Abstract
Clinical registries are health information systems, which have the mission to collect multidimensional real-world data over the long term, and to generate relevant information and actionable knowledge to address current serious healthcare problems.
This article provides an overview of clinical registries and their relevant stakeholders, focussing on registry structure and functioning, each stakeholder’s specific interests, and on their involvement in the registry’s information input and output.
Stakeholders of clinical registries include the patients, healthcare providers (professionals and facilities), financiers (government, insurance companies), public health and regulatory agencies, industry, the research community and the media.
The article discusses (1) challenges in stakeholder interaction and how to strengthen the central role of the patient, (2) the importance of adding cost reporting to enable informed value choices, and (3) the need for proof of clinical and public health utility of registries.
In its best form, a registry is a mission-driven, independent stakeholder–registry team collaboration that enables rapid, transparent and open-access knowledge generation and dissemination.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180077
Keywords: health information system, registry, stakeholder
Introduction
A clinical registry – such as a joint replacement registry – is a type of health information system.1 A registry’s mission is to provide real-world monitoring and quasi real-time reporting that helps to produce reassurance and solutions for healthcare problems. The positive impact of registries on healthcare outcomes and healthcare processes has been demonstrated.2
Clinical registries are an essential part of the learning healthcare system.3 They collect pre-specified structured data longitudinally and derive information, evidence and actionable knowledge to improve the patient’s healthcare. They also monitor whether a change in care results in improvement, no change, or deterioration over time. Improvement in health and care can result from reduction of mortality, morbidity or complications, maintenance or improvement of quality of life, more equal distribution of healthcare (e.g. can prevent over- and under-treatment) and better distribution and sustainability of available resources. Another use of registry data relates to the prevention of adverse events and threats to patients and to public health. Tissue responses from cobalt ions produced from excessive wear in metal-on-metal hip arthroplasty or implant-related infections are examples of serious adverse events which impact on patient outcome. Moreover, the evidence derived from clinical registries has an important role in guiding the innovation process towards relevant, usable and sustainable innovations.4
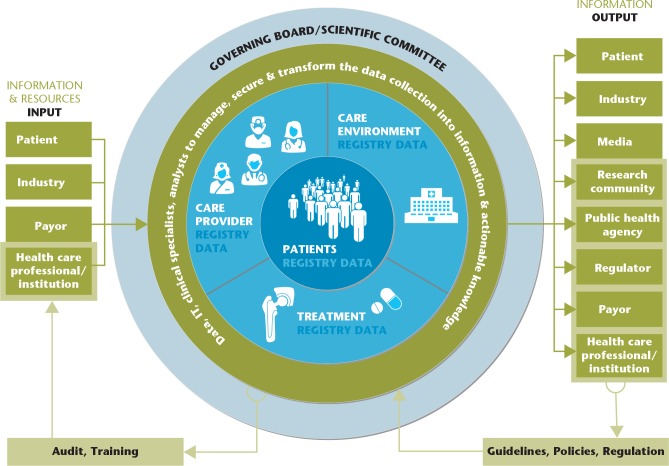
The registry itself is an organization consisting of the governing board/scientific committee and the data handling team (Fig. 1). The latter group is a team of people specializing in data collection, management and linkage, information technology (IT), and communication, as well as in clinical research methodology (clinical specialists, data analysts, epidemiologists). Data are transformed into information through the data collection, management, analyses and visualization phases. Information can be used for descriptive purposes: in a cross-sectional manner such as number of arthroplasties performed per year and the correlation between BMI and age at surgery; or longitudinally such as trends in patient characteristics, implant use and complication occurrence over time.5 It can further be used for analytic purposes, such as in the case of identification of risk and protective factors for a given outcome (e.g. revision surgery) and comparison of treatment outcomes. And third, the information can be used for evaluation of the effectiveness of public health efforts in areas such as, e.g., adequate performance of arthroplasty surgery, prevention of revision, regulation of implant use, or improvement of quality of life after surgery.
Fig. 1.
Registry stakeholders: relevant and interested parties and their relationships with the registry.
The governing board(s) guides and guarantees the purpose/mission of the registry, is responsible for the appropriate use of the data,6 and communicates with the data team and the stakeholders.
