Abstract
In malunion cases, restoration of anatomy is a key factor in obtaining a good functional outcome, but this can be technically very challenging.
Three-dimensional printed bone models can further improve understanding of the malunion pattern.
The use of three-dimensional (3D) computer planning, and the assembly of patient-specific instruments and implants, especially in complex deformities of the upper limb, allow accurate correction while reducing operation time, blood loss volume and radiation exposure during surgery.
One of the major disadvantages of the 3D technique is the additional cost because it requires specific computer software, a dedicated clinical engineer, and a 3D printer.
Further technical developments and clinical investigations are necessary to better define the added value and cost/benefit relationship of 3D in the treatment of complex fractures, non-unions, and malunions.
Cite this article: EFORT Open Rev 2019;4 DOI: 10.1302/2058-5241.4.180074
Keywords: fracture complications, 3D technology, application
Introduction
Upper limb fractures are common injuries and affect people of all ages. As clinical studies have shown a significant correlation between anatomical reduction and joint function, reconstruction of normal anatomy is one of the key factors in achieving a successful clinical outcome.1–7,11 For simple fractures, treatment decisions can be based on two-dimensional imaging modalities (radiographs and cross-sectional CT and MRI images). For more complex injuries, such as comminuted, displaced or intra-articular fractures, the use of three-dimensional (3D) technology allows surgeons to understand injury characteristics and fragment displacement better, assist in an accurate pre-operative planning and surgical simulation, and select the most appropriate implants for internal fixation.8,10–12 Based on this, patient-specific bone models can be 3D printed, sterilized and used in the operating room.
Non-union and malunion are the most common serious complications of upper limb fractures, and often lead to chronic pain, functional impairment and late degenerative changes.2,18–22 Further treatment is often indicated, and reconstruction of normal anatomy is the preferred treatment option but can be technically challenging. Three-dimensional technology allows precise visualization and quantification of the deformity and virtual planning of reconstructive procedures, based on the normal anatomy of the contralateral side. Patient-specific instruments and implants can be designed, allowing computer-planned correction in the operating room and leading to a more predictable outcome.
In this article we describe the surgical techniques and our experience with 3D technology in the treatment of non-unions and malunions of the upper limb, illustrated through four specific cases.
Pre-operative planning
When a patient presents with a non-union or a symptomatic malunion of the upper limb, high resolution bilateral computed tomography (CT) scans of both limbs are obtained according to a specific protocol (slice thickness 0.4 mm, slice increment 0.2 mm, pixel size 0.29 mm, 120 kVp). The DICOM images of the CT scan are loaded into medical image processing software (Mimics Medical, Materialise NV, Technologielaan 15, 3001 Leuven, Belgium) and the individual bones are segmented and converted into 3D STL (stereolithography) files. A mirrored version of the normal contralateral bone is superimposed over the non-united or malunited bone, which enables us to three-dimensionally evaluate the deformity intuitively and quantitatively. Subsequently, for non-union cases, a virtual reduction of the fracture fragments is performed, and the most appropriate implant for fixation is selected. Anatomical models are 3D printed in medical grade material and sterilized to be used during the surgical procedure. These models can also help patients to understand their condition, which improves patient compliance.
For malunited fractures, a virtual corrective procedure is planned using specific computer software (3-Matic, Materialise NV, Technologielaan 15, 3001 Leuven, Belgium). Osteotomies are simulated, and bone fragments are reduced to their anatomical position. Furthermore, the most appropriate implant for fixation is selected and positioned onto the re-positioned bone fragments, and the ideal orientation and length of screws can be determined. To reproduce the virtual surgical plan in the operating room, patient-specific guides and, if necessary, patient-specific implants, are designed and manufactured through 3D printing.
