Abstract
Total hip arthroplasty (THA) registers are established in several countries to collect data aiming to improve the results after THA. Monitoring of adverse outcomes after THA has focused mainly on revision surgery, but patient-reported outcomes have also been investigated.
Several surgery-related factors influencing the survival of the THA have been thoroughly investigated and have changed clinical practice. These factors include surgical approach, specific implants, the size of the components, type of fixation and different bone cements.
Register data have been used to examine the risk of venous thromboembolism and bleeding after THA. These investigations have resulted in shorter duration of thromboprophylaxis and a reduced frequency of blood transfusion.
Registers may provide specific information to surgeons on the outcome of all THAs that they have performed with a detailed analysis of revisions rates and reasons for the revisions.
A number of other stakeholders can use register data to provide benchmarks. The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man supplies data to the Orthopaedic Device Evaluation Panel (ODEP), which provides benchmarks at 3, 5, 7, 10, and 13 years graded from A*, A, B and C.
Future perspectives: National registers have to play a major role in documenting the quality of THA in order to describe best practice and report implant outliers. The registers have to be used for research and post-market surveillance and register data may be a source for intelligent decision tools.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180091
Keywords: total hip arthroplasty, register, prosthetic survival, outlier, thromboprophylaxis, bleeding, surgeon feedback, stakeholders
Background
The establishment of total hip arthroplasty (THA) registers started in the Scandinavian countries in 1979.1 Later on, several countries outside Scandinavia followed with the establishment of nationwide or regional THA registers. The aim of the registers was to collect patient- and surgery-related data to influence surgeons’ choice of implants, fixation method, patient selection and peri-operative management and to improve the outcome after THA. This impact of the registers on orthopaedic practice can be obtained by open access transparent publication of results in scientific journals and annual reports, discussion of results and the role of registers at meeting in the orthopaedic community, feedback mechanisms to the hospitals and regulatory bodies and so on. The impact of registers may be evaluated by measuring the influence on mortality, quality of care including patient safety (complications) and effectiveness (adherence to clinical guidelines), healthcare utilization and healthcare costs.2
Each register contains data that make it possible to investigate rare events; this may be even more obvious with collaboration of registers.3,4 Data from hip arthroplasty registers have mainly been used to investigate adverse outcomes of primary THA leading to revision surgery.5-7 In some countries, a unique personal identification number makes it possible to combine healthcare data at a personal level and investigate adverse outcomes other than those related to surgery, e.g. medical outcomes and mortality.8,9
To our knowledge, only Malchau et al have reported on the development and impact of arthroplasty implant registers based on two American and two Swedish registers.10 The focus of this paper is to report examples of some of the European and the Australian THA quality registers that had had an impact on clinical practice and to give future perspectives of the registers’ role.
Examples of impact
The Swedish Hip Arthroplasty Register
Established in 1979, the Swedish Hip Arthroplasty Register (SHAR) was the world’s first national quality register collecting data on hip arthroplasty.1 A few years after its inception, all hospitals performing hip arthroplasty in Sweden contributed and have continued to do so. Orthopaedic surgeons soon learnt to appreciate the feedback of results. The first large long-term follow-up study based on the register highlighted the importance of implant selection and cementing technique.11 Several underperforming implants associated with worse implant survival were identified resulting in change of practice. This study was among the first to demonstrate the importance of systematic implant surveillance through an arthroplasty register. For example, owing to Swedish surgeons’ cautious attitude towards undocumented new concepts, Sweden avoided the catastrophes associated with metal-on-metal (MoM) implants. Today, six different stem designs account for > 92% of all femoral components used in THA in Sweden. Likewise, for acetabular components, ten different cup designs account for > 82% of the production.12
Thein et al investigated design-related features of the three most commonly used stems in Sweden. Although the most common stem had the lowest crude revision rate, the smallest stem size and extended femoral head were associated with increased risk of revision.13 After presenting the findings, there was a marked decrease in the use of components with these particular attributes.
The inclusion of hemiarthroplasties in the Swedish Hip Arthroplasty Register in 2005 was yet another pioneering work. Very few national arthroplasty registers comprise hemiarthroplasties. Due to the established data collection routines, all hospital units immediately joined with a completeness at case level of 95%.12 Early findings on surgical approaches and the risk of dislocation dramatically influenced the choice of surgical technique in favour of the direct lateral approach.14,15 Doubtless, Swedish register data support the exceptionally high use of cemented fixation in hemiarthroplasties: uncemented stems carry an increased risk of re-operation in general and due to peri-prosthetic fracture, in particular, without differences in mortality.15 Subsequently, Sweden has withstood the increasing use of uncemented fixation for hemiarthroplasty observed internationally.
