Abstract
Haemophilia is a group of coagulation disorders inherited in an X-linked recessive pattern.
Nearly three-quarters of all haemorrhages in haemophilia occur in the musculoskeletal system, usually in the large muscles and joints of the lower extremity.
While prevention of bleeding with active prophylaxis is the recommended optimal therapy for severe haemophilia, there are many patients suffering from musculoskeletal system complications subsequent to uncontrolled bleeding.
Recombinant clotting factor concentrates led to home treatment of acute bleeding episodes as well as allowing for minor and major surgical interventions.
Avoiding of further complications by radiosynoviorthesis is the first-line recommendation, and arthroplasty is regarded as the effective salvage procedure for patients presenting with severe disability.
Physiotherapy and rehabilitation in haemophilia patients are important to return the normal status of joint motion, to regain the muscle strength, to obtain the optimal functional levels and to improve patients’ quality of life.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180068
Keywords: haemophilia, joint bleeding, musculoskeletal complications, rehabilitation
Introduction
Haemophilia is a rarely seen, X-linked recessive coagulation disorder in which deficiencies of certain clotting factors are the cause of haemorrhage with minor trauma or surgery. The three types of haemophilia are haemophilia A, B and C. The most common form, haemophilia A, occurs in 1 in 5000 male births. The deficient factor (f) is fVIII. Haemophilia B affects only 1 in 50 000, where there is fIX deficiency. Although bleeding can occur at almost any age, it usually comes to clinical attention by the age of one year with unexpected bruising over the extremities or haemarthrosis as the most common clinical manifestations.
Large joints of the lower extremity and elbows are the most common target joints. Repeated episodes of haemorrhage lead to subsequent degenerative arthritis with pain, limited range of motion (ROM), deformity and severe contractures (Fig. 1). The severity of haemophilia depends on the amount of clotting factor that is missing: patients with a circulating factor < 1% have ‘severe’ disease; those with 1% to 5% are ‘moderate’; and those who have > 5% are classified as ‘mild’. Patients with severe haemophilia may bleed once or twice spontaneously per week in contrast to those with moderate and mild haemophilia in whom trauma or surgery is the cause of haemorrhage. Children with target joints which bleed frequently have a gradual decrease in sharing activities with their friends and cannot maintain their quality of life and eventually become disabled. Palazzi et al have classified the clinical presentation of affected joints in a simple system shown in Table 1. The end stage of the disease is manifested by fibrous contracture, loss of joint space and substantial disorganization of the joint structures, and pain due to degenerative arthritis.1
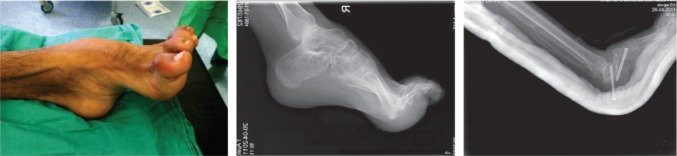
Fig. 1.
Clinical presentation and radiographs of a 27-year-old male with left ankle equinus and forefoot clawing due to compartment syndrome. This patient has severe haemophilia and suffered from a previous untreated calf-bleeding episode. Immediate post-operative radiographs demonstrate correction of the equinus deformity by triple arthrodesis.
Table 1.
Palazzi classification
| Stage | Clinical presentation |
|---|---|
| I. Transient synovitis | Secondary prophylaxis is indicated for 6 months Following therapy, the presenting synovitis should resolve |
| II. Persistent synovitis | Increase in synovial membrane thickness, joint effusion and decreased ROM and peri-articular muscle atrophy |
| III. Chronic haemophilic arthropathy | Significant muscle atrophy and contractures |
| IV. Ankylosis | Fibrous or bony ankylosis |
The availability of concentrated fVIII provides primary prophylaxis and replacement therapy when indicated. When the child is young, the parents or an immediate family member are trained to give them injections; older children will be taught how to inject themselves to prevent episodes of bleeding (prophylactic treatment) or to treat an episode of bleeding (on-demand treatment). Prophylaxis is the key to prevent clinical deterioration of the disease; however, it is expensive and frequently cannot be followed in many countries because of economic reasons. Additionally, many patients develop decreased compliance with regular injections for infusion of factor and its inherent need for venous access. Currently, administering higher doses of factors immediately after a bleeding episode is a more frequent method of preventing further damage (secondary on-demand prophylaxis). Since hypervascularity and a fragile state of the synovium last about six weeks after a bleed, a similar duration of secondary prophylaxis is recommended after each episode of haemarthrosis.
About 15% to 20% of patients with haemophilia develop an inhibitor antibody that prevents the clotting factor from being able to develop blood clots and stop bleeding. Different kinds of clotting factors are then needed to by-pass the normal clotting sequence and induce clotting. This is recognized as the most challenging complication that delays rehabilitation. Its frequency is reported as approximately 25% to 30% of severe haemophilia A patients and 3% to 5% of haemophilia B patients who are under prophylactic treatment.2
Improvements in factor replacement safety and effectiveness have made the performance of major surgical procedures increasingly possible in recent years, including major surgeries such as total joint arthroplasty.3,4
Haemorrhage into the muscle or joints accounts for 80% to 90% of all bleeding episodes in people with haemophilia.2,5 The knee joint is the most frequent target, thought to be due to the large size of the synovial membrane and large rotational forces present. Bleeds are best detected by the patient themselves as overt bruising and swelling or described as an aura of warmth or tingling within the joint preceding the clinical signs. Immediate self-administration of factor is then recommended before any other diagnostic approach.