Registry stakeholders
Stakeholders are ‘A person, group or organization that has interest or concern in an organization. Stakeholders can affect or be affected by the organization’s actions, objectives and policies’.7 In healthcare the main stakeholders are Patients, Providers (professionals and institutions), Payors, and Policymakers (‘The four Ps’ in healthcare).8 Moreover, industry (e.g. medical device, pharmaceutical, biotechnology), regulators, research community, and media are also important. Stakeholders largely differ in interest and need, in support and attitude, and in influence; the latter in the positive as well as the negative sense. This makes their identification and the analysis of their points of view and interests crucial for the success of a mission-driven long-term collaborative effort, such as a clinical registry. It is of utmost importance that the governing board/committee and the registry data team are independent from any single stakeholder interest.
The mission of a registry needs to be clearly defined so that all stakeholders strive towards a common goal. The Australian Joint Replacement Registry (AOANJRR), for example, states their aims as follows:
‘Establish demographic data related to joint replacement surgery in Australia.
Provide accurate information on the use of different types of prostheses.
Determine regional variation in the practice of joint surgery.
Identify the demographic and diagnostic characteristics of patients that affect outcomes.
Analyse the effectiveness of different prostheses and treatment for specific diagnoses.
Evaluate the effectiveness of the large variety of prostheses currently on the market by analysing their survival rates.
Educate orthopaedic surgeons on the most effective prostheses and techniques to improve patient outcomes.
Provide surgeons with an auditing facility.
Provide information that can instigate tracking of patients if necessary.
Provide information for the comparison of the practice of joint replacement in Australia and other countries.’9
The mission influences the granularity of the information contained in the registry, and should address the different requirements of each of the involved partners. The fact that a multi-partner association was needed to get the Swiss National Hip and Knee Arthroplasty Registry (SIRIS) off the ground and flying, signified that more than one point of view had to be taken into consideration if success was to be achieved.10 Although the motivations pertaining to the significance of registries apply to all the partners involved, each partner tends to focus more on a particular aspect.
‘… Patients are the single most important stakeholder group with regard to the learning healthcare system. They are both the donors of personal clinical data and the ultimate beneficiaries from the knowledge gained.’11
In the case of joint replacement, patients expect their implants to provide them with a long-lasting, functional and pain-free result.12,13 The operation should be tissue sparing and complication-free, followed by rapid rehabilitation. The registry data should be presented in such a way as to be readily comprehensible, allowing patients to distinguish between fact and fiction in the ‘information jungle’ of orthopaedic arthroplasty implants. At the same time the registry must be built in a way that respects and protects the patient’s data privacy and takes into account his/her ownership.14–16
Surgeons are primarily concerned with avoiding complications and shortcomings for their individual patients. The implants must be of high quality in their manufacture, versatile and avoid problems such as early loosening, particle disease, heavy-metal poisoning (cobalt), breakage, dislocation, infection, stiffness, or chronic pain. A long, problem-free implant life with a minimum amount of wear of the bearing surfaces is the ultimate goal. The registry should identify in a relatively short time frame the problematic implants as well as the reliable and safe ones. Surgeons are essentially motivated by their own individual clinical results to enter proper and complete information into the data collection system with minimal interference in their daily activities. Surgeons will also want to benchmark their own results as compared with the overall results for each implant, technique, and patient or disease category. A moot question is the public availability of information at the individual surgeon level. This may lead to selection bias by encouraging some surgeon groups to avoid complex or complication-prone patients, who are then left to seek treatment in publicly funded institutions.