In 2002, a prospective patient-reported outcome measures (PROMs) follow-up programme was introduced in the SHAR and became nationwide in 2008. Patients are asked to complete the PROMs, including the EQ-5D-3L and EQ VAS, pre-operatively and at different time-points after THA surgery. Since the introduction, a small improvement in the PROMs has been demonstrated.16,17 Hence, the impact of the SHAR on arthroplasty practice in Sweden is not attributable to isolated major discoveries. It is based on continuous in-depth analyses, persistent communication with the profession and open reporting of results at hospital unit level. The resulting homogeneous use of well-documented implants and methods has yielded outstanding long-term implant survivorship.18
The Finnish Arthroplasty Register
The Finnish Arthroplasty Register (FAR) was founded in 1980. Based on FAR data, Puolakka et al reported a nine-year survival rate of only 65% (95% confidence interval (CI) 61 to 69) for the cementless Biomet THA which was due to the inferiority of the cup and the hexaloc locking mechanism of polyethylene liner. The authors recommended that Biomet cups with hexaloc liners should be abandoned.19
In 2005, Eskelinen et al evaluated the population-based survival of THA fixation methods in osteoarthritis patients aged < 55 years and the factors affecting survival. It was shown that, for younger patients, uncemented proximally circumferentially porous- and hydroxyapatite-coated stems are the implants of choice.20 The results for patients aged < 55 years with rheumatoid arthritis were similar.21 These publications notably supported that uncemented THA became very popular in Finland.
The Norwegian Arthroplasty Register
The Norwegian Arthroplasty Register started as a hip register in 1987; from the start, it followed patients from primary operation to revision of the implant or death using the unique 11-digit person identification of Norwegian inhabitants. The unique catalogue number of implants was entered into an implant library using the implant stickers, thus enabling the register to document poor-performing implants from an early stage. After five years of service, the register identified that cemented hip implants had better short-term results, both in young and older patients, than uncemented implants.22-24 This was mainly due to poor fixation of uncemented cups without roughened surface and stems without circumferential roughened surfaces. With longer follow-up to ten years, the uncemented cups with initial good performance performed poorly due to wear of the polyethylene liner.25,26 Several bone cements were also identified as poorly performing after only three years of follow-up, especially the Boneloc cement and a low viscosity cement.27,28 With a longer follow-up, three cemented implants—the Titan stem, the Spectron EF stem and the non-crosslinked EtO sterilized all-polyethylene Reflection cup—had inferior performance.29,30 Some uncemented hemispherical cups and fully coated uncemented stems had good long-term fixation.31,32 The surgeons instantly stopped using the implants if a specific implant was identified as poorly performing and, in the Nordic countries, the use of cement containing antibiotics was established as routine after publications of fewer revisions due to infections.33,34
Documentation of poorly performing implants, cements and techniques have led to lower revision rates, reduced healthcare cost and reduced suffering for patients.35-37
The Danish Hip Arthroplasty Register
The Danish Hip Arthroplasty Register (DHR) was established in 1995.38,39 Besides important monitoring in annual reports and specific studies on patient- and implant-related risk factors for adverse outcome,40-42 other adverse outcomes including venous thromboembolism (VTE) and blood transfusion have also been thoroughly investigated.
Pharmacological thromboprophylaxis is a standard and well-accepted peri-operative treatment. Despite treatment, risk of symptomatic VTE has been reported to be up to 6% within 90 days of THA surgery.43-45 In Denmark, the risk of symptomatic VTE within 90 days is 1%46 and has been stable during the last 15 years despite change in prophylaxis practice. The duration of the treatment has been a matter of debate for years and there is no consensus in guidelines regarding duration of thromboprophylaxis. The 2012 American College of Chest Physicians guidelines recommended using anticoagulation drugs for a minimum of 10 to 14 days with grade 1B evidence and suggested extending prophylaxis for up to 35 days with grade 2B evidence.47 The 2012 guidelines from the National Institute for Health and Care Excellence recommended prophylaxis for 28 to 35 days.48 In contrast, the 2011 guidelines from the American Academy of Orthopedic Surgeons recommended individual assessment of the optimal duration of thromboprophylaxis without