Surgical technique25
The choice of surgical approach depends on the fracture site. For example, for distal radius malunions, a modified Henry approach is used. A clear exposition of the malunion site is necessary to fit the patient-specific drilling guide closely onto the bone at the pre-determined location. The guides are then secured with K-wires. Fluoroscopy, as well as the printed surgical bone model, can be useful to confirm this planned position. Especially for corrections of the forearm, this can be challenging due to the pronounced cylindrical shape of both the radius and ulna. All the trajectories for later screw insertion are pre-drilled. In cases of malunion, the drilling guide is removed and replaced by the cutting guide, over the retained K-wires, to perform the osteotomy along the pre-planned plane. A standard, pre-bent, or custom-made plate is fixed with screws into the pre-drilled holes, automatically leading to the planned reduction. Autologous bone grafts are used to fill the defect at the osteotomy site. A patient-specific instrument can also be designed and printed to reproduce the pre-determined size of the graft accurately. Arthroscopy can be performed to check the anatomical reduction of intra-articular fragments.
Post-operative treatment
The post-operative management depends on the type of deformity and correction as well as the bone quality and fixation method.
Clinical cases
Case 1
A 24-year-old motorcyclist sustained a fracture at the waist of the scaphoid, which was treated with cast immobilization for 12 weeks. He suffered from activity-related pain and continuous swelling of the wrist. Examination showed limited motion and weakness. As shown in Fig. 1a, a non-union of the scaphoid was present on the CT-scan six months after the injury. To avoid long-term wrist problems, further surgical treatment was planned.
Fig. 1a.
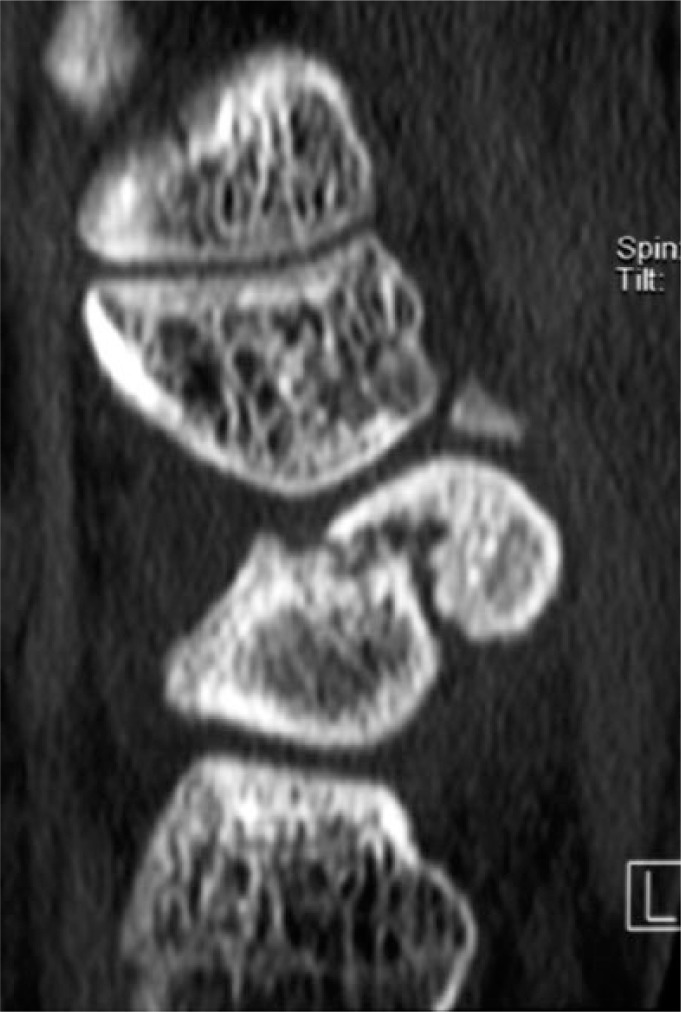
CT-scan showing a non-union of the scaphoid.