Physiotherapy and rehabilitation in haemophilic patients are important to return to the normal status of joint motion, to regain the muscle strength, to obtain the optimal functional levels and to improve the patients’ quality of life.6,7 Primary prophylaxis is the gold standard for treating the haemophilia patients and rehabilitation should always be performed under the cover of prophylactic treatment.
Physiotherapy and rehabilitation treatment can be divided into three sub-categories:
1) Muscular haematomas
2) Haemarthroses
a) Acute haemarthrosis
b) Chronic arthropathy
3) Rehabilitation after surgery
Rehabilitation of acute haemarthrosis
Haemarthrosis develops within a few hours and the typical signs in the clinical examination are: an inflamed, warm joint; a very limited ROM; and an antalgic flexion position of the affected joint (Fig. 2).8,9
Fig. 2.
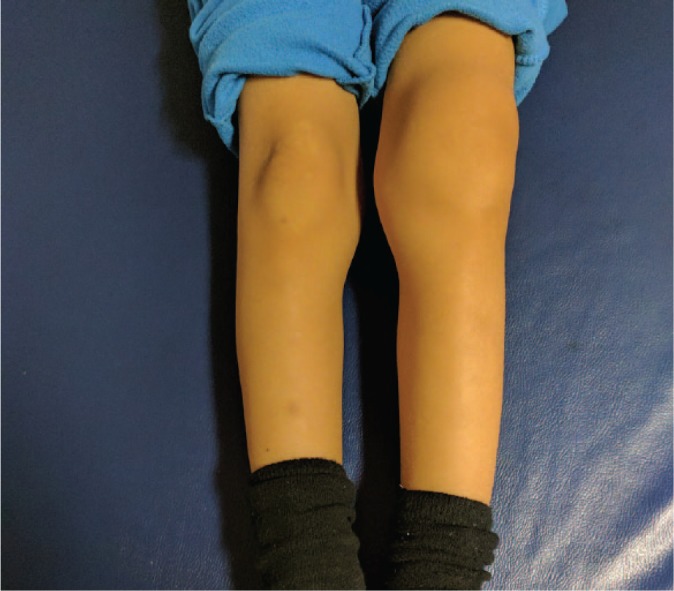
Clinical presentation of a five-year-old male with left knee acute haemarthrosis.
As in acute haematomas, the R-RICE regime is the first option for treatment of acute haemarthrosis. Immobilization of the joint and avoidance of weight-bearing during the first week is necessary to prevent re-bleeding and cartilage damage. Ravanbov et al10 showed detrimental effects of the blood on the overall cartilage function under loading. In addition, it is important to provide a proper balance between rest, early mobilization and weight-bearing to prevent the negative effects of immobilization while minimizing the re-bleeding.11
Bleedings in the lower extremity require at least one day of bed rest and protected weight-bearing on the affected side during the following five to seven days. The extremity should be elevated when sitting and crutches must be used when walking. In knee joint haemarthrosis, applying a compressive bandage is valuable. If the joint bleeding is in the upper extremity, similarly, lifting and carrying heavy objects are contraindicated for five to seven days following haemarthrosis. To provide a sufficient rest position, a supportive sling for the shoulder and a ‘long-arm’ posterior plaster splint for the elbow are adequate.6,9
Haemarthrosis causes severe disability and reduces the quality of life of the haemophilic patient.9 Recurrent haemarthrosis results in chronic inflammation of the synovium. This inflammation is the main cause of the vicious cycle of haemarthrosis-synovitis-haemarthrosis. First, the synovium becomes unable to re-absorb the intra-articular blood and causes synovial hypertrophy. Then, impingement of the hypervascularized tissues triggers the bleeding again.5,9,12 Therefore, the principal approach is to avoid acute joint bleedings; if there is bleeding, it is paramount to manage it as rapidly and efficiently as possible.
The recurrent intra-articular haemorrhages cause chondrocyte apoptosis (cartilage cell death) which eventually results in haemophilic arthropathy. Since more than two to three bleeds into the same joint may cause permanent joint damage, it is important to control the intra-articular bleeding as early as possible to prevent the cartilage and joint destruction.9,11
Rehabilitation of muscular haematoma
Muscle haemorrhages occur in 10% to 20% of patients. Although bleeding into the central nervous system is < 5%, it is generally life-threatening.8,9,11 Bleeds into the muscle cause a ROM limitation, disability and reduce the quality of life of the haemophilia patient.9 Muscle haematomas develop when the muscle is over-stretched or when there is direct trauma to the muscle, which leads the fibres to tear and bleed. The haematomas in the gluteal or thigh regions and calf muscles can reach large sizes; conversely, subcutaneous haematomas rarely grow to a large size. Depending on the location, some muscle haematomas can be hazardous, for example:
bleeding into the thigh or gluteal muscles may lead to severe anaemia;
bleeding into forearm, palm or calf muscles may cause compartment syndrome (neurovascular compression);
bleeding into gluteal muscles or the popliteal fossa may compress the sciatic nerve;
bleeding into the anterior compartment of the forearm may compress median or ulnar nerves and cause Volkmann’s ischaemic contracture;
bleeding into the iliopsoas muscle may compress the femoral nerve.
Iliopsoas haematoma is one of the most commonly seen muscular haematomas in haemophilic patients. Since the haematoma grows gradually, it may cause a delayed diagnosis. As a result, the diagnosis is usually made when symptoms of femoral nerve compression or anaemia is observed.11
Muscular bleeding in haemophilia carries the important risks of developing compartment syndrome and pseudotumours, which have led to a series of serious debilitating consequences including death.