any elaboration on the THA patients who may benefit from extended prophylaxis.49 Administration of extended thromboprophylaxis after THA has proven difficult in many clinical settings; concerns about treatment benefit and risk in routine clinical practice have remained. In 2015, a study was published based on data from the DHR, examining risk of symptomatic VTE and major bleeding associated with short-term (1 to 6 days), medium-term (7 to 27 days) compared with extended (⩾ 28 days) therapy. The 90-day risks of VTE were 1.1% (short), 1.4% (medium) and 1.0% (extended), yielding an adjusted hazard ratio of 0.83 (95% CI 0.52 to 1.31) and 0.82 (95% CI 0.50 to 1.33) for short and standard versus extended treatment. Risk of bleeding was similar in the three groups.50 This study was the key piece of evidence for making national Danish guidelines regarding thromboprophylaxis: 1) thromboprophylaxis for THA patients should not exceed ten days; and 2) prophylaxis for THA performed in fast-track settings should not exceed length of stay when hospitalized for a maximum of five days.51
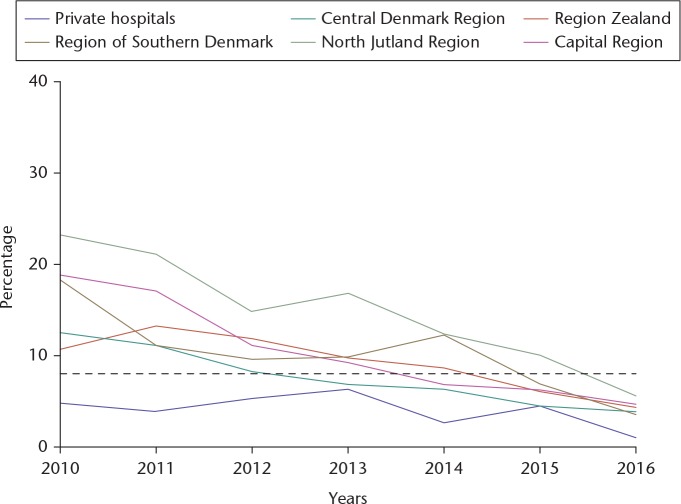
In 2009, a study based on DHR data reported on substantial differences in the use of red blood cell transfusion among THA patients in Danish orthopaedic departments (16% to 64%). The differences in the use of blood transfusions could not be explained by a range of patient- and surgery-related factors, which suggested that the variation did not reflect differences in the patients' need for blood transfusion, but rather true differences in transfusion policy.52 In addition, the same group published that red blood cell transfusion was associated with an adverse prognosis following primary THA, in particular with increased odds of death and pneumonia.53 Subsequently, a new quality indicator was initiated in the DHR: ‘Use of blood transfusion within 7 days of THA surgery’ linking DHR with data from the Danish Transfusion Database. During the period 2010 to 2016, the average use of blood transfusion decreased from 16% in 2010 to 4% in 2016, and variation between the five Danish regions was reduced from 7% to 24% in 2010 to 1% to 6% in 2016 (Fig. 1).
Fig. 1.
The percentage of primary THA patients diagnosed with osteoarthritis of the hip receiving blood transfusion within seven days from surgery during years from 2010 to 2016.5
The Australian Orthopaedic Association National Joint Replacement Registry
Provision of information to orthopaedic surgeons is one of the most effective means of influencing outcomes of joint replacement surgery as they are best placed to influence change. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), established in 1999, provides information in multiple ways, including the Annual Report, regular Scientific Presentations of register data and the provision of individual surgical outcomes. Surgeons use this information to improve the outcomes of THA; examples include the reduction in use after identification of outlier prostheses such as the ASR,54,55 a reduction in the overall use of resurfacing hip replacement and its use largely in males aged < 65 years, and encouraging the use of cemented femoral stems in older patients.56
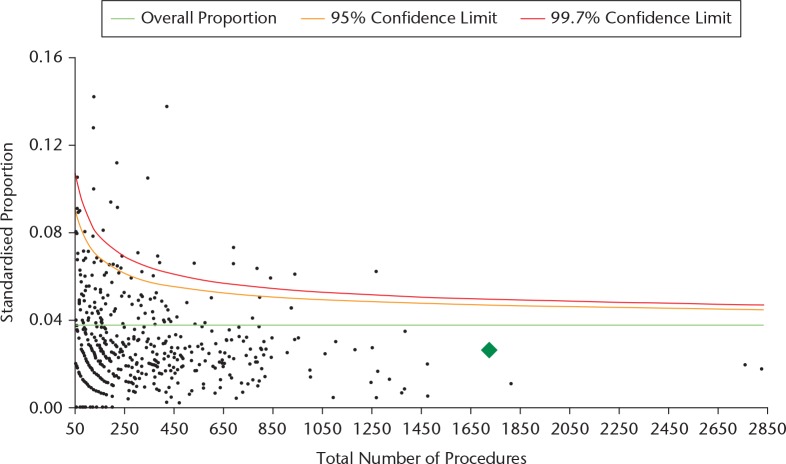
The AOANJRR has continually worked to improve the quality and timing of the information provided to surgeons. All Australian orthopaedic surgeons who have performed joint replacement surgery can access their personal information through a secure Web portal which was first made available in 2009. This information is up to date within four to six weeks of procedures being recorded in the register. Surgeons can download a complete report of the outcome of all joint replacements that they have performed with a detailed analysis of the rates of revision of the prostheses they have used with tables of the types and reasons for the revisions. They also are given a funnel plot that displays the variation in revision rate by volume of procedures performed by the surgeon, with the national average and the 95% and 99.7% upper confidence limits displayed. The individual surgeon is represented by a green diamond that demonstrates their performance with respect to their peers (Fig. 2). Funnel plots are provided for several options associated with THA including overall outcomes for all diagnoses and all types for revisions, and outcomes for specific revision diagnoses such as prosthesis dislocation or revision for infection within two years. This enables surgeons to identify their performance, compare themselves to the national average and examine the reasons for revision. The Australian Orthopaedic Association has recommended access of a surgeon’s individual reports with funnel plot data be counted as a specific requirement of ongoing Continuous Professional Development for those surgeons performing joint replacement.