To correct the angular and rotational deformity of the scaphoid (Fig. 1b 3D technology was used to simulate the reduction of the fracture fragments and restore normal anatomy, based on the mirrored contralateral side (Fig. 1c). An additional informed consent for the use of 3D technology was signed by the patient. The residual bony defect of the scaphoid was measured three-dimensionally, and a vascularized medial femoral condyle graft was designed accordingly (Fig. 1d). A classic volar approach of the scaphoid, next to the flexor carpi radialis (FCR) tendon, was performed. The information obtained using 3D technology facilitated precise anatomical reconstruction of the scaphoid in the operating room. A scaphoid splint was provided for six weeks. After cast removal the patient could start with passive and active range-of-motion exercises. Bony consolidation was acheived at three months post-operatively. The pain had decreased, and symptoms improved further with rehabilitation (Fig. 1e).
Fig. 1b.
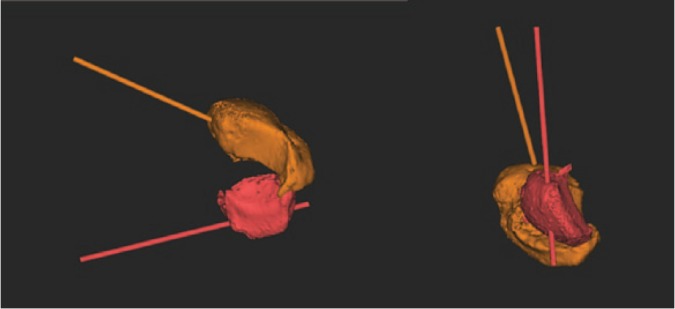
Three-dimensional simulation of reduction of the scaphoid non-union allows measurement of the angulatory and rotational correction that is needed to restore normal anatomy.
Fig. 1c.
Comparison of the scaphoid non-union with the mirrored contralateral side.
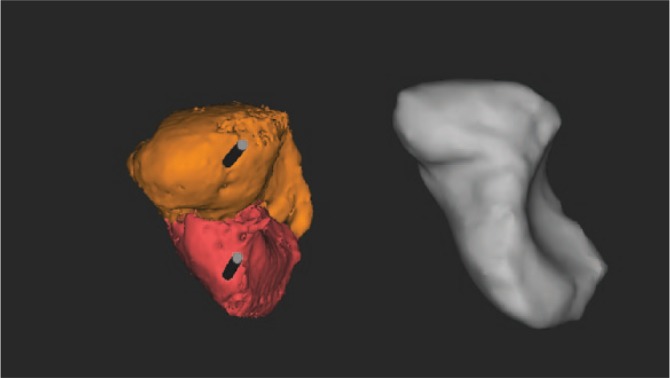
Fig. 1d.
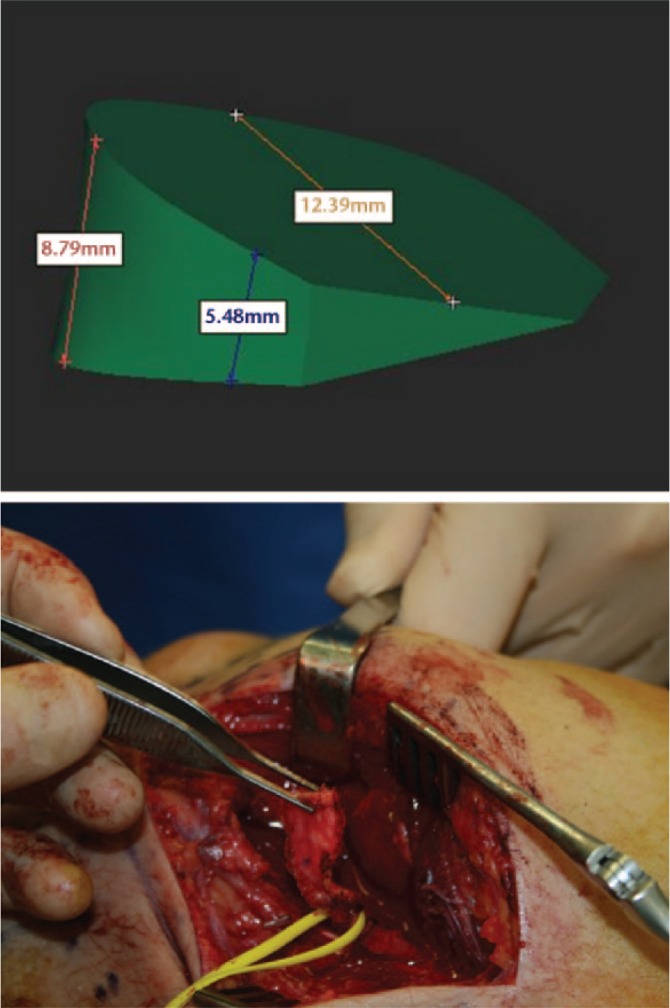
Precise planning of the three dimensions of the bone graft and prelevation of the graft at the medial femoral condyle.
