Abstract
The National Joint Registry (NJR) was established in 2002 as the result of an unexpectedly high failure rate of a cemented total hip replacement.
Initial compliance with the Registry was low until data entry was mandated. Current case ascertainment is approximately 95% for primary procedures and 90% for revision procedures.
The NJR links to other data sources to enrich the reporting processes. The NJR provides several web-based and open-access reports to the public and detailed confidential performance reports to individual surgeons, hospitals and industry bodies.
A transparency and accountability process ensures that device and surgical performance are actively monitored on a six-monthly basis, and adverse variation is dealt with in an appropriate way that underpins patient safety.
The NJR also manages a comprehensive research-ready database and data protection compliant access system that enables external researchers to use the dataset and perform independent analyses for patient benefit.
Moving forwards, the NJR intends to look at factors that lead to better outcomes so that good practice can be embedded into routine care.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180084
Keywords: joint replacement, outcomes, patient safety, quality improvement, registry
Introduction
Sir John Charnley recommended in 1972 that, ‘Serious consideration should be given to establishing a central registry to keep a finger on the pulse of total implant surgery on a nationwide basis. Surgeons should not be permitted to perform total hip implant work (especially those involving the use of cement) unless prepared to have weekly returns made of the operations as they are performed and thereafter to have patients questioned annually by circular from the Registry’. However, he went on to say, ‘Obviously this will be fiercely resisted as an encroachment on professional liberty but if the work from a general Orthopaedic Department is good there will be no grounds for dissatisfaction – the existence of scrutiny will be a powerful factor in dissuading consultants in General Hospitals in the event of the unpredicted absence from an operating session’.1
The first national arthroplasty registry was established by the Swedish Knee Arthroplasty Registry in 19752 followed by the Swedish Hip Arthroplasty Registry in 1979.3 In the UK two regional registries were established in the early 1990s; the Trent Regional Arthroplasty Study4 and the North West Arthroplasty Register.5 Although there was a clinical recommendation that a national joint register should be established in 1996,6 this did not happen. The following year a cemented total hip replacement, the 3M Capital Hip, was reported as having a higher than expected incidence of femoral component loosening7 and in February 1998 a hazard notice was issued by the Medical Devices Agency8 which recommended that all patients implanted with this device should be called for clinical review. A total of 4,669 prostheses were supplied to 95 clinical centres in the UK9 but many of the patients with the Capital Hip could not be readily identified. This caused considerable public concern and associated media interest. A report produced by the Royal College of Surgeons of England10 concluded that ‘If a national hip registry had been in place to collect appropriate information then poorly performing hip replacements could have been detected at a much earlier stage. This would have reduced the pain, anxiety and potential immobility of patients. It is my hope that this report will play its part in helping to bring such a registry into being’. The National Joint Registry of England and Wales (NJR) was established in 2002 on the recommendation of Lord Hunt, the Parliamentary Under-Secretary of State for Health, and started to collect data in April 2003. It was envisaged that the Registry would have the benefit of providing a major research database, would allow comparative audit of hospitals and prostheses as well as monitoring of new joint replacement prostheses and would make it possible to identify patients requiring urgent clinical recall.11 Since then the NJR has evolved considerably. This review will outline the development and current status of the NJR with particular emphasis on how it has made an impact on clinical practice.
NJR expansion and development
Compliance with the NJR was mandated for hip and knee replacement surgery carried out in the independent sector from the outset, but not for the NHS until 2011. As a result, buy in and compliance were low in the early years. In 2004 compliance was just over 43%, 80% in 2006 and approximately 95% in 2015. The NJR was linked to a routinely collected NHS dataset, the Hospital Episode Statistics (HES) that enriched the data-reporting process. In 2008 the NJR started to look at variation in revision outcomes for implants and individual surgeons and developed a process to manage potential adverse variation. In 2010 the NJR started to collect information on ankle joint replacement surgery and in the same year the Registry was linked to the Department of Health Patient Reported Outcome Measures (PROMs) data collected on patients undergoing hip and knee replacement surgery. In 2011 the NJR launched its Supplier Feedback system and also published the Public and Patient Guide and established an NJR Patient Network. In 2012 the NJR started to collect information on elbow and shoulder replacements. In the same year annual reports were provided to hospitals containing confidential information about their performance (Annual Clinical Report) as well as publicly available data on hospital volumes and revision rates. In 2013 the NJR extended to include Northern Ireland and in 2015 the Isle of Man was added, the NJR now being the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man.
How is the NJR funded?
When the NJR was set up it was funded by applying a levy of about £25 to the cost of every hip and knee replacement procedure carried out whether the operation was registered or not. The levy was added on to the cost of the implant, paid by the hospital and collected by the supplier and then forwarded to the Registry. This funding method had the advantage that the Registry received the funding even if the procedure was not submitted to the Registry. Total funding was over two million pounds per annum and has increased as the volume of surgery has increased. Over recent years the costs have been reduced by obtaining additional income from industry for access to the Supplier Feedback service. Current costs equate to about £15 per procedure, although the money is now collected as an annual subscription payment from the hospital rather than a levy. The more procedures the hospital carries out, the higher the subscription. To put these costs in perspective they represent about 0.25% of total procedural costs for NHS cases and about 0.2% for cases carried out in the independent sector.
