and women who had a family history of stroke with those who had no family history of stroke. Ever-regular smokers excludes occasional smokers (n=22,620) and ex-smokers who stopped by choice (n=13,141). Tests for heterogeneity include only ever-regular smokers. Horizontal lines represent 95% confidence intervals.
Among ex-smokers, no statistically significant heterogeneity was observed in the associations between family history of stroke and IS by years of smoking cessation (Table 4). Nevertheless, the associations between family history of stroke and IS seemed to be less evident in ex-smokers with longer years since quitting. Among men, the HRs were 1.32 (95% CI, 1.09 to 1.59) among those who had stopped for less than 10 years, and 1.01 (95% CI, 0.85 to 1.20) among those who had stopped for 10 years or more before baseline (P for interaction=0.05). Similar patterns were observed among women, with HRs of 1.01 (95% CI, 0.55 to 1.84) and 0.92 (95% CI, 0.51 to 1.65), respectively (P for interaction=0.86).
Table 4.
Risk of incident ischemic stroke according to family history of stroke, stratified by smoking stopped years
| Variable | Family history of stroke |
Pinteraction | |||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| Total no. | Cases | HR (95% CI) | Total no. | Cases | HR (95% CI) | ||
| Men | 0.05 | ||||||
| Never-smokers | 23,611 | 2,222 | Ref | 4,666 | 578 | 1.11 (1.01–1.23) | |
| Ex-smokers | |||||||
| Stopped for <10 yr | 5,692 | 471 | Ref | 1,268 | 157 | 1.32 (1.09–1.59) | |
| Stopped for ≥10 yr | 4,958 | 643 | Ref | 1,223 | 175 | 1.01 (0.85–1.20) | |
| Women | 0.86 | ||||||
| Never-smokers | 225,929 | 14,453 | Ref | 47,891 | 4,615 | 1.19 (1.15–1.23) | |
| Ex-smokers | |||||||
| Stopped for <10 yr | 394 | 63 | Ref | 95 | 17 | 1.01 (0.55–1.84) | |
| Stopped for ≥10 yr | 454 | 70 | Ref | 101 | 20 | 0.92 (0.51–1.65) | |
Adjusted for age; study area; level of education; marital status; household income; alcohol consumption; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; menopause status (only for women); body mass index; prevalent hypertension; prevalent diabetes.
HR, hazard ratio; CI, confidence interval.
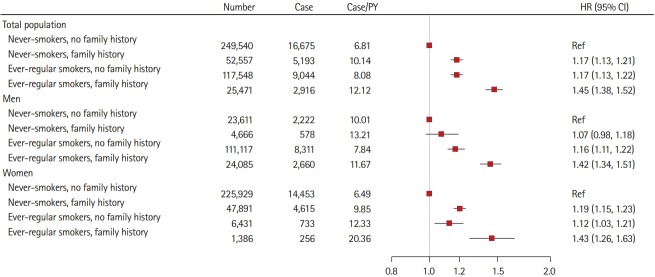
Joint association of family history of stroke and smoking with IS risk
We further examined the joint association of family history and smoking with IS risk (Figure 2). The strongest association was observed in the combination of family history with ever-regular smoking. Compared with never-smokers who reported no family history of stroke, ever-regular smokers who reported a family history of stroke showed a 42% risk increasing in IS (HR, 1.42; 95% CI, 1.34 to 1.51) among men and a 43% risk increasing (HR, 1.43; 95% CI, 1.26 to 1.63) among women, respectively.
Figure 2.
Risk of incident ischemic stroke according to family history of stroke and smoking status. Family history refers to family history of stroke. Model was stratified by age and study area and adjusted for level of education; marital status; household income; alcohol consumption; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; menopause status (only for women); body mass index; prevalent hypertension; prevalent diabetes. Horizontal lines represent 95% confidence intervals (CIs). HR, hazard ratio; PY, person-year.
Discussion
In this large prospective cohort of Chinese adults, we observed a significant interaction between smoking and family history of stroke on IS incidence risk among men. The association between family history of stroke and IS was more pronounced among ever-regular smokers than among never-smokers; whereas the association between family history and IS risk among ex-smokers after more than 10 years of cessation appeared similar to that among never-smokers. The interactions between smoking and family history of stroke were consistent across the smoking characteristic subgroups including types of tobacco smoked, age at starting, smoking duration, smoking amount, or depth of inhalation. Participants with a family history of stroke and reported ever-regular smoking had the greatest risk of IS.