The industry’s main focus is on manufacturing and sales. Designing and providing a first-rate, problem-free, economically sustainable implant system is its primary goal. Progress and technical innovation are also powerful motivators for an industry dedicated to providing high-performance implants.13 The registry is seen as an essential tool of post-market surveillance and clinical control that justifies improvements in materials, design and concepts. In their view the downside is that monitoring and regulation may hinder efforts at innovation, thereby restricting opportunities to create new products that may have the potential to be better, safer and more profitable than existing ones. The industry works with/and depends on other stakeholders such as healthcare providers, regulators, and payors/insurance companies for pre- and post-market authorization, reimbursement and procurement processes.17,18
Hospitals aim to provide excellent and safe care, at a reasonable cost, to a large number of patients. Hospitals want to avoid the expenditures and hazards related to healthcare delivery using implant systems of uncertain reliability, value and safety. Teaching hospitals also look for not-too-steep learning curve systems thereby avoiding complications and early revisions. The registry is perceived as a quality control instrument, not only of the implants used, but of the whole chain of its clinical organization ranging from purchase and procurement decisions to the management of the care delivery process (e.g. pre-operative consultation, patient consent, procedures in the operating room, post-operative period). Hospitals, being healthcare-providing institutions in today’s competitive environment, are also very keen to uphold their reputations, and a registry is an invaluable tool for this purpose.19
Payors including government (e.g. the National Health Service in the UK), insurance companies and third-party payors, want minimal delays and waiting times for patients, short hospitalization times, no expensive re-admissions for complications (including revisions) and a quick return to work. Insurers are very cost-conscious when it comes to implant pricing, medical honoraria and hospital bills. The insurers’ wish is to provide equal benefits for all their clients within the budget available to them. The registry is perceived as an instrument for quality control of care providers and institutions and also as a cost-control tool. In particular, registries provide information and evidence for reimbursement decisions (e.g. coverage of a new device or technique conditional on evidence development).20
Policymakers including public health agencies and regulators are concerned with the welfare of the whole population. They therefore need data on the overall clinical activity for public health purposes, needs assessments and for planning the macro-economic policies related to healthcare. Moreover, they rely on registry information among others for health technology assessment and subsequent reimbursement policies.13,21 Public health agencies are also keen to ensure that the institutions under their supervision provide high-quality and complication-free healthcare to the overall population. The agencies will also have an interest in benchmarking hospitals and in keeping insurance and third-party payor costs down to a minimum. National and international regulatory bodies play an important role in supervising implant systems, as they seek to guarantee that the industrial specifications of nationally manufactured and imported implants are safe, efficient and reliable for public usage. In this endeavour they strongly rely on the evidence generated from international, national and regional registries.22–24
The research community (academia) is interested in advancing knowledge and generating high-quality evidence so that solutions for current healthcare challenges can be found and future problems can be anticipated or prevented. Moreover, research goes beyond the health domain and extends to societal, political and economic perspectives. The research community has the ability and responsibility to bring stakeholders together and translate the information generated in registries into understandable and actionable knowledge for all. The International Society of Arthroplasty Registries (ISAR) is an example of a research community in the field of joint replacement registries with ‘… a shared purpose of improving outcomes for individuals receiving joint replacement surgery worldwide. The focus of the society is to utilize the strength of cooperation and sharing of information and further enhance the capacity of individual registries to meet their own aims and objectives. The society is involved in the development of frameworks to encourage collaborative activities and provides a support network for established and developing registries’.25
The media are a tremendously important source of health information for the public. They play a strategic role in disseminating accurate information understandable for lay people26 and in educating the public regarding health issues.27 They have a unique potential in communicating health risks and benefits of new treatments to the public.28 However, inaccurate reporting (e.g. exaggeration of benefits and under-reporting of risks), hidden conflicts of interest, and misleading promotion are constant threats to be aware of.29 Finally, the media can influence decision-makers and politicians by giving a public forum to multi-stakeholder debates.
Stakeholders and registry input
In the context of clinical registries, stakeholder input consists of financial and data contribution (Fig. 1). Payors, in particular the government (e.g. Sweden, the UK), often entirely or partly contribute to the costs of creating and/or maintaining the registry. Other additional or sole registry cost contributors can be the hospitals (e.g. SIRIS, Switzerland) or the industry (e.g. AOANJRR, Australia).
Registry data input can be mandatory or voluntary. Most of the time data input is achieved by healthcare professionals and the patients (e.g. patient-reported outcomes), and this contribution is in general unpaid and not academically recognized. The data input consists first of all of a standardized long-term data contribution in a defined population for a specific purpose(s).1 The data can be provided on an institutional, regional, national or supranational level. The unit of this kind of data is typically the patient or the intervention. In addition, other types of data input can be temporary and more broad (unit-unrelated) such as detailed implant information provided to the registry by the industry. Harmonization of data input (in content and methods of data transmission), high coverage and completeness as well as audits and training of those who provide the data, are paramount for high registry quality. Moreover, the easier and shorter the input, the higher the likelihood of continuous reliable participation.