The first 48 hours after a muscular bleed is defined as the ‘acute phase’ of muscle hematoma. The management of an acute haematoma includes: immediate Replacement of clotting factors; Rest (immobilization); Ice; Compression (except iliopsoas muscle); and Elevation (R-RICE regime).11
Although the current role of the ice treatment in haemophilia remains controversial, it is believed that cooling of blood and tissues can facilitate the control of coagulation and shorten bleeding.9 Ice can be applied for five to 15 minutes only during the first six hours to avoid skin damage.
The second stage, the ‘sub-acute phase’, of muscular haematoma is three to 12 days. Rehabilitation during this stage includes: pain-free stretching exercises; functional massage; and movement to prevent muscle fibrosis.
At the third stage (depending on the patient’s pain and performance), gradual muscle strengthening exercises can be added to the rehabilitation programme. Hydrotherapy is a safe and effective method for both the second and third stages of muscular haematomas. Proprioceptive neuromuscular facilitation techniques can be applied in a gentle way, depending on the patients’ tolerance.
Rehabilitation of chronic arthropathy and management of deformities of upper and lower limbs
Severe limitation of the joint motion and muscle atrophy are the major complications in haemophilic patients with chronic arthropathy (Fig. 1). Physiotherapy and rehabilitation sessions should be introduced and performed daily, as tolerated by the patient, to improve the joint mobility as well as the muscle strength.2 The rehabilitation programme should be patient-specific and include four main exercise treatments such as stretching, strengthening, balance, proprioception and aerobic exercises to increase physical fitness.2,13
Although the shoulder has never been considered a target joint in haemophilic patients, many adult haemophiliacs suffer from rotator cuff impingement or arthritis. In the management of shoulder pain, conservative treatment with physiotherapy should be considered first.14,15 Persistence of symptoms despite effective conservative treatment may indicate the need for arthroscopic synovectomy.14 Arthroscopic synovectomy in the shoulder controls bleeding and reduces pain, but it is less effective in improving articular function.14,15
The elbow is the most frequently affected upper limb joint in haemophilia. Chronic haemophilic synovitis of the elbow eventually leads to enlargement of the radial head and arthropathy (Fig. 3). Radial head hypertrophy is regarded as the source of pain, with recurrent bleeding, and loss in forearm rotation. Excision of the radial head is usually required to improve the forearm rotation but minimal or no improvement in flexion–extension should be anticipated.16
Fig. 3.
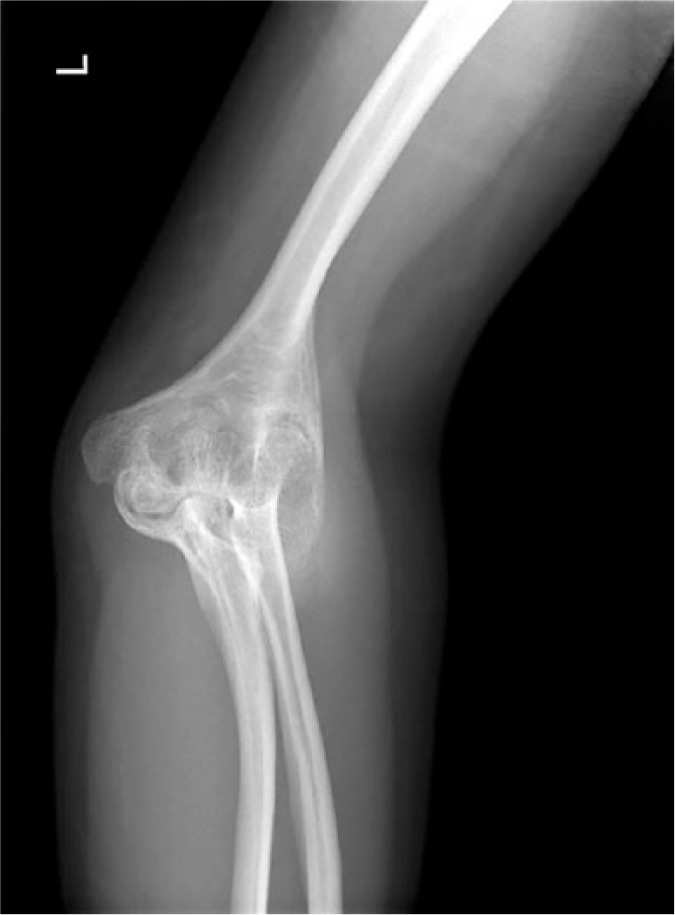
A 28-year-old male patient suffering from a severe left elbow deformity presenting with total loss of joint motion and neurological impairment, due to advanced haemophilic arthropathy.
In comparison with other large joints of the lower extremity, the hip presents fewer bleeding episodes in haemophiliacs while the iliopsoas muscle is frequently responsible for bleeding around the hip joint. This is a critical site of bleeding which may cause femoral nerve palsy. Early identification and proper management consisting of hospitalization, strict bed rest, factor replacement and sometimes haematoma evacuation should be implemented.