Fig. 2.
Funnel plot of surgeon’s primary THA (excluding large head metal-on-metal) for all diagnoses with revision for any reason.
Australian orthopaedic surgeons are making increasing use of register data; feedback from surgeons indicates that the process is an important element in the way they practice and will often motivate change, especially if they are outside the norms of national outcomes. Examples where surgeons use register data on a regular basis include tables of outcomes of specific prostheses and figures of revision rates by age or gender. Surgeons can interact with patients in consultations to provide them with accurate, up-to-date and unbiased information and register data can be used to show each patient the typical outcomes for other patients with similar demographic and co-morbidity profiles.
The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man
The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) was established in 2002 with the intention of identifying implants with a higher than expected failure rate and in the event of a ‘recall’ to be able to notify patients so that appropriate action could be taken. Over the years, the NJR has evolved considerably and now provides specific data and feedback, and reports to a number of ‘stakeholders’ (Table 1).
Table 1.
The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) supports performance and regulation.
| Activity | Objective | How does it do this? | Other bodies | Reporting period |
|---|---|---|---|---|
| Clinician performance | To provide feedback | Clinician feedback portal | Clinician and Hospital | Biannual |
| Hospital performance | To alert on adverse performance | Annual clinical report and NHS Choices website | Hospital, CQC and NHS Improvement | Biannual |
| Implant performance | To identify implants with inferior performance | Implant performance committee | MHRA | Biannual |
| New Technology | Safe introduction of new devices | Providing data | Beyond Compliance | Daily |
| Benchmarking | Safe use of implants | Providing data | ODEP | Annual |
| Safety | Component mismatch | Web-based alerts | NJR and Hospital | Daily |
| Service Development | Service improvement | GIRFT programme | GIRFT programme and NHS Improvement | Annual |
NHS, National Health Service; CQC, Care Quality Commission; MHRA, Medicines and Healthcare Products Regulatory Agency; OPED, Orthopaedic Device Evaluation Panel; GIRFT, Getting it Right First Time.
Before the NJR, there was very little useful information on national activity and performance in relation to hip replacement surgery. The data and reports provide insight and reflection and the regulatory components drive accountability and patient safety. In relation to implant monitoring two levels of outlier have been defined:57
Level 1: An implant has a patient time incidence rate (PTIR) of at least twice the overall PTIR, for implants that have been used in at least 100 primary operations in the NJR.
Level 2: An implant has a PTIR of at least twice the overall PTIR, for implants that have been used in < 100 primary operations in the NJR. Or when an implant has a PTIR of at least one and a half times the overall PTIR, for implants that have been used in at least 100 primary operations in the NJR.
As of January 2017, 2806 different combinations of hip stem/cup implants have been monitored with 17 Level 1 outliers and 40 Level 2 outliers.
The NJR did not prevent the problems with MoM bearings but it did identify the failure at an early stage and reported this to the Medicines and Healthcare Products Regulatory Agency. This resulted in regulatory action and a significant change in practice. It was recognized that enhanced post-market surveillance would be useful and an initiative called ‘Beyond Compliance’ was introduced in 2012.58 It was named Beyond Compliance because this was voluntary and beyond the European regulatory requirements.
The NJR supplies data to the Orthopaedic Device Evaluation Panel (ODEP).59 The panel consists of clinicians and other members who invite the industry to provide data from a variety of sources (not just register data) on the implants. The ODEP then consider the submission and provide a benchmark at 3, 5, 7, 10 and 13 years. The strength of the submission is graded from A*, A, B and C depending on the quality of the data submitted at the appropriate benchmark period.
The NJR monitors potential component mismatches as the data are submitted. Examples of this would be incompatible head and liner combination.60 If these data are entered, the hospital is alerted to check for a potential data entry error or address the clinical situation.
Future perspective
Data completeness and quality
There is a constant need for validation of data regarding completeness and quality. Completeness requires that all patients can be identified in more than one register in the region or country. Several registers do these evaluations routinely, but others are not able to do so.
Regarding data quality, there is a need for the use of implant barcodes and catalogue numbers to identify specific implants at risk including sizes, offset etc. Regarding revision surgery, there is a discrepancy in the definition between registers which is why harmonization is needed. Several registers have shown that only around 60% of revisions due to infections are captured in national registers, which is a clear limitation.61 By merging with other databases holding information on bacteria cultures, registers can capture around 90% of the infections. Thus, merging of databases is a way to go for better data quality on infections.