Stakeholders and registry output
The registry output consists of peer-reviewed publications, presentations at scientific meetings, detailed annual reports on implant performance (up to several hundreds of pages!); the latter in the field of joint replacement. The National Joint Registry (NJR) of England, Wales, Northern Ireland and the Isle of Man also provides information specifically for patients on a patient website and a patient blog.30 Moreover, registries may produce specific reports for industry, public health agencies (e.g. national quality agencies), and the media. The information generated is used in shared decision-making between patients and healthcare providers, providing evidence for post-marketing surveillance, health technology assessment, reimbursement and procurement decisions, and allows healthcare provider benchmarking. Registries can also generate risk alerts, which are relevant for all stakeholders. The Australian registry for example flags implants that are identified as having a higher than anticipated rate of revision.9 The case of metal-on-metal hip arthroplasty illustrates the importance of registry reporting.31
Registry reporting can be quasi real time, periodic or on demand. Annual reports from the national arthroplasty registries are open access. In general, there is no direct academic reward for researchers to publish annual reports or specific patient information. The stakeholders use the ‘registry output’ to produce guidelines, policies and regulation, which in turn can influence the future work of the registry (Fig. 1). Different stakeholders often publish on the same topic. This is a great resource, often under-acknowledged by other stakeholder groups. The recent publications on medical device regulation and evaluation from multiple stakeholders such as governmental organizations, public health agencies, regulators, academia, and the media are an enlightening example of this richness.32–38
Challenges
There are numerous challenges for registries and their stakeholders. Although every member of every stakeholder group is highly likely to become a patient once or more during her/his lifetime, and although all stakeholders seem to agree that patients are the single most important group,13 the reality is currently otherwise. Between the different stakeholder groups there exist ties of a financial, personal and/or structural nature that sometimes compete with the mission to provide necessary – as opposed to unnecessary – safe, efficient, and sustainable healthcare for all patients.34 There are also divergent motivations and hidden or unrecognized conflicts of interest. Moreover, the amount and quality of information that the patients receive from clinical registries – although improving – is still far too low. Stronger patient involvement in registries is needed for more patient-relevant information output.39 Patients’ data co-ownership and their influence on the registry mission and data collection priorities are part of this. These steps need to be supplemented by greater personal and time investment from the registry team and improved collaboration with the media.40–42
Costs are often unknown and/or unmeasured in registries. This is a major drawback. Knowing the costs of the care or treatment options is important for all stakeholders in order to make informed decisions about what is a valuable choice and what is not. However, a study among orthopaedic surgeons in the US reported that they rarely know the costs of implants, although the majority thought it would be important for the selection process.43
Registries may struggle to find and retain skilled team members such as data managers, statisticians and clinician-epidemiologists on a long-term basis. They particularly face difficulties in securing long-term funding streams when government funding is not available.19 Academically and clinically rewarding registry participation on the input as well as on the output side may be part of the solution for both problems.44
Finally, registries are adept at monitoring complex real-world situations because they can record multiple exposures, co-variates and outcomes over the long term. The ever-increasing pace of introduction of new treatments makes sole reliance on randomized controlled trials (RCTs) as the standard for demonstrating efficacy to support marketing authorizations almost impractical.11 However, registries can ‘host’ all types of research designs including RCTs to the advantage of both.45 Registries as part of Big Data have created high hopes. It will be of critical importance to check whether the knowledge generated by the registries and their stakeholders is implemented and proves to be of clinical and public health utility.19,46
Conclusions
In its best form, a registry is a mission-driven independent stakeholder–registry team collaboration enabling rapid, transparent, open-access knowledge generation and dissemination. As part of knowledge management systems, registries help ‘remembering the past, handling the present, preparing the future’.47 As society ages and healthcare costs escalate, the healthcare system faces serious challenges including the question of how to provide necessary – as opposed to unnecessary – safe, efficient, and sustainable healthcare for all and how to finance this. Registries and their stakeholders have the ability and the responsibility to contribute to addressing these challenges.
Footnotes
ICMJE Conflict of interest statement: PH declares grants from Fondation pour la recherche ostéoarticulaire. He is a board member of Fondation pour la recherche ostéoarticulaire and the Swiss Foundation for Innovation and Training in Surgery (SFITS), and he is the Editor-in-Chief of EFORT Open Reviews.
AL is an editorial board member of EFORT Open Reviews and a board member of Fondation pour la recherche ostéoarticulaire.