Fig. 1e.
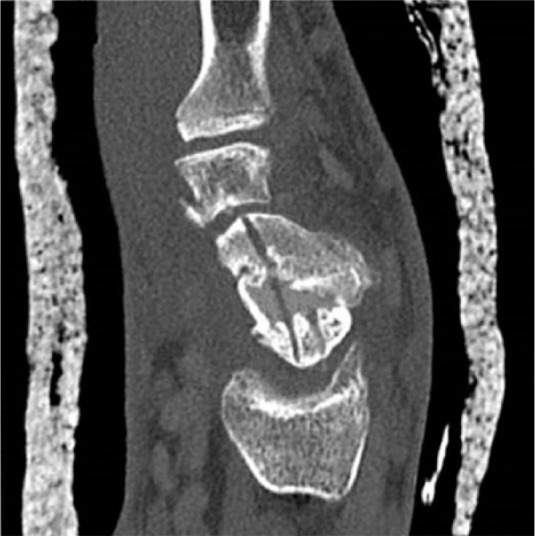
Interposition of the autograft and complete bony consolidation at three months post-operatively; previous K-wire trajectories are still visible.
Case 2
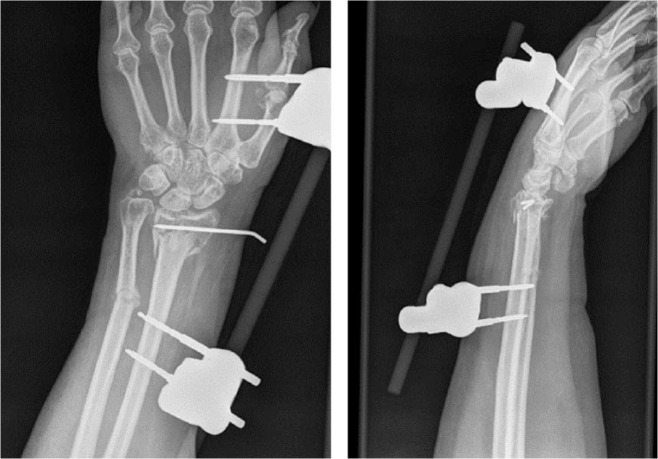
A 48-year-old woman had a comminuted and highly displaced intra- and extra-articular fracture of the distal radius. An external fixation device and K-wires had been placed elsewhere as initial treatment. Residual deformity on radiographs had been accepted because of concomitant injuries (Fig. 2a). Although the fracture healed completely, both intra- and extra-articular deformities were present. The patient suffered from significant pain (VAS 8/10) and had a pre-operative Quick-DASH score of 62. Given the complexity of the fracture, the decision was made to perform a corrective osteotomy using 3D technology. An additional informed consent for the use of 3D technology was obtained.
Fig. 2a.
Comminuted, displaced and intra-articular fracture of the distal radius as the result of a high energy trauma. An external fixator and K-pinning was used as initial treatment.