How NJR data are made available
NJR data, and analyses based upon this data, are made available to a wide range of stakeholders in a secure and managed way. Due to the high-profile nature of the NJR and its increasing recognition as a valuable resource, demand for data and indicators from the NJR continues to increase year on year. The NJR data are used in line with the terms to which patients consent when they agree to their data being recorded in the Registry. Anonymized data are made available to authorized stakeholders, allowing them to answer questions about why patients need joint replacement, comparing different treatments and how they work, and by studying the outcomes and complications of joint diseases and their treatment. Our first priority is to protect those who have given their details to the Register. Examples of NJR outputs are given in the sections below divided into two groups ‘open/public access’ and ‘secure/restricted access’.
Open/public access
Annual report
The NJR Annual Report12 presents analysis of the data submitted. It profiles key trends in surgical practice, activity levels; implant usage and patient demographics, along with chosen specialist research topics. Over the years the amount of information in the annual report has increased and reports over longer time periods (now 15 years). Critically, hip replacements are listed as constructs rather than just as brands. This means that the many different types of stem/socket combinations can be assessed and, where numbers allow, the effect of the bearing used and age and gender of the patient can be considered. The online Annual Report provides interactive web access to content from the NJR Annual Report, providing visitors with the ability to analyse and compare data across years, and to filter and segment results to a greater extent than possible through the printed report. The NJR website receives around 5,000 visitors per month.
Patient perspective
The NJR is aware how important it is for patients and members of the public to understand its purpose and potential benefit. To aid this the Registry supplies hospitals with patient information leaflets that explain how a patient’s data are used and why their consent is vital for the monitoring process. It also publishes a Public and Patient Guide13 in addition to its main Annual Report every year. The guide is produced with input and advice from the NJR Patient Network and provides information in a format that is focused on patients rather than surgeons.
NJR Surgeon and Hospital Profile website
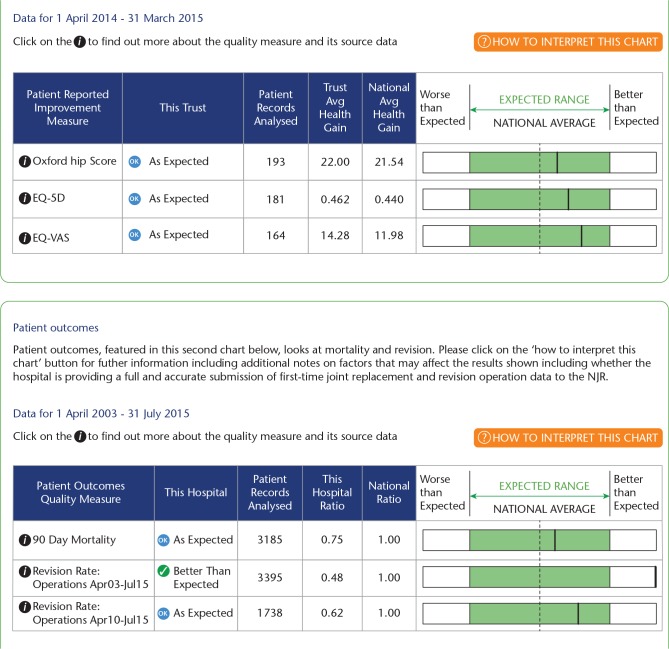
This is a public website14 profiling surgeon and hospital-level activity and outcomes based upon NJR data (Fig. 1). It was developed as part of the NHS England Consultant Outcomes Publication initiative and launched in 2013. It allows patients and the public to look up any hospital or surgeon and review the number and type of cases performed and, for hospitals, the outcomes achieved. The hospital outcomes include revision rates over the entire NJR period and over the last five years. It also shows the mean gain in the Oxford Hip or Oxford Knee Score compared to the national average gain, the EQ-5D and the EQ-VAS. Thermometer plots demonstrate whether the performance by the hospital is within the expected range, or better or worse than expected (Fig. 2).
Fig. 1.
National Joint Registry Surgeon and Hospital Profile homepage.
Fig. 2.
Hospital outcome performance displayed within the National Joint Registry Surgeon and Hospital Profile website.
Secure/restricted access
Clinician Feedback
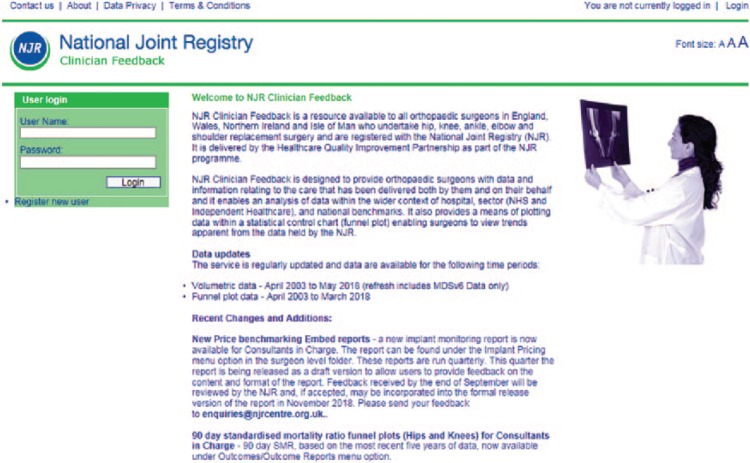
Clinician Feedback is a web-based portal that can be accessed by surgeons to review activity and personal outcome data.15 Access is password protected and confidential to the surgeon (Fig. 3). Clinician Feedback allows clinicians to review their data recorded on the NJR through a series of interactive graphs, charts, reports and data tabulations. It allows surgeons to review their outcomes data and to assess whether this is within the expected range, and enables them to preview their data prior to publication on the NJR Surgeon and Hospital Profile website.
Fig. 3.
National Joint Registry Clinician Feedback portal.