In patients with haemophilia the knees tend to bleed from an age, as early as two to five years, and it is the most affected joint. Management of bleeding into the knee and treatment of its deformities are discussed in detail in related sections. Patients with advanced arthropathy of the ankle may benefit from arthrodesis. Corrective osteotomy may be beneficial in axial lower limb deformities, around the hip, knee and ankle. This may be an option for patients in whom joint replacement is not available or not desirable.13
Radiosynoviorthesis
In the presence of chronic synovitis and recurrent haemarthrosis the first-line treatment of the chronic synovitis unresponsive to haematological treatment is radiosynoviorthesis (RS).17 In practice, more than three bleeding episodes into the target joint during the last six months despite factor replacement are regarded as an indication to proceed with synoviorthesis. It can be performed at any age (ideally after the first year of life).
Synoviorthesis is an effective method to reduce the frequency of haemarthrosis in joints presenting with chronic synovitis. This is a highly effective treatment for reducing pain, discomfort and slowing down the arthritis progression.
There are two basic types of synoviorthesis: chemical and radiation synoviorthesis. They are realized with corticosteroid, rifampicin or with Beta-emitting isotopes consecutively. Beta-emitting agents are preferred due to low penetration rates and their safety for body exposure to radiation when compared with gamma irradiation. Yttrium-90, Rhenium-186 and Phosporus-32 have different tissue penetration rates compatible with the variable synovial thickness of different joint sizes of the upper and lower extremity. Large colloid particle forms are effective to avoid vascular contamination.7,17,18 Chemical synovectomy is outdated because it requires weekly painful injections until the synovitis is controlled.19,20 On average, radiosynovectomy has a 75% to 80% satisfactory outcome in the long term. However, for the remaining 25%, a sequence of repeated injections is required for an eventual success. If RS is repeated three times with three- to six-month intervals without significant clinical success, an arthroscopic synovectomy should be considered, which is seldom necessary today.9,11,20 Specific protocols are developed in centres to ascertain the factor replacement, suitable anaesthesia, degree of immobilization and patient management.18,19 After 30 years of using radiation synovectomy worldwide, no systemic adverse reaction has been reported in relation to the radioactive materials.
Radionuclide synovectomy is performed in outpatient clinics, it is a cost-effective and highly efficient way and two joints can be injected in a single session. Today it is the procedure of choice for chronic synovitis.13,18
Fractures
Fractures are relatively less frequent in this disease, due to patients’ protected lifestyle and lower level of activities. However, a person with haemophilic arthropathy should still be considered at risk for fractures around joints that have significant loss of motion and in bones that are osteoporotic.18 Caviglia et al21 reported the largest series of 151 fractures in haemophiliacs. They have underlined a changing pattern of fractures, becoming more common in the upper extremity, with occurrence at younger ages but being less frequent overall.
The neurological and vascular status of the region distal to the fracture should be monitored and recorded before and after dealing with the fracture.18 Compartment syndrome is more likely to occur in haemophilic patients, so documenting the neurovascular function is vital.18,21 However, complications are now less frequent due to the advent of new and more feasible treatments, consequently producing less impairment of the patient’s quality of life.18,21
According to the 2012 guidelines of World Federation of Hemophilia (WFH), fracture management should include immediate factor concentrate replacement to a level of at least 50%, maintained for three to five days and then maintained for ten to 14 days for the upper extremity and one week longer for lower extremity fractures, i.e. while the fracture becomes stabilized and to prevent soft-tissue bleeding.18
The fracture management plan should be appropriate for the specific fracture and follow the same principles as used for standard modern operative treatment techniques under appropriate coverage of clotting factor concentrates. Expectations for healing and functional outcome should be the same as in non-haemophiliacs.18
When a conservative treatment is indicated, splints are preferred over circumferential casting.22 Internal fixation with nails and plates are the first line of treatment for surgical fracture management. Fractures presenting with skin breakdown or necrosis due to excessive haematoma formation or late presentation of fractures in an infected state may require external fixators. In patients with nonunion, total knee arthroplasty (TKA) is another possible surgical treatment (Fig. 4).13,18
Fig. 4.
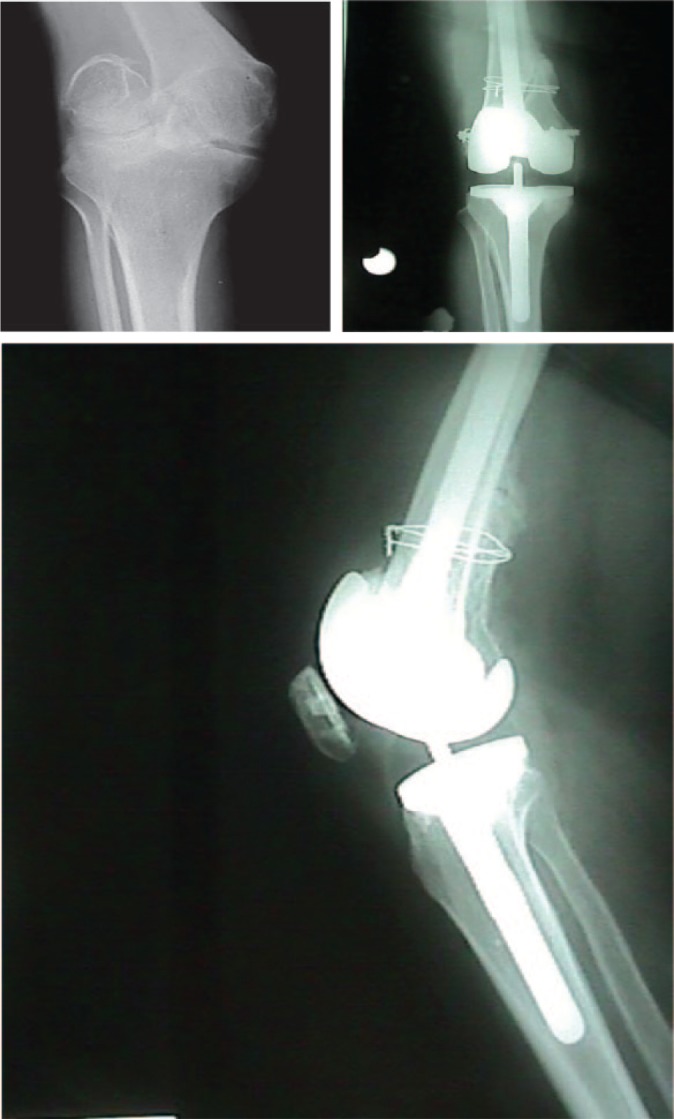
A 24-year-old male patient with neglected intracondylar nonunion of his right knee. Severe deformity required one stage reduction, fracture fixation and joint replacement by a centrally constrained condylar TKA.