Research
The registers have been used in research for years.62 Research using the national registers as a source of data will continuously play a major role in future improvement of practice. The focus of register research should not only be on the implants, where differences may be small, but also on patient selection and complications. Patient selection is a major task and the registers only holds information on those who have had surgery. However, in some countries there are registers on patients with osteoarthritis treated non-operatively.63 Registers may also play a more important role in executing randomized controlled trails (RCT) designed for a register (RRCT). Such a RRCT may be much more powerful than traditional RCTs with an increased efficiency and cost-effectiveness.64 By adding a randomization feature in a register, unselected consecutive inclusion can be done. This may overcome the limitations of RCTs where generalizability and statistical power in particular can be problematic. Other possibilities are to perform cluster randomization at department level using register parameters as primary or secondary outcomes or merging with other databases.
Collaboration with health authorities
The registers provide data and produce reports but other bodies, typically health authorities, provide the clinical governance and regulation. In Europe, the rules are based on European Union (EU) Council Directives. Health authorities receive information about implant performance and recalls from the industry. In many countries, there is no direct collaboration between the registers and health authorities, which may delay crucial information to reach the surgeons and have severe consequences for the patients. This is exemplified by the ASR recall from DePuy in 2010: the AOANJRR published concerns regarding this implant in the annual report 2006. The year after, the ASR was identified as an outlier. Thus, it took three years and many thousands of operations before the information came to the authorities and resulting in withdrawal of the implant from the market. This underlines that there is a need for better collaboration between registers and health authorities.
Collaboration between registers
There is a need for the sharing of results between registers. Some registers are too small to study rare events such as implant fracture or outcome for rare patient groups, which is why the merging of databases between countries can be attractive. The focus should be implant outliers and observation of new implants on the market. This was the motivation for the Nordic countries where hip, knee and shoulder arthroplasty registers have been merged.65
Post-market surveillance
Post-market surveillance is the long-term monitoring of safety and efficacy of the implants for the benefit of the patients. Europe has a common regulatory environment and the new EU device regulation will enforce post-market surveillance.66 Industry has to react to poor results together with the health authorities in order to have safe implants on the market and, further, industry has to deliver documentation for their products in order to keep them on the market necessitating collaboration with the registers. One important role for the registers is to collect patient characteristics and outcomes other than revision procedures (e.g. co-morbidity, use of medication, smoking status and PROMs) in order to improve analyses on register data. This information might be achieved by linkage to other data sources including national patient registers and prescription databases.67 Data have to be read and understood by industry which may require consultancy with register experts.
Development of intelligent decision tools
With the rapidly growing literature, we cannot expect that every surgeon is updated on risk factors for revision and inferior outcome of THA. Moreover, it is not likely that clinicians can put all these data together and extrapolate the results. The use of artificial intelligence by extraction from big databases followed by machine-learning through identifying risk factors will have a future within the treatment of the patients.68 It is obvious that registers that hold the results of the average surgeon and hospital deliver data for such intelligent systems that can automatically be updated. Indeed, it is an attractive vision for the future that these technologies can play a role in the decision-making for treatment which may make the treatment safer, more efficient and cost-effective. However, there are significant challenges for the development and use of intelligent decision tools, including the need for evidence of effectiveness and safety, methodological issues (e.g. data quality and patient privacy and consent), and clinical implementation and utility.69
Conclusion
THA registers have influenced the orthopaedic practice in many aspects, e.g. changes in choice of implants and fixation method, peri-operative management and patient selection. Examples of impact from many THA quality registers have been demonstrated; however, more registers provide relevant information than those mentioned (e.g. the Dutch Arthroplasty Register and the New Zealand National Joint Register). Although open access survivorship data are important for implant outlier detection, peer-reviewed scientific papers with adjustment and discussion are still vital to change treatment practices and several important papers have been published during recent years. Further, register data have been used in the concept of stepwise introduction of new implant technology70,71 and in the future, THA registers may focus on extending the collaboration between registers to develop a stronger foundation for post-market surveillance and intelligent decision tools.
Footnotes
ICMJE Conflict of interest statement: CV reports travel/accommodations/meeting expenses to his institution from Zimmer Biomet, Denmark unrelated to activities listed.
OR reports payment for lectures from Zimmer Biomet, outside the submitted work.
CR reports payment for lectures from Zimmer Biomet, Depuy, Johnson & Johnson and Swemac, outside the submitted work.
RDS reports grants/pending grants from National Health and Medical Research Council Australia, outside the submitted work.
SO reports grants/pending grants from Zimmer Biomet and Viking Medical, outside the submitted work.
MP reports payments to his institution by the National Joint Registry of England and Wales through previous employment, outside the submitted work.
GH reports payments for lectures from Ortomedic AS and Link Norway, outside the submitted work.