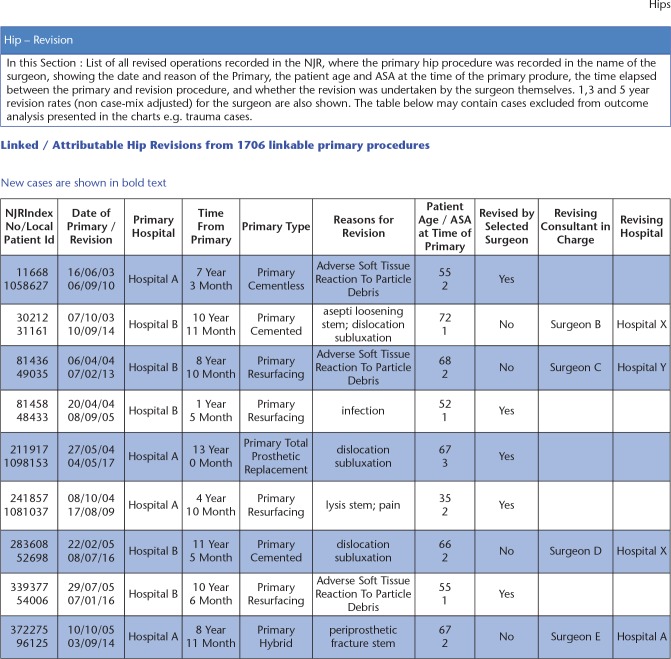
Some data are interactive and can be filtered by patient demographics, procedure type, time period and location of activity. Other reports are downloadable including the Consultant-Level Report (Surgeon Report), Annual Clinical Report (Hospital Report), and procedural-level data related to primary and revision surgery (Fig. 4). The primary procedure report enables the surgeon to download all primary procedures and the endpoint for each, i.e. viable, revised, or patient deceased. It can be filtered by joint type, procedure type, patient case-mix, endpoint, and time. It can be viewed either on screen or downloaded as a PDF or spreadsheet. The spreadsheet provides the most detailed data including, for example, details of what was revised, the indications for revision, and (for all procedures) the time from the primary procedure to the first change in endpoint. Where a procedure has been revised by another surgeon, the surgeon’s name and hospital are now included in the report. The report does not necessarily provide the ‘current’ end state but the first change since the primary took place. For example, if a patient has died after the revision of a primary procedure, the end state will be reported as ‘Revised’, not ‘Deceased’. There is a similar report for revision procedures.
Fig. 4.
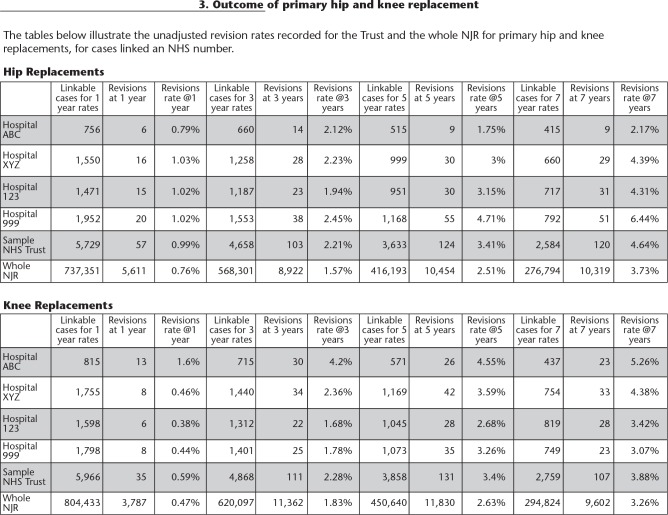
Details of primary cases that have been revised are listed in a table in the Consultant-Level Report (mock data displayed).
Consultant-Level Report
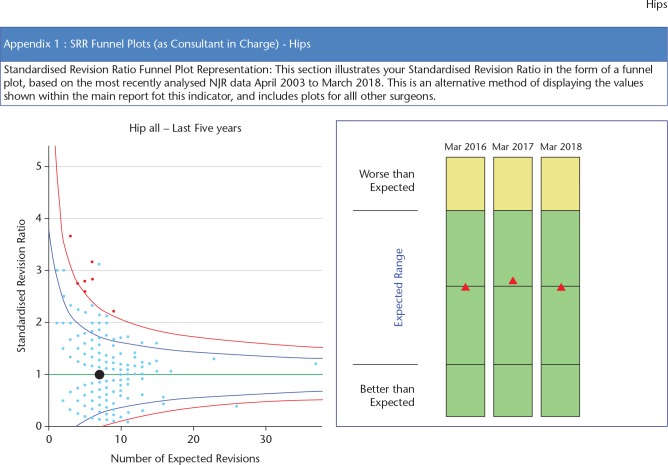
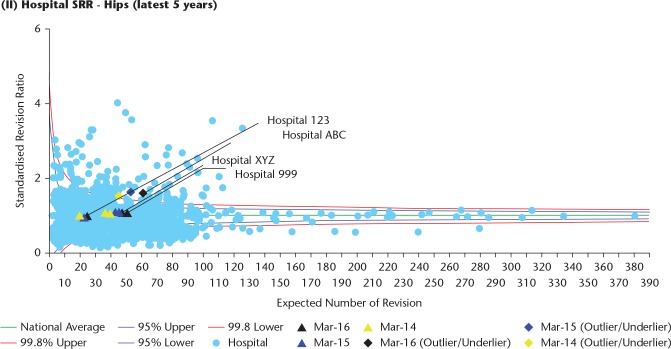
This is accessed via the Clinician Feedback portal. The Consultant-Level Report16 provides clinicians with a downloadable PDF report summarizing their activity and outcomes. The report has been designed specifically to provide supporting information in annual appraisal and five-yearly revalidation. The surgeon is able to review all activity related to hip and knee replacement surgery by fixation, bearing and, for hips, use of ODEP-rated components (Orthopaedic Data Evaluation Panel). Outcome data are displayed as unadjusted revision rates at various time periods, observed compared to expected number of revisions and data are displayed as funnel plots with 95% and 99.8% confidence boundaries (Fig. 5). Separate charts are available for different types of hip and knee replacement (Table 1).