Fractures adjacent to joints with existing haemophilic joint degeneration deserve special attention. Prolonged immobilization is prone to lead to significant limitation of ROM and should be avoided. Physiotherapy should be started as soon as the fracture is stabilized to restore motion, muscle strength and function. Regular exercise, fall-prevention strategies, and optimization of calcium and vitamin D intake are all recommended for further fracture prevention.18
Pseudotumours
Starker described this potentially limb-threatening and life-threatening condition unique to haemophilia in 1918. It is most commonly seen in the long bones or pelvis. It occurs as a result of inadequately treated and incompletely resorbed soft-tissue clots, usually in a muscle or adjacent to the periosteum where the bleeding is encapsulated and erodes into the bone. Pseudotumours produce recognizable vascularization and, if not treated, can reach an enormous size causing pressure on the neurovascular structures and pathological fractures that mimic malignant tumours. A fistula can develop through the overlying skin (Fig. 5).13,18
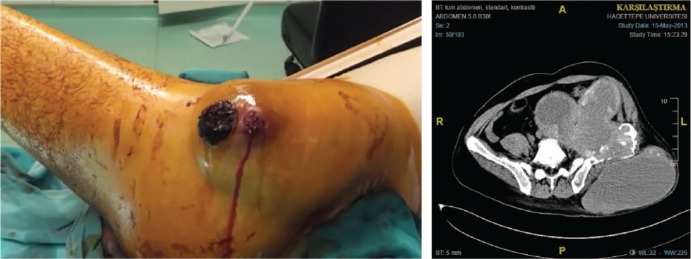
Fig. 5.
A 46-year-old male patient with a large pelvic pseudotumor that has protruded through the skin. This lesion was infected and presented with recurrent bleeding episodes. MRI of the same patient demonstrates massive iliac bone destruction and a large intrapelvic mass with external communication. The patient died due to consequent sepsis.
Generally, they are observed in patients who live far from treatment centres or who have no follow-up of their treatment, so they present late for adequate treatment. The incidence of haemophilic cysts and pseudotumours is 1.56%; both appear frequently in the second or third decade of life in patients with severe fVIII or fIX deficiency.23
Diagnosis is made by the physical finding of a localized mass. Femoral location is the most frequent, followed by the pelvis and, less frequently, the tibia. Radiographs are usually enough, although complementary studies may be needed to evaluate the soft tissues. MRI and three-dimensional CT are recommended.18 Management depends on the site, size, rate of growth and effect on adjoining structures. Options recommended by WFH Guidelines include an initial conservative approach with at least six weeks of factor administration at 25% to 30% and immobilization until radiographic evidence of healing occurs.13,18
A follow-up MRI is recommended at the end of six weeks. If the tumour is decreasing, factor replacement is continued and MRI is repeated for three cycles. Percutaneous treatment is indicated when the cyst continues to increase in size despite preventive treatment, when the rupture of the haematoma is imminent, for the prevention of skin necrosis, and when there is associated neurovascular injury. Aspiration followed by injections of fibrin glue, arterial embolization or radiotherapy may heal some lesions. Radical open surgery, if necessary, will be much easier if the tumour has shrunk.23
Surgical excisions, including limb amputations, may be necessary for large pseudotumours, particularly if they erode long bones. Large abdominal pseudotumours present a special challenge in surgical management of haemophilia; surgery must only be performed by teams with appropriate experience in haemophilia management.23
Elective orthopaedic surgery for advanced severe arthropathy
Surgery in patients with haemophilia is classified as ‘major’ or ‘minor’ regarding the difficulty they present in haemostasis with minor surgery including only superficial soft-tissue procedures requiring a small incision.
A major surgical procedure is defined as one requiring factor replacement for more than five consecutive days.24 Administration at a peak level of factor of 50% to 80% for minor surgery and 80% to 100% for a major procedure is recommended. Major orthopaedic surgical procedures include arthroscopic release of intra-articular adhesions or open extra-articular contracture release, removal of pseudotumours, osteotomy to correct existing angular deformities, arthrodesis of smaller joints and prosthetic joint replacement of large joints (hip, knee, shoulder, elbow and ankle).13,18
Current guidance recommends that all surgical procedures in patients with haemophilia should ideally be managed in a haemophilia treatment centre with collaboration of a multidisciplinary team consisting of a haematologist, surgeon, anaesthetist, nurse, physical therapist, laboratory technicians, social worker and pharmacist.11,13,18 Effective communication and coordination between these healthcare providers and the patient/patient’s family is essential to the success of surgical interventions.