AJC has nothing to declare.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for evaluating patient outcomes: a user’s guide. Third ed. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [Internet] [PubMed] [Google Scholar]
- 2. Hoque DME, Kumari V, Hoque M, et al. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One 2017;12:e0183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen LA, Aisner D, McGinnis JM, eds. The learning healthcare system: workshop summary. Institute of Medicine (IOM) Roundtable on Evidence-Based Medicine. Washington, DC: National Academies Press, 2007 [PubMed] [Google Scholar]
- 4. Lehoux P, Williams-Jones B, Miller F, Urbach D, Tailliez S. What leads to better health care innovation? Arguments for an integrated policy-oriented research agenda. J Health Serv Res Policy 2008;13:251–254. [DOI] [PubMed] [Google Scholar]
- 5. Cnudde P, Nemes S, Bülow E, et al. Trends in hip replacements between 1999 and 2012 in Sweden. J Orthop Res 2018;36:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Data custodianship guidelines for the government of British Columbia. Version 1.1. 2017. (date last accessed 20 August 2018). [Google Scholar]
- 7. Post JE, Preston LE, Sauter-Sachs S. Redefining the corporation: stakeholder management and organizational wealth. Palo, Alto, CA: Stanford University Press, 2002. [Google Scholar]
- 8. Ritz D, Althauser C, Wilson K. Connecting health information systems for better health: leveraging interoperability standards to link patient, provider, payor, and policymaker data. Seattle, WA: PATH and Joint Learning Network for Universal Health Coverage, 2014. [Google Scholar]
- 9. Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, knee & shoulder arthroplasty: 2017 annual report. Adelaide: AOA, 2017. [Google Scholar]
- 10. Swiss National Joint Registry (SIRIS) Report 2012–2015. Bern 2017. http://www.swissorthopaedics.ch/images/content/SIRIS/170516_SIRISAnnualReport2015_Finalcopie.pdf (date last accessed 12 October 2018).
- 11. Eichler HG, Bloechl-Daum B, Broich K, et al. Data rich, information poor: can we use electronic health records to create a learning healthcare system for pharmaceuticals? Clin Pharmacol Ther 2018;September 4. doi: 10.1002/cpt.1226. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott CEH, Bugler KE, Clement ND, MacDonald D, Howie CR, Biant LC. Patient expectations of arthroplasty of the hip and knee. J Bone Joint Surg [Br] 2012;94:974–981. [DOI] [PubMed] [Google Scholar]
- 13. Henshall C, Schuller T. HTAi policy forum: health technology assessment, value-based decision making, and innovation. Int J Technol Assess Health Care 2013;29:353–359. [DOI] [PubMed] [Google Scholar]
- 14. Kish LJ, Topol EJ. Unpatients: why patients should own their medical data. Nat Biotechnol 2015;33:921–924. [DOI] [PubMed] [Google Scholar]
- 15. Evans BJ. Barbarians at the gate: consumer-driven health data commons and the transformation of citizen science. Am J Law Med 2016;42:651–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mouton Dorey C, Baumann H, Biller-Andorno N. Patient data and patient rights: swiss healthcare stakeholders’ ethical awareness regarding large patient data sets – a qualitative study. BMC Med Ethics 2018;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Ana FJ, Umstead KA, Phillips GJ, Conner CP. Value driven innovation in medical device design: a process for balancing stakeholder voices. Ann Biomed Eng 2013;41:1811–1821. [DOI] [PubMed] [Google Scholar]
- 18. Gilman BL, Brewer JE, Kroll MW. Medical device design process. Conf Proc IEEE Eng Med Biol Soc 2009;2009:5609–5612. [DOI] [PubMed] [Google Scholar]
- 19. Stey AM, Russell MM, Ko CY, Sacks GD, Dawes AJ, Gibbons MM. Clinical registries and quality measurement in surgery: a systematic review. Surgery 2015;157:381–395. [DOI] [PubMed] [Google Scholar]
- 20. Garber AM. Evidence-based coverage policy. Health Aff (Millwood) 2001;20:62–82. [DOI] [PubMed] [Google Scholar]
- 21. OECD. New health technologies: managing access, value and sustainability. Paris: OECD Publishing, 2017. [Google Scholar]
- 22. International medical device regulator forum. Principles of international system of registries linked to other data sources and tools, 2016. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-160930-principles-system-registries.pdf (date last accessed 12 October 2018).
- 23. Methodological principles in the use of international medical device registry data, 2017. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-170316-methodological-principles.pdf (date last accessed 15 October 2018).
- 24. Tools for assessing the usability of registries in support of regulatory decision-making, 2018. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-180327-usability-tools-n46.pdf (date last accessed 15 October 2018).