Fig. 5.
Standardized revision rates are displayed in a funnel plot. The black dot is the surgeon, the pale blue dots are all other surgeons in the Registry. The dots in red display surgeons with higher than expected revision rates.
Table 1.
Data contained in the Consultant-Level Report
| Activity over last 12 months and 36 months by hospital site and type of surgery | |
|---|---|
| Hip activity by subtype of fixation and primary/revision and bearing combination | |
| Patient | ASA BMI and age |
| Outcomes | Standardized Revision Ratios (SRR) and Standardized (90-day) Mortality Ratios (SMR) presented as observed rate to expected rate and also as funnel plots for both hip and knee replacement surgery. |
| Hip subtypes | All primary hip procedures over the lifetime of the National Joint Registry (NJR). |
| All primary hip replacement procedures over the lifetime of the NJR (less withdrawn/excluded implants. | |
| All primary hip replacements over the last five years. | |
| Primary cemented hip procedures over the lifetime of the NJR. | |
| Primary uncemented hip procedures over the lifetime of the NJR. | |
| Primary hybrid hip procedures over the lifetime of the NJR. | |
| Primary metal-on-metal hip replacements over the lifetime of the NJR. | |
| Hip resurfacing over the lifetime of the NJR. | |
| List of hip revisions linked to a primary procedure | NJR index number and local ID number, date of primary, date of revision, primary hospital, time from primary, primary type, reasons for revision, patient age and ASA grade at time of primary, revised by selected surgeon, revising consultant in charge, revising hospital. |
| Counts of revised primaries by year. | |
| Unadjusted revision rates at 1, 3 and 5 years. | |
| Number of attributable revisions unadjusted revision rate and national average. | |
| Linkable/attributable hip revisions of revisions from linkable revision procedures. | |
| Details of any 90-day mortality events. | |
| Hips only: Implant usage by Orthopaedic Data Evaluation Panel (ODEP) rating acetabular and femoral component. | |
| Knee subtypes | Similar to hips but groupings are: |
| All primary knee procedures. | |
| Primary cemented knee replacement. | |
| Primary uncemented knee replacement. | |
| Unicondylar knee replacement. | |
| Patella-femoral replacements. | |
Annual Clinical Report for hospitals
In England and Wales NHS organizations are known as Trusts. These Trusts often have more than one hospital within the Trust organization. Normally this comprises a large acute site hospital and a smaller hospital for elective or planned surgery. Most private or independent hospitals have one site but most are affiliated with a larger business organization. The majority of independent hospitals carry out some NHS-funded activity. The NJR send an Annual Clinical Report to every Trust or independent hospital that has recorded NHS activity. The report contains information on the quality of NJR data submissions and details of unadjusted revision rates at various time points for hip, knee, shoulder, elbow and ankle surgery (Fig. 6). More detailed information in the form of funnel plots is provided for hip and knee replacement surgery for the hospital and how the hospital compares with all other hospital in the Registry (Fig. 7).17
Fig. 6.
In the Trust Report (Annual Clinical Report) unadjusted revision rates of the hospitals within the Trust are displayed over various time points with comparative data for the whole National Joint Registry.
Fig. 7.
Standardized revision rates are displayed as funnel plots for the hospitals. The different colours represent different endpoints so that performance over the years can be seen.
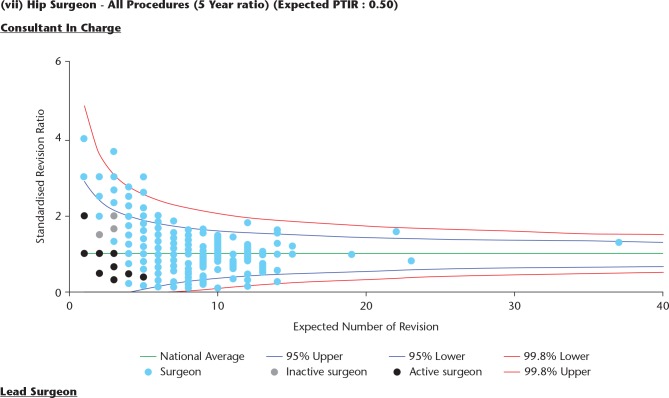
Individual surgeons are not identified in the plots by name but they represented by a number to provide confidentiality (Fig. 8). The NJR recommends that the group of surgeons working within the hospital collectively reviews the report on an annual basis so that outcomes and procedure techniques can be compared and discussed.
Fig. 8.
Within the hospital report all the surgeons working in the hospital are shown as black dots. This plot shows standardized revision rates for all hip procedures over the last five years.
Implant pricing data
Implant pricing data are provided at hospital and individual surgeon level.18 This information can be downloaded from the Clinician Feedback portal. Visibility of this costing data is intended to provoke discussion and reflection between individual surgeons and the hospital management team. Information is given about product usage for primary and revision hip and knee replacement. For hips this includes a breakdown of fixation and bearing choices within different age groups of patients. The costs within these groupings are displayed as well as comparative costs within the hospital between surgeons and comparative costs between the hospital and national averages.