Total joint arthroplasty
Many haemophilic patients experience multiple haemarthrosis episodes in a target joint and develop severe joint destruction as early as during their second or third decade of life. Joint preservation is no longer possible and the remaining available treatment is total joint arthroplasty, especially for major joints of the lower extremity.
End-stage haemophilic arthropathy in the hip or knee joint can be successfully treated with total joint arthroplasty. However, the success rate compared with a primary arthritis cohort may not be as good and certain pre-operative knee characteristics may affect the functional results. Haemophiliac patients with advanced knee arthritis frequently present with complex knee deformities. The most common deformity is flexion contracture; it is usually associated with external rotation and posterior subluxation of the tibia on the femur to the lateral side with a valgus deformity.1,25 The co-existence of limited ROM and soft-tissue contractures due to fibrosis present technical difficulties during TKA (Fig. 6). During the course of the operation, extensive soft-tissue releases are required to correct the malalignment and contractures as well as some proximalization of the joint line with increased resection of the distal femur. The femur and tibia may display peri-articular osteopenia and may be further weakened by large cysts. A review of the literature reveals a common practice of resecting the posterior cruciate ligament and implanting a posterior cruciate-substituting total knee prosthesis design for better deformity correction.26,27 Existing joint destruction may require bone grafting and utilization of revision components such as stems, augments or wedges. Associated hip and ankle arthritis with deformities are frequently addressed with additional surgery.
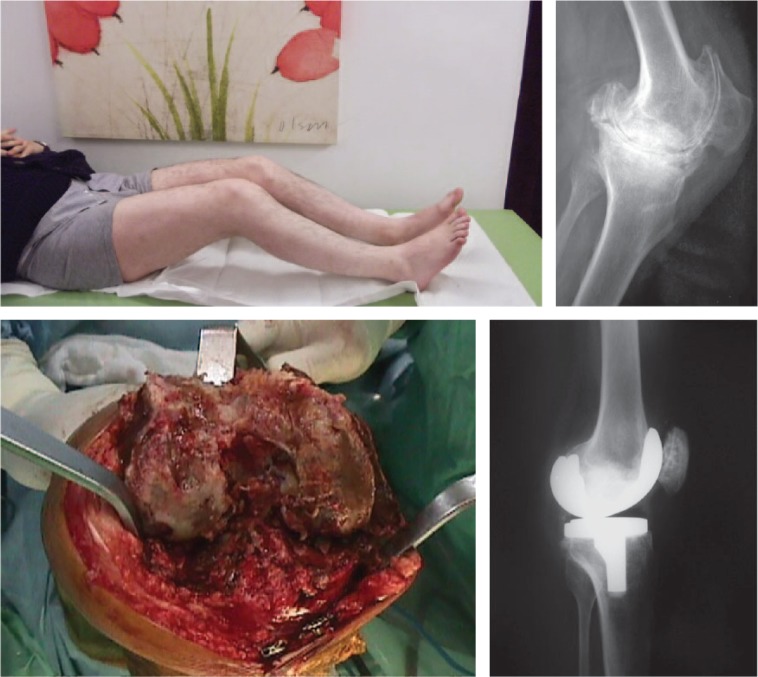
Fig. 6.
Clinical presentation of a haemophiliac patient with Palazzi Stage III arthritis of both knees. The patient was suffering from severe deformity consisting of flexion contractures, valgus angulation and joint stiffness. Lateral radiograph of his left knee demonstrates destructive arthritis. Intra-operative radiography of the same patient shows severe bony destruction, cyst formation, haemosiderosis, and dark-coloured/fibrotic nature of the synovial tissue. Correction of severe flexion contracture was achieved by a TKA at the five-year follow-up visit.
Several authors have reported that patients tend to have less favourable knee functional scores and increased post-operative complications related to existing pre-operative contractures.25,27 Silva et al27 anticipated less-than-optimal knee motion after total knee replacement in haemophilic arthropathy because of arthrofibrosis, but emphasized the high patient satisfaction due to excellent pain control despite the restricted ROM. Kagaya et al,28 in their computer analysis of human gait, have stated that hip or knee flexion contracture of > 15° and absence of ankle equinus is required to maintain positive step-length and forward movement of the centre of gravity. Our observation indicates that 27.5° of pre-operative flexion contracture is the threshold for avoiding post-operative knee flexion contractures in 98.8% of the cases (specificity 85.7%, sensitivity 100%, p < 0.001).29,30 Despite the possible long-term complications of TKA in younger age groups, we suggest that surgical intervention in haemophiliacs should not be delayed for better functional knee scores and fewer bleeding episodes during the course of follow-up.
TKA in haemophiliac individuals carries a considerably higher risk for complications, in the range of 0% to 31.5%.30,31 Anticipated complications include: high risk of infection; co-existing diseases that compromise the immune system such as hepatitis and HIV positivity; frequent intra-articular bleeding and haematoma formation; which are more prominent in patients with inhibitors to fVIII and fIX; and patients receiving immunosuppression for its treatment.32–35
Moore at al30 reviewed studies published between 1980 and 2015 in a meta-analysis to guide clinicians regarding outcome expectations after TKA in patients with haemophilia. A total of 336 TKAs in 254 patients were analysed with a mean follow-up of 6.3 years. Statistically significant ROM improvements were found with a 9.72° improvement of flexion contracture and an average of 15.69° increase in flexion. Knee scores showed statistically significant improvements in both clinical and functional scores. Despite an inevitably high complication rate of 31.5%, the authors have concluded that TKA is an effective procedure for improving ROM and decreasing functional deficits in advance haemophilic arthropathy.