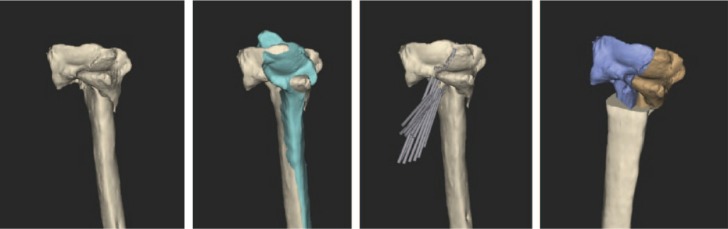
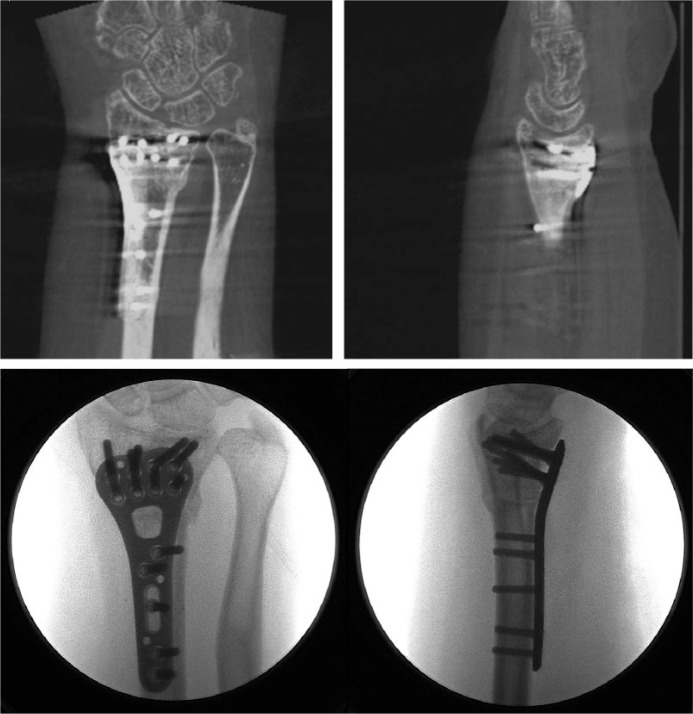
Three-dimensional STL files of the malunited distal radius were produced, and the mirrored version of the healthy contralateral side was superimposed on the malunited side. A virtual intra- and extra-articular corrective osteotomy was planned to restore normal anatomy (Fig. 2b). To reproduce this virtual osteotomy, patient-specific guides were designed, 3D printed and sterilized (Fig. 2c). A modified Henry approach was performed. During surgery, the drill and saw guides were placed on the bone at the appropriate location and fixed with K-wires (Fig. 2d). Then, the final screw holes were already pre-drilled using a printed guide. The intra-articular osteotomy was prepared by making several guided 1.2 mm drill holes along the deformity which was completed with a small osteotome. The extra-articular component of the osteotomy was made with an oscillating saw using a 3D-printed guide (Fig. 2e). A standard plate was fixed into the pre-drilled holes, automatically producing the planned reduction. A splint was provided for two weeks, followed by a removable brace for four weeks. After cast removal, the patient could start with passive and active range-of-motion exercises. Post-operative radiographs and CT scan confirmed a good anatomic reduction (Fig. 2f). The pain improved significantly after surgery (VAS 1/10) and the patient-rated QuickDASH decreased to 12.
Fig. 2b.
Three-dimensional images of the malunited radius are superimposed on a mirrored version of the healthy contralateral side and the correction is planned.
Fig. 2c.
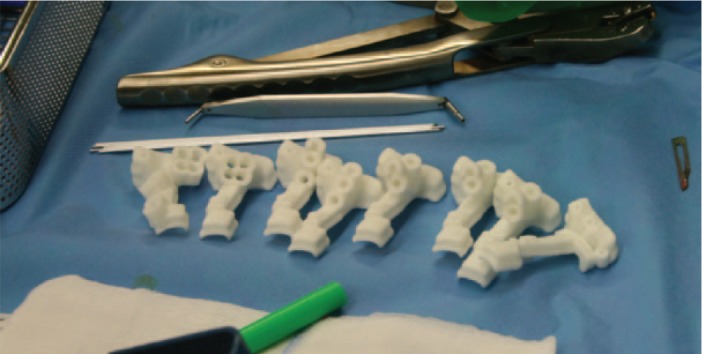
Patient-specific drill and cutting guides were manufactured.