All other authors have nothing to declare.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Herberts P, Ahnfelt L, Malchau H, Stromberg C, Andersson GB. Multicenter clinical trials and their value in assessing total joint arthroplasty. Clin Orthop Relat Res 1989;249:48-55. [PubMed] [Google Scholar]
- 2. Hoque DME, Kumari V, Hoque M, et al. Impact of clinical registries on quality of patient care and clinical outcomes: A systematic review. PLoS One 2017;12:e0183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop 2009;80:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furnes O, Paxton E, Cafri G, et al. Distributed analysis of hip implants using six national and regional registries: comparing metal-on-metal with metal-on-highly cross-linked polyethylene bearings in cementless total hip arthroplasty in young patients. J Bone Joint Surg [Am] 2014;96-A:25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danish Hip Arthroplasty Register. Annual Report 2018. 2018. http://danskhoftealloplastikregister.dk/wp-content/uploads/2015/11/DHR-årsrapport-2018_til-offentliggørelse.pdf (date last accessed 24 March 2019).
- 6. Seppanen M, Karvonen M, Virolainen P, et al. Poor 10-year survivorship of hip resurfacing arthroplasty. Acta Orthop 2016;87:554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varnum C, Pedersen AB, Kjaersgaard-Andersen P, Overgaard S. Comparison of the risk of revision in cementless total hip arthroplasty with ceramic-on-ceramic and metal-on-polyethylene bearings. Acta Orthop 2015;86-4:477-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541-9. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen AB, Mehnert F, Sorensen HT, et al. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J 2014;96-B:479-85. [DOI] [PubMed] [Google Scholar]
- 10. Malchau H, Garellick G, Berry D, et al. Arthroplasty implant registries over the past five decades: Development, current, and future impact. J Orthop Res 2018;36:2319-30. [DOI] [PubMed] [Google Scholar]
- 11. Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978-1990. Acta Orthop Scand 1993;64:497-506. [DOI] [PubMed] [Google Scholar]
- 12. Kärrholm J, Mohaddes M, Odin D, et al. The Swedish Hip Arthroplasty Register - Annual Report 2017. 2018. https://registercentrum.blob.core.windows.net/shpr/r/Eng_Arsrapport_2017_Hoftprotes_final-Syx2fJPhMN.pdf (date last accessed 24 March 2019).
- 13. Thien TM, Kärrholm J. Design-related risk factors for revision of primary cemented stems. Acta Orthop 2010;81(4):407-12. doi: 10.3109/17453674.2010.501739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leonardsson O, Garellick G, Karrholm J, Akesson K, Rogmark C. Changes in implant choice and surgical technique for hemiarthroplasty. 21,346 procedures from the Swedish Hip Arthroplasty Register 2005-2009. Acta Orthop 2012;83:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leonardsson O, Kärrholm J, Åkesson K, Garellick G, Rogmark C. Higher risk of reoperation for bipolar and uncemented hemiarthroplasty. Acta Orthop 2012;83:459-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cnudde P, Nemes S, Bulow E, et al. Trends in hip replacements between 1999 and 2012 in Sweden. J Orthop Res 2018;36:432-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rolfson O, Kärrholm J, Dahlberg LE, Garellick G. Patient-reported outcomes in the Swedish Hip Arthroplasty Register: results of a nationwide prospective observational study. J Bone Joint Surg [Br] 2011;93-B:867-75. [DOI] [PubMed] [Google Scholar]
- 18. Makela KT, Matilainen M, Pulkkinen P, et al. Countrywise results of total hip replacement. An analysis of 438,733 hips based on the Nordic Arthroplasty Register Association database. Acta Orthop 2014;85:107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puolakka TJ, Pajamaki KJ, Pulkkinen PO, Nevalainen JK. Poor survival of cementless Biomet total hip: a report on 1,047 hips from the Finnish Arthroplasty Register. Acta Orthop Scand 1999;70:425-9. [DOI] [PubMed] [Google Scholar]
- 20. Eskelinen A, Remes V, Helenius I, et al. Total hip arthroplasty for primary osteoarthrosis in younger patients in the Finnish arthroplasty register. 4,661 primary replacements followed for 0-22 years. Acta Orthop 2005;76:28-41. [DOI] [PubMed] [Google Scholar]
- 21. Eskelinen A, Paavolainen P, Helenius I, Pulkkinen P, Remes V. Total hip arthroplasty for rheumatoid arthritis in younger patients: 2,557 replacements in the Finnish Arthroplasty Register followed for 0-24 years. Acta Orthop 2006;77:853-65. [DOI] [PubMed] [Google Scholar]
- 22. Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. Early failures among 14,009 cemented and 1,326 uncemented prostheses for primary coxarthrosis. The Norwegian Arthroplasty Register, 1987-1992. Acta Orthop Scand 1994;65:1-6. [DOI] [PubMed] [Google Scholar]
- 23. Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. Early aseptic loosening of uncemented femoral components in primary total hip replacement. A review based on the Norwegian Arthroplasty Register. J Bone Joint Surg [Br] 1995;77-B:11-7. [PubMed] [Google Scholar]
- 24. Havelin LI, Vollset SE, Engesaeter LB. Revision for aseptic loosening of uncemented cups in 4,352 primary total hip prostheses. A report from the Norwegian Arthroplasty Register. Acta Orthop Scand 1995;66:494-500. [DOI] [PubMed] [Google Scholar]