Our study is the first to examine the interaction between smoking and family history of stroke on incident IS in a prospective cohort. Our findings are in line with several previous cross-sectional studies that reported the interactions between smoking and single genetic variants on IS [20-22]. Similar smoking-gene interaction in relation to IS was also found in Chinese populations [23-25]. In our study, we found that the interaction between smoking and family history of stroke was particularly significant among men, even though a similar but not significant interaction pattern was also observed among women. The gender difference may partly due to the extremely small number of ever-regular smokers among women.
For the first time, we further provided data about the interplay between family history and different smoking characteristics on IS among ever-regular smokers. The associations between family history and IS were not significantly differ across different smoking characteristics subgroups. Our findings were weakened by the fact that the effect of family history on IS risk did not increase with greater amount smoked and increased smoking duration. Although numbers are small in the stratified analyses among smokers, our findings may imply that the smokers themselves differ from the never-smokers, and that in general, the effects of family history are minimal in neversmokers.
Our observations, together with previous reports, suggest that smoking may strengthen the deleterious effects of genetic variants on the development of IS. The potential mechanisms underlying the smoking-gene interactions remain unclear. Smoking is associated with endothelial cell dysfunction [26-29], increased activation of inflammation and coagulation [30], increased fibrinogen concentration and platelet aggregability [31], as well as reduced fibrinolytic capacity [29], which may cause a procoagulant state and subsequently promote atherosclerosis and increase IS risk. Of note, all these alternations may overlap with pathways linking the genetic variations to the development of IS, making the interaction between smoking and genetic factors biologically feasible.
One of the novel findings of the present study is that the associations between family history and IS risk among ex-smokers after more than 10 years of cessation were similar to the level of never-smokers, indicating the risk conferred by an interaction may be lowered by quitting smoking. The present results strongly suggest that although the best public health advice remains not to start smoking at all, encouraging smokers to stop is crucial to reduce smoking-attributed IS risk, particularly among those with a family history of stroke. On the basis of the high smoking prevalence among Chinese, widespread smoking cessation is one of the most effective and cost-effective strategies to reduce IS risk over the next few decades [32].
The major strengths of the present study included prospective design, large sample size, a population geographically spread across urban and rural China, and careful adjustment for nongenetic familial factors. Several limitations need to be acknowledged. The participants consisted of Chinese middleaged and older men and women. Thus the results may not be generalizable to other age or racial/ethnic groups. Moreover, smoking status and family history was self-reported, which might lead to some degree of misclassification. However, measurement errors in the prospective study design are more likely to be non-differential and therefore, would have biased the results toward the null. In addition, the family history of stroke is a relatively crude marker for genetic susceptibility. Future studies need to examine the gene-smoking interaction by using well-established IS-associated genetic variants. Finally, although we have adjusted various confounding variables, residual confounding is still possible.
Conclusions
In summary, we found the impact of family history on IS was accentuated by tobacco smoking; and such accentuation tended to be reversible after more than 10 years of cessation. Our findings reemphasize the importance of counseling of family members regarding smoking behavior, and considering geneenvironment interaction in the prevention of IS.
Acknowledgments
The most important acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford, and the 10 regional centres.
This work was supported by grants (2016YFC0900500, 2016YFC0900501, 2016YFC0900504) from the National Key R&D Program of China. The CKB baseline survey and the first resurvey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (202922/Z/16/Z, 088158/ Z/09/Z, 104085/Z/14/Z), National Natural Science Foundation of China (81390540, 81390544, 81390541), and Chinese Ministry of Science and Technology (2011BAI09B01). Lu Qi is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616). Mengyu Fan is a recipient of a scholarship under the China Scholarship Council (201706010313).
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2018.03566.
Members of the China Kadoorie Biobank collaborative group:
Multivariable-adjusted HRs (95% CIs) for incident ischemic stroke according to family history of stroke
Associations between each type of family history and ischemic stroke risk, according to smoking status
References
- 1.Bennett DA, Krishnamurthi RV, Barker-Collo S, Forouzanfar MH, Naghavi M, Connor M, et al. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart. 2014;9:107–112. doi: 10.1016/j.gheart.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Causespecific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Wen X, Li W, Li X, Wang Y, Lu W. Risk factors for stroke in the Chinese population: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2017;26:509–517. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese Center for Disease Control and Prevention 2015 Chinese adults tobacco survey report. http://www.notc.org.cn/gzdt/201512/t20151228_123959.html. 2015. Accessed April 23, 2019.