Research Data Access Portal
The NJR is a resource that is available to external researchers conducting new and clinically relevant research. Approved researchers are provided with access to research-ready data through the NJR Data Access Portal.19 Information on existing projects can be found on the NJR website.20
Supplier Feedback
Supplier Feedback is a portal that provides medical device suppliers access their own product data and is available subject to subscription cost.21 Data are updated on a monthly basis and provide information on the use of their implants, and outcomes for patients receiving their implants. This allows suppliers to assure the on-going safety, quality and appropriate usage of their implants and, where applicable, supports post-market surveillance.
Transparency and accountability
The NJR is funded as part of the NHS. This leads to inevitable tensions between the politicians who want all outcome data to be published, and the surgeons who have concerns about the effects of publishing detailed outcome data at surgeon level. For now, we continue to publicly publish unit outcomes and mortality data, and surgeon data on patient demographics, diagnoses and mortality but not on revision as an outcome. With the support of the NHS medical director in 2017 we published our Accountability and Transparency Model.22 This has five limbs underpinned by a software management system. These comprise: management of hospital,23 surgeon24 and implant outliers,25 appraisal26 and implant mismatch notification.27 Stakeholders (including purchasers, regulators, surgeon and hospital representatives and quality improvement teams) have been extensively consulted. For each topic there are a published methodology, a series of flow charts detailing every step of each process, together with Responsible, Accountable, Consulted and Informed (RACI) charts clarifying who is responsible for, accountable for, consulted and informed about each of the steps, and detailing relevant timeframes for the outlier process. Memoranda of understanding and service level agreements have been drawn up with all the stakeholders.
Management of the unit or hospital
All 147 NHS Trusts and all independent hospitals in England and Wales are provided with an annual report (Annual Clinical Report) so that they can reflect on their activity and outcomes in relation to hip and knee replacement surgery. In addition to this the NJR data are analysed twice a year to identify potential adverse performance. The surgical performance committee of the NJR reviews this data and where there are concerns about adverse performance the medical director of the hospital is informed. The hospital is asked to undertake an audit and provide an action plan to the NJR surgical performance committee. If this does not happen or if the action plan is considered inadequate by the NJR then the UK regulator the CQC (Care Quality Commission)28 is informed and takes any necessary further action. The British Orthopaedic Association (BOA)29 is available to undertake an invited elective service review if an external review is considered to be of benefit by the hospital. Members of NHS Improvement30 and the Getting It Right First Time initiative (GIRFT)31 are also informed.
Individual surgeon performance
The process for assessing and managing surgical performance has been an evolutionary one, with close collaboration between experienced clinicians, the Bristol University statistical team, and Northgate Public Services (data storage contractor).32 Data were first analysed to assess implant and surgical performance when five years’ worth had been accumulated. It was clear that there was a huge variety in the type and volumes of practice between the surgeons and units submitting data. Funnel plots were the method chosen to compare performance, with those lying outside of the 99.8% confidence limit being deemed at outlier or ‘alarm’ level. An expected number of revisions was generated from the overall patient time incidence revision rate (PTIR), this allowed for the construction of plots for surgeons and units which from the outset were carried out for all hips, and separately for each of the subtypes (cemented, cementless, hybrid, resurfacing) to facilitate detailed scrutiny. Similarly, for knee replacements with all types together and the corresponding subtypes. Ninety-day mortality was similarly studied. Further refinement was added in after three years with the introduction of case-mix adjustment. Revision risk was adjusted for age, diagnosis and gender, and mortality for age gender and American Society of Anaesthetists (ASA) grade. This then became expressed as a standardized revision/mortality ratio.
Once a surgeon crosses the 95% threshold they are notified, and recommended to review their data and share with local colleagues. Once the 99.8% threshold is crossed, the data are peer reviewed within the Surgical Performance Committee, and then usually both the surgeon and his unit medical director are notified.
Initial concerns over data accuracy and completeness meant that identification of surgeons and units at this level were primarily an indication for an internal audit to be triggered, and the data carefully verified before internal action was taken – this being the responsibility of the unit. The data are refreshed every six months. They are analysed for linked revisions and 90-day mortality for the last five years of data and for revisions on the last 10 years also. Beyond 10 years the data are still analysed, but not used for outlier assessment.
To date there are just over 6,000 surgeons with data for hip or knee replacement, or both. Of these, 130 have appeared at some point as outliers for hip replacement surgery and 131 for knee replacement. Currently there are 47 hip surgeons with outlier data at 10 years and 9 at five years. In relation to knee surgery there are 57 surgeons with outlier data at 10 years and 18 at five years. The causes of performance variation are variable and at times complex. In some instances, adverse performance may be related to the use of a poorly performing implant or bearing material. In others there may be a problem with infection and/or instability indicating potential issues with surgical technique. Sufficient data have not yet accumulated to enable analysis for shoulders, elbows or ankles.
In 2012 there was political pressure for the Registry to provide revision rates for individual surgeons and make this information available for public scrutiny. The profession was concerned that this, amongst other things, would drive a risk-averse culture and others questioned whether the validity of the NJR data was sufficient to allow robust and fair reporting. It could also be open to legal challenge. As a result, a compromise was reached whereby data were published on individual surgeons in relation to type and volume of activity and 90-day mortality but not for revision rates. Instead revision rates and PROMs data were published at the hospital level but not for individual surgeons. It is possible that there will be pressure in the future to publish revision rates for surgeons. If this does occur it is likely to be at a time when the Registry data have been fully validated, compliance rate is high (over 99%) and when there have been several years of internal reporting of adverse performance.