- 25. Havelin LI, Engesaeter LB, Espehaug B, et al. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000;71:337-53. [DOI] [PubMed] [Google Scholar]
- 26. Havelin LI, Espehaug B, Engesaeter LB. The performance of two hydroxyapatite-coated acetabular cups compared with Charnley cups. From the Norwegian Arthroplasty Register. J Bone Joint Surg [Br] 2002;84-B:839-45. [DOI] [PubMed] [Google Scholar]
- 27. Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. The effect of the type of cement on early revision of Charnley total hip prostheses. A review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg [Am] 1995;77-A:1543-50. [DOI] [PubMed] [Google Scholar]
- 28. Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE. The type of cement and failure of total hip replacements. J Bone Joint Surg [Br] 2002;84-B:832-8. [DOI] [PubMed] [Google Scholar]
- 29. Espehaug B, Furnes O, Engesaeter LB, Havelin LI. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register: concerns about some newer implants. Acta Orthop 2009;80:402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hallan G, Espehaug B, Furnes O, et al. Is there still a place for the cemented titanium femoral stem? 10,108 cases from the Norwegian Arthroplasty Register. Acta Orthop 2012;83:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hallan G, Lie SA, Furnes O, et al. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg [Br] 2007;89-B:1574-80. [DOI] [PubMed] [Google Scholar]
- 32. Hallan G, Dybvik E, Furnes O, Havelin LI. Metal-backed acetabular components with conventional polyethylene: a review of 9113 primary components with a follow-up of 20 years. J Bone Joint Surg [Br] 2010;92-B:196-201. [DOI] [PubMed] [Google Scholar]
- 33. Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg [Br] 1997;79-B:590-5. [DOI] [PubMed] [Google Scholar]
- 34. Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:644-51. [DOI] [PubMed] [Google Scholar]
- 35. Furnes A, Lie SA, Havelin LI, Engesaeter LB, Vollset SE. The economic impact of failures in total hip replacement surgery: 28,997 cases from the Norwegian Arthroplasty Register, 1987-1993. Acta Orthop Scand 1996;67:115-521. [DOI] [PubMed] [Google Scholar]
- 36. Furnes O, Havelin LI, Espehaug B, et al. The Norwegian registry of joint prostheses–15 beneficial years for both the patients and the health care. Tidsskr Nor Laegeforen 2003;123:1367-9. [PubMed] [Google Scholar]
- 37. Fevang BT, Lie SA, Havelin LI, Engesaeter LB, Furnes O. Improved results of primary total hip replacement. Acta Orthop 2010;81:649-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand 2000;71:433-9. [DOI] [PubMed] [Google Scholar]
- 39. Gundtoft PH, Varnum C, Pedersen AB, Overgaard S. The Danish Hip Arthroplasty Register. Clin Epidemiol 2016;8:509-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnsen SP, Sorensen HT, Lucht U, et al. Patient-related predictors of implant failure after primary total hip replacement in the initial, short- and long-terms. A nationwide Danish follow-up study including 36,984 patients. J Bone Joint Surg [Br] 2006;88-B:1303-8. [DOI] [PubMed] [Google Scholar]
- 41. Thillemann TM, Pedersen AB, Johnsen SP, Soballe K. Implant survival after primary total hip arthroplasty due to childhood hip disorders: results from the Danish Hip Arthroplasty Registry. Acta Orthop 2008;79:769-76. [DOI] [PubMed] [Google Scholar]
- 42. Paulsen A, Pedersen AB, Johnsen SP, et al. Effect of hydroxyapatite coating on risk of revision after primary total hip arthroplasty in younger patients: findings from the Danish Hip Arthroplasty Registry. Acta Orthop 2007;78:622-8. [DOI] [PubMed] [Google Scholar]
- 43. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S-453S. [DOI] [PubMed] [Google Scholar]
- 44. Huo MH, Spencer DL, Borah BJ, et al. Post-discharge venous thromboemolism and bleeding in a large cohort of patients undergoing total hip or knee arthroplasty. J Clin Outcomes Manag 2012;19:355-63. [Google Scholar]
- 45. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology 2003;99:552-60. [DOI] [PubMed] [Google Scholar]
- 46. Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg [Am] 2010;92-A:2156-64. [DOI] [PubMed] [Google Scholar]
- 47. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e278S-e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. National Clinical Guideline Centre. Acute and chronic conditions. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. 2010. https://www.ncbi.nlm.nih.gov/books/NBK116518/pdf/Bookshelf_NBK116518.pdf (last assessed 24 March 2019) [PubMed]
- 49. American Academy of Orthopaedic Surgeons. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty - Evidence-based guideline and evidence report. 2011. https://www.aaos.org/research/guidelines/vte/vte_full_guideline.pdf (last accessed 24 March 2019). [DOI] [PubMed]
- 50. Pedersen AB, Sorensen HT, Mehnert F, Johnsen SP, Overgaard S. Effectiveness and safety of different duration of thromboprophylaxis in 16,865 hip replacement patients–a real-word, prospective observational study. Thromb Res 2015;135:322-8. [DOI] [PubMed] [Google Scholar]
- 51. RADS. Baggrundsnotat: Tromboseprofylakse til ortopædkirurgiske patienter. 2014. http://www.rads.dk/media/2084/210514-baggrundsnotat-tromboseprofylakse.pdf (last accessed 24 March 2019).