Regarding patients with inhibitors, FEIBA (factor eight by-passing activity) and recombinant active fVIII concentrations have made major orthopaedic surgery possible.35 However, in patients with inhibitors, it is desirable to defer surgery until the antibody titre is < 5 Bethesda Units.36
Physiotherapy and rehabilitation play an important role in restoring the knee ROM and functional outcomes after TKA (Table 2).11,13,37
Table 2.
Rehabilitation after TKA in patients with haemophilia
| Inpatient rehabilitation | • Pain evaluation with visual analogue scale • Ice (3 times per day) with lower extremity elevated 20 to 30 minutes • Continuous passive motion (first week after surgery) • Weight-bearing as tolerated • Ambulation with walker • Observation of the incision • Increase the knee flexion gradually |
|
| Exercises Post-operative day 1 |
• Ankle pumping • Isometrics for quadriceps, gluteal and hip abductor muscles • Heel-slide exercises • Weight-bearing exercises • Transvers to chair or to bathroom from bed |
|
| Post-operative day 2 | • Continue the previous exercises • Active assistive range of knee motion exercises • Active assistive (AA) straight leg raises (SLR) • Knee extension exercises |
|
| Post-operative days 3 to 5 | • Continue the previous exercises • Increase the walking distance • Stair ascending and descending |
|
| Post-operative | Goals | Exercises |
| Weeks 1 to 4 | • Progression of the early phase exercises • Full knee extension and 90° knee flexion (depends on the pre-operative levels) • Improvement of the gait pattern • Improvement of the weight-bearing on the surgery side • Improvement of balance • Improvement of the neuromuscular function of quadriceps and gluteus maximus |
• Postural exercises while sitting and standing • Closed kinetic chain exercises for quadriceps, hip abductors, and hamstring muscles • AA knee flexion–extension exercises while sitting • Gentle stretching for gastro-soleus muscles • Patellar mobilization • Hip abduction while standing or side-lying |
| Weeks 4 to 12 | • Normal gait pattern • Progression of ROM exercises • Progression of hip abduction and adduction exercises • Gait training on soft surfaces • Balance and proprioceptive exercises |
• Treadmill walking • Heel strike, toe-off exercises • Bridge exercises • Terminal knee extension • Open kinetic chain exercises in different knee ranges such as 90°-60°-45°etc. • Proprioceptive neuromuscular facilitation (PNF) techniques • Hip strengthening exercises with ‘TheraBand’ • Side walking • Proprioceptive exercises • Exercises on balance board |
Conclusions
Haemophilia management requires a team approach, of which the patient and the family are a part because compliance has ultimate importance. With the administration of National Joint Registries, every child with haemophilia has to be followed closely with regular multi-team visits. Musculoskeletal affections are the most common problems for this particular group of patients to seek healthcare. Effective collaboration with orthopaedic departments and haemophilia services and societies have a significant contributing role in this lifelong management.
Footnotes
ICMJE Conflict of interest statement: BA reports a consulting fee or honorarium from Shire.
HG-D reports no conflict of interest.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg [Am] 1977;59-A:287–305. [PubMed] [Google Scholar]
- 2. Lobet S, Pendeville E, Dalzell R, et al. The role of physiotherapy after total knee arthroplasty in patients with haemophilia. Haemophilia 2008;14:989–998. [DOI] [PubMed] [Google Scholar]
- 3. McCollough NC, III, Enis JE, Lovitt J, et al. Synovectomy or total replacement of the knee in hemophilia. J Bone Joint Surg [Am] 1979;61-A:69–75. [PubMed] [Google Scholar]
- 4. Small M, Steven MM, Freeman PA, et al. Total knee arthroplasty in haemophilic arthritis. J Bone Joint Surg [Br] 1983;65-B:163–165. [DOI] [PubMed] [Google Scholar]
- 5. Balkan C, Kavakli K, Karapinar D. Iliopsoas haemorrhage in patients with haemophilia: results from one centre. Haemophilia 2005;11(5):463–467. [DOI] [PubMed] [Google Scholar]
- 6. Carcao M, Hilliard P, Escobar MA, et al. Optimising musculoskeletal care for patients with haemophilia. Eur J Haematol 2015;95(suppl 81):11–21. [DOI] [PubMed] [Google Scholar]
- 7. Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol 2007;136:777–787. [DOI] [PubMed] [Google Scholar]
- 8. Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis 2012;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez-Merchan EC. Articular bleeding in hemophilia. Cardiovasc Hematol Disord Drug Targets 2016;16:21–24. [DOI] [PubMed] [Google Scholar]
- 10. Ravanbod R, Torkaman G, Esteki A. Biotribological and biomechanical changes after experimental haemarthrosis in the rabbit knee. Haemophilia 2011;17:124–133. [DOI] [PubMed] [Google Scholar]