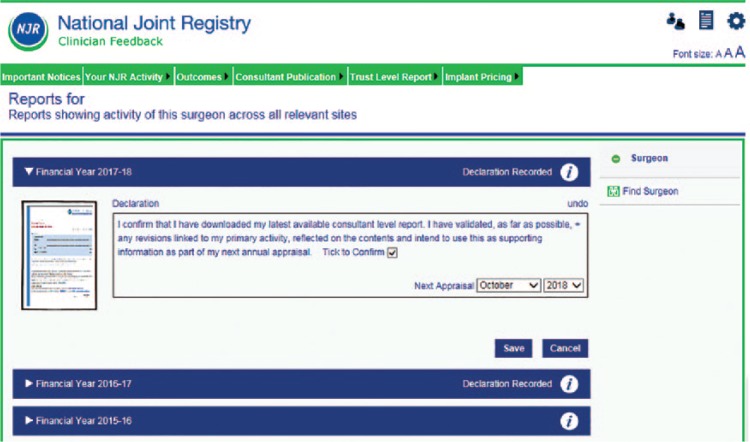
Using NJR data in appraisal
In 2017 the appraisal notification system was made available. This appends to a statement to the annual downloadable Consultant-Level Report that states: ‘I confirm that I have downloaded my latest available consultant level report. I have validated, as far as possible, any revisions linked to my primary activity, reflected on the contents and intend to use this as supporting information as my next annual appraisal’. This box may only be ticked after downloading the report (Fig. 9). For the first time this year the Annual Report to Hospitals included the names of all consultants contributing data from that hospital, and if they have downloaded their individual reports and have confirmed the statement.
Fig. 9.
Appraisal declaration made online.
Monitoring of devices
All implants are monitored on a six-monthly basis and assessed using outlier methodology. If the revision rate (PTIR) is twice that of the group average (with separation of confidence intervals) (an alarm) then the UK regulator the MHRA (Medicines and Healthcare products Regulatory Agency) are informed. It is the regulator (and not the NJR) that decides on the appropriate action. Where the PTIR is between 1.5 and 2 times the group PTIR this represents alert level (level 2). Level 2 also includes implants with twice the group PTIR but less than 100 implants. In this case a notification is sent to the company to inform them of a potential performance issue but the regulator is not informed. As of January 2017, 163 brands of knee implant have been monitored with the identification of 4 Level 1 outliers and 12 Level 2 outliers. Some 2,806 different combinations of hip stem/cup implants have been monitored with 17 Level 1 outliers and 40 Level 2 outliers.
Most of the larger suppliers have access to a monthly download about their own products (Supplier Feedback). They do not have access to competitor information but they have enough information to monitor the performance of their own devices. This promotes a degree of self-regulation and also supports post-market surveillance on recently introduced devices.
Monitoring implant performance was the prime reason for the establishment of the NJR as the result of the 3M Capital Hip failure. Prior to 2003 we had very little knowledge of joint replacement activity or outcome in England and Wales but within five years we were starting to see robust data on the revision rates and survivorship of most of the pertinent implants that were being used. Adverse outcomes were identified with several metal-on-metal articulations which resulted in several Medical Device Alerts form 2008 onwards with reporting of the Articular Surface Replacement (ASR) resurfacing in 2010. Without the NJR these devices would have been identified at a much later stage and as such the value of a Registry is not to prevent device failure put to pick up adverse performance at an early stage in order that expedient action can be taken to reduce patient harm.
Data are supplied to the ODEP33 which provides benchmarking of implants within the UK and worldwide. The panel consists of clinicians and other members who invite industry to provide data from a variety of sources (not just Registry data) on the implants. The ODEP then consider the submission and provide a benchmark at 3, 5, 7 and 10 years. The strength of the submission is graded A*, A, B or C depending on the quality of the data submitted at the appropriate benchmark period. The ODEP works independently from the NJR.
In relation to how new and innovative products could be introduced to market, it was recognized that closer post-market surveillance would be useful and an initiative ‘Beyond Compliance’34 was introduced in 2012. It was named Beyond Compliance because this was voluntary and beyond the European regulatory requirements. Beyond Compliance uses NJR data but also obtains more granular data such as PROMs and radiographs to monitor more closely new products.
Implant mismatch notification
The real-time (at time of data entry) alert and follow up of possible implant mismatch notification is new and has identified 21 genuine implant mismatches in the first year. We are currently investigating the possibility of developing a stand-alone app for use on a mobile or computer in the operating theatre that will scan implants in real time and immediately alert staff of any incompatible combinations, to supplement the current checks carried out in the operating theatre.
How does the National Joint Registry support research?
The NJR is the world’s largest research-active joint replacement registry with over 2.5 million registered procedures. As it continues to mature in its second decade it has an ever-increasing ability to provide data that help researchers answer important questions and improve the lives of patients undergoing joint replacement. At the same time, the issue of security of person-identifiable data (PID) continues to make media headlines. We have also witnessed substantial changes to the way our national bodies control access to PID, most recently through the European General Data Protection Regulations. In this brief review of our recent and current activities we outline how the NJR has evolved to meet these dual responsibilities.
How is research using the NJR resource managed?