- 52. Pedersen AB, Mehnert F, Overgaard S, Moller B, Johnsen SP. Transfusion practice in total hip arthroplasty in Danish departments of orthopaedic surgery. Ugeskr Laeger 2009;171:973-7. [PubMed] [Google Scholar]
- 53. Pedersen AB, Mehnert F, Overgaard S, Johnsen SP. Allogeneic blood transfusion and prognosis following total hip replacement: a population-based follow up study. BMC Musculoskelet Disord 2009;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Steiger RN, Miller LN, Davidson DC, Ryan P, Graves SE. Joint registry approach for identification of outlier prostheses. Acta Orthop 2013;84:348-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg [Am] 2011;93-A:2287-93. [DOI] [PubMed] [Google Scholar]
- 56. Tanzer M, Graves SE, Peng A, Shimmin AJ. Is cemented or cementless femoral stem fixation more durable in patients older than 75 years of age? A comparison of the best-performing stems. Clin Orthop Relat Res 2018;476:1428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Joint Registry Accountability and Transparency Model Implant outlier process standard operating procedure. http://www.njrcentre.org.uk/njrcentre/NJRAccountabilityandTransparencyModel/ImplantOutlierProcess/tabid/1473/Default.aspx (last accessed 24 March 2019).
- 58. Beyond Compliance. www.beyondcompliance.org.uk (last accessed 24 March 2019).
- 59. ODEP. www.odep.org.uk (last accessed 24 March 2019).
- 60. Whittaker RK, Hexter A, Hothi HS, et al. Component size mismatch of metal on metal hip arthroplasty: an avoidable never event. J Arthroplasty 2014;29:1629-34. [DOI] [PubMed] [Google Scholar]
- 61. Gundtoft PH, Pedersen AB, Schonheyder HC, Overgaard S. Validation of the diagnosis 'prosthetic joint infection' in the Danish Hip Arthroplasty Register. Bone Joint J 2016;98-B:320-5. [DOI] [PubMed] [Google Scholar]
- 62. Varnum C, Pedersen AB, Gundtoft PH, Overgaard S. The what, when and how of orthopaedic registers: an introduction into register-based research. EFORT Open Rev 2019;4: S126-S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. No authors listed. Better management of patients with OsteoArthritis. https://boa.registercentrum.se/boa-in-english/better-management-of-patients-with-osteoarthritis-boa/p/By_o8GxVg (last accessed 24 March 2019).
- 64. James S, Rao SV, Granger CB. Registry-based randomized clinical trials—a new clinical trial paradigm. Nat Rev Cardiol 2015;12:312-6. [DOI] [PubMed] [Google Scholar]
- 65. Mäkelä KT, Furnes O, Hallan G, et al. The benefits of collaboration: the Nordic Arthroplasty Register Association. EFORT Open Rev 2019;4: S180-S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. No authors listed. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1499958093461&uri=CELEX:32017R0745 (last accessed 24 March 2019).
- 67. Lubbeke A, Silman AJ, Prieto-Alhambra D, et al. The role of national registries in improving patient safety for hip and knee replacements. BMC Musculoskelet Disord 2017;18:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones LD, Golan D, Hanna SA, Ramachandran M. Artificial intelligence, machine learning and the evolution of healthcare: A bright future or cause for concern? Bone Joint Res 2018;7:223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rumsfeld JS, Joynt KE, Maddox TM. Big data analytics to improve cardiovascular care: promise and challenges. Nat Rev Cardiol 2016;13:350-9. [DOI] [PubMed] [Google Scholar]
- 70. Malchau H. On the importance of stepwise introduction of new hip implant technology: assessment of total hip replacement using clinical evaluation, radiostereometry, digitised radiography and a national hip registry. Gothenburg: University of Gothenburg, 1995. [Google Scholar]
- 71. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. [DOI] [PubMed] [Google Scholar]