Fig. 2d.
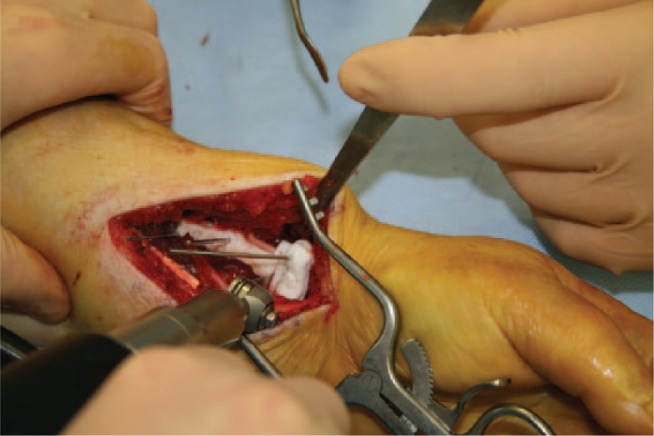
The patient-specific guides are precisely positioned on the distal radius via an open distal Henry approach.
Fig. 2e.
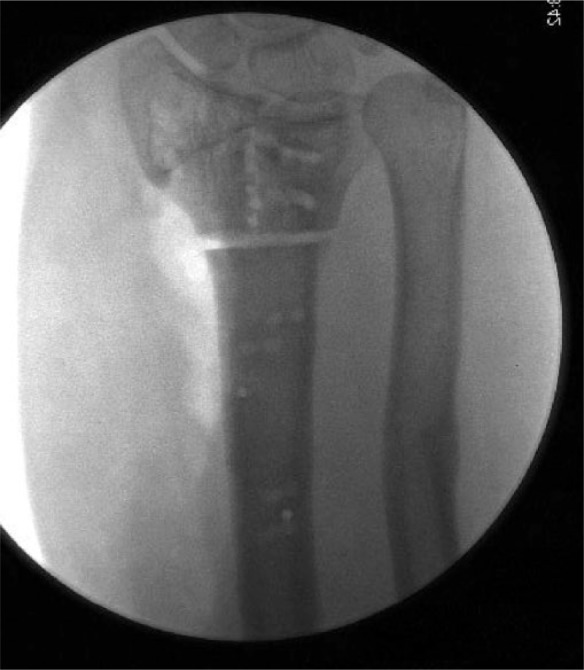
Fluoroscopy images following intra- and extra-articular osteotomy and pre-drilling of the screw holes for later plate and screw fixation.
Fig. 2f.
Post-operative fluoroscopy images and CT scans confirm good anatomical reduction.
Case 3
A 14-year-old boy sustained a midshaft fracture of the radius and ulna at the age of nine years, and was treated with closed reduction and cast immobilization for six weeks. He developed a malunion, with severe pain at the distal radioulnar joint (DRUJ) and a significant loss of forearm rotation (total arc of forearm motion 36°) (Fig. 3a). A corrective osteotomy to restore normal anatomy was the treatment of choice. An additional informed consent from both the patient and the parents, for the use of 3D technology, was obtained.
Fig. 3a.