The Research Committee is responsible for delivering the NJR research agenda. The Committee’s aims are to maximize data access for researchers and to promote the profile and branding of NJR outputs, but also to protect the NJR dataset and strengthen its governance through safe, effective, and efficient data management in line with European legislation. We aim to help research applicants successfully navigate the ‘legislative waters’, and generate high-impact research outcomes for both patients and the profession. These developments include a single entry point for all research applications arising within or external to the NJR, use of an annually updated build of the research dataset, and the recent development of a bespoke online Data Access Portal for researchers.
The NJR research pathway
The first step when submitting an application is to check that the topic of interest is not already being studied by checking the NJR research library. The next step is the download and completion of a simple Expression of Interest (EOI) form that outlines the area of research proposed. The EOI is then reviewed within the Research Committee to determine the complexity and feasibility of the proposed project.35 Applications requesting unlinked and fully anonymized NJR data are directed down the ‘external’ route, whilst applications requesting access to linked datasets or PID are directed down the ‘internal’ or ‘collaborative’ project route. Internal projects require participation of an NJR Steering Committee member co-applicant to guarantee UK data governance requirements for traceable PID.
What areas of research does the NJR support and how do we share the data?
Proposed research must be of potential benefit to patients, be feasible, relevant, novel, and ethically sound. Accordingly, we have established a broad set of themed areas within which we will consider applications for data access. Data must be shared within the prevailing legal framework in a way that is, where possible, cost-neutral to NJR. To address this need we have introduced the online Data Access Portal to maximize safe access to, whilst retaining ownership of, the dataset. Access to the portal for approved projects is made using an access key unique to each project applicant. We have also developed a ‘research-ready’ annual data build, enabling researchers to access a pre-cleaned dataset ready to ‘plug and play’. These resources will facilitate more comfortable engagement with the broader national and international research agenda, reducing the burden on researchers by providing a single, ‘clean’ source of data, updated each year with a new cumulative dataset, and a safe online environment within which to work using the resource.
Research Fellows
The NJR also fosters a Research Fellowship scheme in partnership with the Royal College of Surgeons of England, designed to build registry-based research capacity within the clinical orthopaedic community. We invite applications for new fellows on an annual cycle, with appointees typically undertaking a two-year fellowship term. These posts are advertised each September through the NJR e-bulletin.
Research outputs
The research output from the NJR has been substantial over several years. A full list of NJR-assisted publications and research requests can be found on the NJR website.36 Taken in combination, these facilities help create a strong foundation for research activity using the NJR dataset.
Conclusions
The NJR has matured over the 15 years it has been in operation. It started off with descriptive data and now contains high-quality detailed outcome data. We have moved from knowing very little about descriptive practice and outcomes to a position where we have extremely detailed and accurate information. The paradigm shift has been to be able to identify implants that are performing poorly at an early stage and take early remedial action and, through ODEP, to provide information to benchmark devices over longer timeframes. Similarly, in relation to surgeons and hospitals, providing regular feedback regarding activity and outcomes has been key in encouraging reflection on performance. The NJR is now integrated with and integral to the joint replacement care package. There are other emerging orthopaedic related condition and procedure-based registries that have the potential to provide much needed evidence on optimal treatment. Presently these registries are under the umbrella of the BOA and are known as the Trauma and Orthopaedic Unifying Structure (TORUS).37 These registries consist of The British Spine Registry (BSR),38 The UK National Ligament Registry (NLR),39 The UK Knee Osteotomy Registry (UKKOR),40 The Non Arthroplasy Hip Register (NAHR),41 The Bone and Joint Infection Register (BAJIR),42 The British Orthopaedic Foot and Ankle Society Registry (BOFAS Registry),43 The UK National Hand Registry,44 and the British Limb Reconstruction Society Registry (BLRS).45 The NJR may have an important role in assisting these registries with technical and infrastructure support.
Footnotes
ICMJE Conflict of interest statement: MP declares salary and travel/accommodations/meeting expenses from the UK National Joint Registry, activity outside the submitted work.
JMW is a member of the NJR steering committee, and his time spent on NJR business is remunerated by the NJR to his employer.
MP is seconded to the NJR for two days a week and his hospital is reimbursed for this time.
All other authors have nothing to declare.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Charnley JC. The organisation of a special centre for hip surgery. Internal Publication Number 39. July 1972. [Google Scholar]
- 2. Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish Knee Arthroplasty Register: a nation-wide study of 30,003 knees 1976–1992. Acta Orthop Scand 1994;65:375–386. [DOI] [PubMed] [Google Scholar]
- 3. Herberts P, Ahnfelt L, Malchau H, Stromberg C, Andersson GB. Multicenter clinical trials and their value in assessing total joint arthroplasty. Clin Orthop Relat Res 1989;249:48–55. [PubMed] [Google Scholar]
- 4. Fender D, Harper WH, Gregg PJ. The Trent Regional Arthroplasty Register. JBJS (Br) 2000:82-B:944–947. [DOI] [PubMed] [Google Scholar]
- 5. Porter ML. The North West Arthroplasty Register. JBJS (Br) 2000:82:Supp II. [Google Scholar]
- 6. Sochart DH, Long A, Porter ML. Joint responsibility: the need for a national arthroplasty register. BMJ 1996:313:66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massoud SN, Hunter JB, Holdworth BJ, Wallace WA, Juliusson R. Early femoral loosening in one design of cemented hip replacement. J Bone Joint Surg (Br) 1997;79:603–608. [DOI] [PubMed] [Google Scholar]
- 8. Medical Devices Agency. Hazard notice. London: MDA, 1998. (MDA9801) [Google Scholar]
- 9. Muirhead-Allwood SK. Lessons learnt of a hip failure. BMJ 1998; 316:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An Investigation of the performance of the 3MTM CapitalTM Hip System. Report by The Royal College of Surgeons of England; July 2001. https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=2ahUKEwju7dDL2I3eAhVmx4UKHY8zCQcQFjABegQICBAB&url=https%3A%2F%2Fwww.rcseng.ac.uk%2Flibrary-and-publications%2Frcs-publications%2Fdocs%2Fcapital-hip-system%2F&usg=AOvVaw2-T_Vglo8dzfFrMvH6FRc3 (date last accessed 17 October 2018). [Google Scholar]
- 11. Joint Approach. The Newsletter of the NJR February 2003. https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwii_5L6hJreAhXCTMAKHZV_C6oQFjAAegQIBhAC&url=http%3A%2F%2Fwww.njrcentre.org.uk%2FNjrCentre%2FPortals%2F0%2FDocuments%2FEngland%2Fnewsletters%2FIssue1.pdf&usg=AOvVaw1NJy-P32daOvMonYrV7itV (date last accessed 22 October 2018).