- 11. Lobet S, Hermans C, Lambert C. Optimal management of hemophilic arthropathy and hematomas. J Blood Med 2014;5:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn AL. Pathophysiology, diagnosis and prevention of arthropathy in patients with haemophilia. Haemophilia 2011;17:571–578. [DOI] [PubMed] [Google Scholar]
- 13. Alhaosawi MM. Guidelines of management of musculoskeletal complications of hemophilia. J Appl Hematol 2014;5:75–85. [Google Scholar]
- 14. MacDonald PB, Locht RC, Lindsay D, Levi C. Haemophilic arthropathy of the shoulder. J Bone Joint Surg [Br] 1990;72-B:470–471. [DOI] [PubMed] [Google Scholar]
- 15. Wiedel JD. Arthroscopic synovectomy: state of the art. Haemophilia 2002;8:372–374. [DOI] [PubMed] [Google Scholar]
- 16. Luck JV, Jr, Kasper CK. Surgical management of advanced hemophilic arthropathy. An overview of 20 years’ experience. Clin Orthop Relat Res 1989;(242):60–82. [PubMed] [Google Scholar]
- 17. Kavakli K, Aydogdu S, Taner M, et al. Radioisotope synovectomy with rhenium186 in haemophilic synovitis for elbows, ankles and shoulders. Haemophilia 2008;14:518–523. [DOI] [PubMed] [Google Scholar]
- 18. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia 2013;19:e1–e47. [DOI] [PubMed] [Google Scholar]
- 19. Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol 2007;1366:777–787. [DOI] [PubMed] [Google Scholar]
- 20. Uğur O, Gedik GK, Atilla B, Rubello D. Radiosynovectomy: current status in the management of arthritic conditions. Nucl Med Commun 2008;29:755–758. [DOI] [PubMed] [Google Scholar]
- 21. Caviglia H, Landro ME, Galatro G, Candela M, Neme D. Epidemiology of fractures in patients with haemophilia. Injury 2015;46:1885–1890. [DOI] [PubMed] [Google Scholar]
- 22. Taşer Ö. Treatment of closed fractures in hemophiliac patients. In: Caviglia HA, Solimeno LP, eds. Orthopedic surgery in patients with hemophilia. Milan: Springer Verlag, 2008:257–262. [Google Scholar]
- 23. Palazzi FF, Rivas S. Surgical treatment of hemophiliac pseudotumors. In: Caviglia HA, Solimeno LP, eds. Orthopaedic surgery in patients with haemophilia. Milan: Springer Verlag, 2008:241–248. [Google Scholar]
- 24. Solimeno LP, Escobar MA, Krassova S, Seremetis S. Major and minor classifications for surgery in people with hemophilia: a literature review. Clin Appl Thromb Hemost 2018;24:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez-Merchan EC. Correction of fixed contractures during total knee arthroplasty in haemophiliacs. Haemophilia 1999;5(suppl 1):33–38. [DOI] [PubMed] [Google Scholar]
- 26. Bae DK, Yoon KH, Kim HS, Song SJ. Total knee arthroplasty in hemophilic arthropathy of the knee. J Arthroplasty 2005;20:664–668. [DOI] [PubMed] [Google Scholar]
- 27. Silva M, Luck JV., Jr Long-term results of primary total knee replacement in patients with hemophilia. J Bone Joint Surg [Am] 2005;87-A:85–91. [DOI] [PubMed] [Google Scholar]
- 28. Kagaya H, Ito S, Iwami T, Obinata G, Shimada Y. A computer simulation of human walking in persons with joint contractures. Tohoku J Exp Med 2003;200:31–37. [DOI] [PubMed] [Google Scholar]
- 29. Atilla B, Çağlar O, Pekmezci M, et al. Pre-operative flexion contracture determines the functional outcome of haemophilic arthropathy treated with total knee arthroplasty. Haemophilia 2012;18:358–363. [DOI] [PubMed] [Google Scholar]
- 30. Moore MF, Tobase P, Allen DD. Meta-analysis: outcomes of total knee arthroplasty in the haemophilia population. Haemophilia 2016;22:e275–e285. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez-Merchan EC. Total knee replacement in haemophilic arthropathy. J Bone Joint Surg [Br] 2007;89-B:186–188. [DOI] [PubMed] [Google Scholar]
- 32. Feng B, Weng XS, Lin J, et al. Outcome of total knee arthroplasty combined patelloplasty for end-stage type A hemophilic arthropathy. Knee 2012;19:107–111. [DOI] [PubMed] [Google Scholar]
- 33. Solimeno LP, Mancuso ME, Pasta G, et al. Factors influencing the long-term outcome of primary total knee replacement in haemophiliacs: a review of 116 procedures at a single institution. Br J Haematol 2009;145:227–234. [DOI] [PubMed] [Google Scholar]
- 34. Norian JM, Ries MD, Karp S, Hambleton J. Total knee arthroplasty in hemophilic arthropathy. J Bone Joint Surg [Am] 2002;84-A:1138–1141. [DOI] [PubMed] [Google Scholar]
- 35. Zülfikar B, Aydogan G, Salcioglu Z, et al. ; FEIBA Investigators Team. Efficacy of FEIBA for acute bleeding and surgical haemostasis in haemophilia A patients with inhibitors: a multicentre registry in Turkey. Haemophilia 2012;18:383–391. [DOI] [PubMed] [Google Scholar]
- 36. Balkan C, Karapinar D, Aydogdu S, et al. Surgery in patients with haemophilia and high responding inhibitors: Izmir experience. Haemophilia 2010;16:902–909. [DOI] [PubMed] [Google Scholar]
- 37. Poonnoose PM, van der Net J. Musculoskeletal outcome in hemophilia: bleeds, joint structure and function, activity, and health-related fitness. Semin Thromb Hemost 2015;41:872–879. [DOI] [PubMed] [Google Scholar]