- 25. International Society of Arthroplasty Registries (ISAR). Mission statement. http://www.isarhome.org/statements (date last accessed 15 October 2018).
- 26. Salita JT. Writing for lay audiences: a challenge for scientists. Medical Writing 2015;24:183–189. [Google Scholar]
- 27. Institute of Medicine (US) Committee on Assuring the Health of the Public in the 21st Century. Chapter 7: media. In: The future of the public’s health in the 21st century. Washington, DC: National Academies Press, 2002. [PubMed] [Google Scholar]
- 28. Moynihan R, Bero L, Ross-Degnan D, et al. Coverage by the news media of the benefits and risks of medications. N Engl J Med 2000;342:1645–1650. [DOI] [PubMed] [Google Scholar]
- 29. Schwitzer G, Mudur G, Henry D, et al. What are the roles and responsibilities of the media in disseminating health information? PLoS Med 2005;2:e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Joint Registry (NJR), United Kingdom. Working for patients. http://www.njrcentre.org.uk/njrcentre/Patients/tabid/74/Default.aspx (date last accessed 8 October 2018).
- 31. Cohen D. Out of joint: the story of the ASR. BMJ 2011;342:d2905. [DOI] [PubMed] [Google Scholar]
- 32. European Commission. The new regulatory frameworks on medical devices, 2017. https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_en (date last accessed 12 October 2018).
- 33. International Medical Device Regulator Forum. http://www.imdrf.org/documents/documents.asp (date last accessed 12 October 2018).
- 34. OECD. Chapter 4: ensuring timely and affordable access to medical devices. In: New health technologies: managing access, value and sustainability. Paris: OECD Publishing, 2017. [Google Scholar]
- 35. World Health Organization. WHO global model regulatory framework for medical devices including in vitro diagnostic medical devices. Geneva: World Health Organization, 2017. [Google Scholar]
- 36. Fuchs S, Olberg B, Panteli D, Perleth M, Busse R. HTA of medical devices: challenges and ideas for the future from a European perspective. Health Policy 2017;121:215–229. [DOI] [PubMed] [Google Scholar]
- 37. Lübbeke A, Silman AJ, Prieto-Alhambra D, Adler AI, Barea C, Carr AJ. The role of national registries in improving patient safety for hip and knee replacements. BMC Musculoskelet Disord 2017;18:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joyce TJ. Failing medical implants are causing hundreds of thousands of people misery. The Conversation, Sep 2017. https://theconversation.com/failing-medical-implants-are-causing-hundreds-of-thousands-of-people-misery-84230 (date last accessed 15 October 2018).
- 39. Nelson EC, Dixon-Woods M, Batalden PB, et al. Patient focused registries can improve health, care, and science. BMJ 2016;354:i3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The Conversation. Who we are. https://theconversation.com/uk/who-we-are (date last accessed 17 October 2018).
- 41. Kaiser Family Foundation. Media internships and fellowships for journalists. https://www.kff.org/media-internships-fellowships/ (date last accessed 15 October 2018).
- 42. HealthNewsReview.org. Gary Schwitzer, publisher & founder of HealthNewsReview.org. https://www.healthnewsreview.org/about-us/reviewers/gary-schwitzer/ (date last accessed 15 October 2018).
- 43. Okike K, O’Toole RV, Pollak AN, et al. Survey finds few orthopedic surgeons know the costs of the devices they implant. Health Aff (Millwood) 2014;33:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berwick DM, Jain SH, Porter ME. Clinical registries: the opportunity for the nation. Health Affairs Blog, 11 May 2011. https://www.healthaffairs.org/do/10.1377/hblog20110511.010833/full/ (date last accessed 17 October 2018).
- 45. Ioannidis JP, Adami HO. Nested randomized trials in large cohorts and biobanks: studying the health effects of lifestyle factors. Epidemiology 2008;19:75–82. [DOI] [PubMed] [Google Scholar]
- 46. Rumsfeld JS, Joynt KE, Maddox TM. Big data analytics to improve cardiovascular care: promise and challenges. Nat Rev Cardiol 2016;13:350–359. [DOI] [PubMed] [Google Scholar]
- 47. Ronald Maier. Knowledge management systems: information and communication technologies for knowledge management. Third ed. Berlin, Heidelberg, New York: Springer, 2007. [Google Scholar]