- 12. NJR Annual Reports. http://www.njrreports.org.uk/ (date last accessed 17 October 2018).
- 13. NJR Public and Patient Guide. http://www-new.njrcentre.org.uk/njrcentre/Reports-Publications-and-Minutes/Public-and-Patient-Guide (date last accessed 22 October 2018).
- 14. NJR Surgeon Hospital Profile Website. http://www.njrsurgeonhospitalprofile.org.uk (date last accessed 22 October 2018).
- 15. NJR Clinician Feedback. http://www.njrcentre.org.uk/njrcentre/Healthcare-providers/Accessing-the-data/Clinician-Feedback (date last accessed 22 October 2018).
- 16. Consultant-Level Report. http://www-new.njrcentre.org.uk/njrcentre/News-and-Events/NJR-Consultant-level-report (date last accessed 22 October 2018).
- 17. Annual Clinical Report. http://www-new.njrcentre.org.uk/njrcentre/Healthcare-providers/Accessing-the-data/Management-Feedback (date last accessed 22 October 2018).
- 18. NJR implant pricing data. http://www.njrcentre.org.uk/njrcentre/Implant-procurement (date last accessed 22 October 2018).
- 19. NJR Data Access Portal. www.njrreports.org.uk/Research (date last accessed 22 October 2018).
- 20. Existing NJR research projects. http://www.njrcentre.org.uk/njrcentre/Research/Research-Portfolio (date last accessed 22 October 2018).
- 21. NJR Supplier Feedback Portal. http://www.njrcentre.org.uk/njrcentre/Implant-suppliers (date last accessed 22 October 2018).
- 22. NJR Accountability and Transparency Model. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model (date last accessed 17 October 2018).
- 23. NJR: Hospital process. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model/Alert-and-Alarm-Unit-Hospital-Process (date last accessed 22 October 2018).
- 24. NJR surgeon process. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model/Alert-and-Alarm-Surgeon-Process (date last accessed 22 October 2018).
- 25. NJR implant process. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model/Implant-Outlier-Process (date last accessed 22 October 2018).
- 26. NJR appraisal enhancement. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model/Surgeon-Appraisal-Enhancement-Process (date last accessed 22 October 2018).
- 27. NJR implant mismatch notification. http://www.njrcentre.org.uk/njrcentre/NJR-Accountability-and-Transparency-Model/Implant-Mismatch-Notification-Process (date last accessed 22 October 2018).
- 28. Care Quality Commission. https://www.cqc.org.uk/ (date last accessed 22 October 2018).
- 29. British Orthopaedic Association. https://www.boa.ac.uk/ (date last accessed 22 October 2018).
- 30. NHS Improvement. https://improvement.nhs.uk/ (date last accessed 22 October 2018).
- 31. Getting it Right First Time. gettingitrightfirsttime.co.uk (date last accessed 22 October 2018). [Google Scholar]
- 32. Northgate Public Services. https://www.northgateps.com/ (date last accessed 22 October 2018).
- 33. Orthopaedic Data Evaluation Panel. www.odep.org.uk (date last accessed 17 October 2018).
- 34. Beyond Compliance. www.beyondcompliance.org.uk (date last accessed 22 October 2018).
- 35. NJR Research Committee. http://www.njrcentre.org.uk/njrcentre/Research/Research-requests (date last accessed 22 October 2018).
- 36. NJR research: list of papers and publications. http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2015th%20AR%20Online%20Appendices%20Part%204.pdf (date last accessed 22 October 2018).
- 37. Trauma and Orthopaedic Registries Unifying Structure. https://www.boa.ac.uk › Professional Practice (date last accessed 22 October 2018).
- 38. The British Spine Registry. www.britishspineregistry.com/ (date last accessed 22 October 2018).
- 39. The UK National Ligament Registry. www.uknlr.co.uk/ (date last accessed 22 October 2018).
- 40. The UK Knee Osteotomy Registry. www.ukkor.co.uk/ (date last accessed 22 October 2018).
- 41. The Non Arthroplasty Hip Registry. https://www.britishhipsociety.com/main?page=NAHR (date last accessed 22 October 2018).
- 42. The Bone and Joint Infection Register. https://bajir.org/ (date last accessed 22 October 2018).
- 43. The British Orthopaedic Foot and Ankle Registry. https://www.bofas.org.uk/ (date last accessed 22 October 2018).
- 44. The UK National Hand Registry. https://www.bssh.ac.uk/about/news (date last accessed 22 October 2018).
- 45. The British Limb Reconstruction Society Registry. blrs.org.uk/ (date last accessed 22 October 2018). [Google Scholar]