- 8.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Ma C, Xi B. Tobacco control in China: still a long way to go. Lancet. 2016;387:1375–1376. doi: 10.1016/S0140-6736(16)30080-0. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 11.Hancock DB, Soler Artigas M, Gharib SA, Henry A, Manichaikul A, Ramasamy A, et al. Genome-wide joint meta-analysis of SNP and SNP-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet. 2012;8:e1003098. doi: 10.1371/journal.pgen.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jousilahti P, Rastenyte D, Tuomilehto J, Sarti C, Vartiainen E. Parental history of cardiovascular disease and risk of stroke. A prospective follow-up of 14371 middle-aged men and women in Finland. Stroke. 1997;28:1361–1366. doi: 10.1161/01.str.28.7.1361. [DOI] [PubMed] [Google Scholar]
- 14.Tian T, Jin G, Yu C, Lv J, Guo Y, Bian Z, et al. Family history and stroke risk in China: evidence from a large cohort study. J Stroke. 2017;19:188–195. doi: 10.5853/jos.2016.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leander K, Hallqvist J, Reuterwall C, Ahlbom A, de Faire U. Family history of coronary heart disease, a strong risk factor for myocardial infarction interacting with other cardiovascular risk factors: results from the Stockholm Heart Epidemiology Program (SHEEP) Epidemiology. 2001;12:215–221. doi: 10.1097/00001648-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Woo D, Khoury J, Haverbusch MM, Sekar P, Flaherty ML, Kleindorfer DO, et al. Smoking and family history and risk of aneurysmal subarachnoid hemorrhage. Neurology. 2009;72:69–72. doi: 10.1212/01.wnl.0000338567.90260.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Bragg F, Yang L, Kartsonaki C, Guo Y, Du H, et al. Smoking and smoking cessation in relation to risk of diabetes in Chinese men and women: a 9-year prospective study of 0·5 million people. Lancet Public Health. 2018;3:e167–e176. doi: 10.1016/S2468-2667(18)30026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CR, North KE, Bray MS, Avery CL, Mosher MJ, Couper DJ, et al. NOS3 polymorphisms, cigarette smoking, and cardiovascular disease risk: the Atherosclerosis Risk in Communities study. Pharmacogenet Genomics. 2006;16:891–899. doi: 10.1097/01.fpc.0000236324.96056.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezzini A, Grassi M, Del Zotto E, Bazzoli E, Archetti S, Assanelli D, et al. Synergistic effect of apolipoprotein E polymorphisms and cigarette smoking on risk of ischemic stroke in young adults. Stroke. 2004;35:438–442. doi: 10.1161/01.STR.0000112973.00867.98. [DOI] [PubMed] [Google Scholar]
- 22.Szolnoki Z, Somogyvári F, Kondacs A, Szabó M, Fodor L, Bene J, et al. Evaluation of the modifying effects of unfavourable genotypes on classical clinical risk factors for ischaemic stroke. J Neurol Neurosurg Psychiatry. 2003;74:1615–1620. doi: 10.1136/jnnp.74.12.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Cheng J, Peng J, Han S, Yu L, Nie S. Effects of polymorphisms of heat shock protein 70 gene on ischemic stroke, and interaction with smoking in China. Clin Chim Acta. 2007;384:64–68. doi: 10.1016/j.cca.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Yang H, ZhiPing T. Association studies of genetic polymorphism, environmental factors and their interaction in ischemic stroke. Neurosci Lett. 2006;398:172–177. doi: 10.1016/j.neulet.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Q, Li YK, Lu F, Yin L, Wang YZ, Wei W, et al. Interactions between ACYP2 genetic polymorphisms and environment factors with susceptibility to ischemic stroke in a Han Chinese population. Oncotarget. 2017;8:97913–97919. doi: 10.18632/oncotarget.18430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blann AD, Kirkpatrick U, Devine C, Naser S, McCollum CN. The influence of acute smoking on leucocytes, platelets and the endothelium. Atherosclerosis. 1998;141:133–139. [PubMed] [Google Scholar]
- 27.Barua RS, Ambrose JA, Saha DC, Eales-Reynolds LJ. Smoking is associated with altered endothelial-derived fibrinolytic and antithrombotic factors: an in vitro demonstration. Circulation. 2002;106:905–908. doi: 10.1161/01.cir.0000029091.61707.6b. [DOI] [PubMed] [Google Scholar]
- 28.Nagy J, Demaster EG, Wittmann I, Shultz P, Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium. 1997;5:251–263. doi: 10.3109/10623329709052590. [DOI] [PubMed] [Google Scholar]
- 29.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 31.Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984;36:389–395. doi: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 32.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med. 2014;370:60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Members of the China Kadoorie Biobank collaborative group:
Multivariable-adjusted HRs (95% CIs) for incident ischemic stroke according to family history of stroke
Associations between each type of family history and ischemic stroke risk, according to smoking status