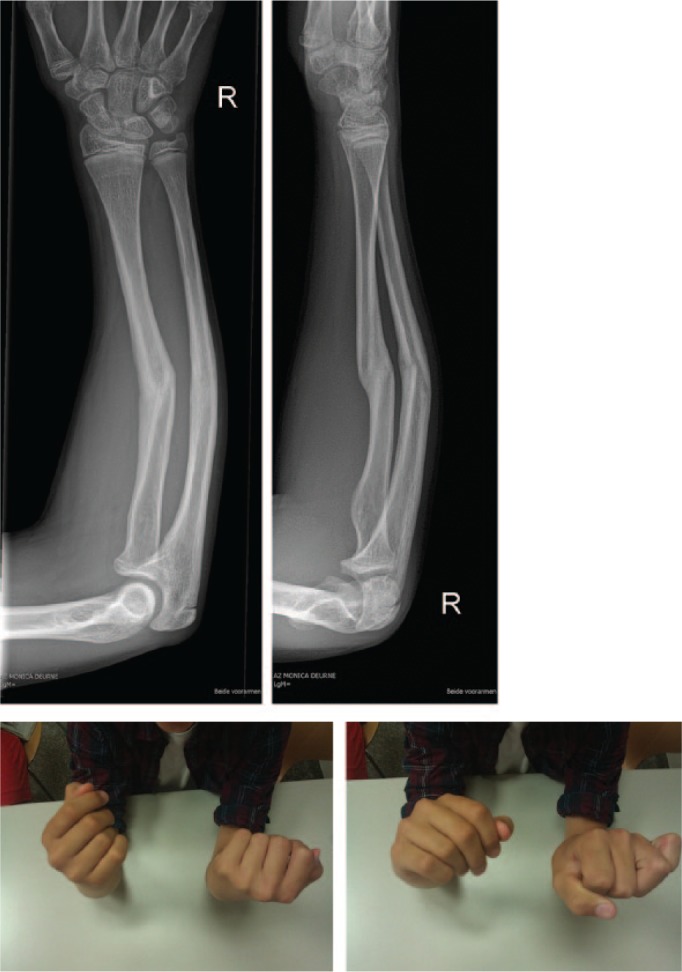
Malunion of the forearm with severe loss of rotation: forearm pro- and supination of 40° and 25°.
Virtual corrective osteotomies of radius and ulna were planned, as well as a simulation of plate fixation (Fig. 3b). Since standard plates did not fit on the corrected forearm bones, patient-specific plates were designed. A modified Henry approach was used for the radius and an approach along the subcutaneous border of the ulna. To reproduce this virtual osteotomy, patient-specific guides, as well as the patient-specific titanium plates, were 3D printed (Fig. 3c). An upper arm cast was provided for two weeks. After cast removal, the patient started with passive and active range-of-motion exercises. Post-operative radiographs showed a good anatomical reduction (Fig. 3d), and consolidation was reached after three months. The patient’s function recovered completely, with pronation of 92° and supination of 98°.
Fig. 3b.
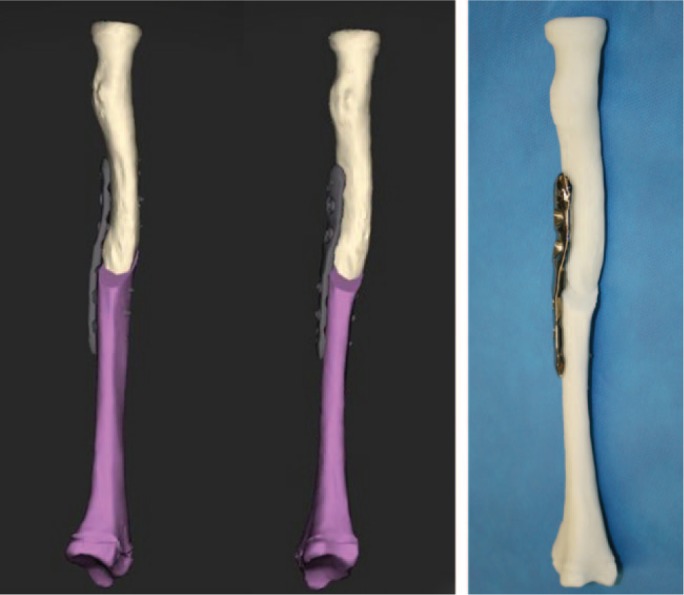
Virtual corrective osteotomy of the radius and simulation of plate fixation with the design of a patient-specific implant.
Fig. 3c.
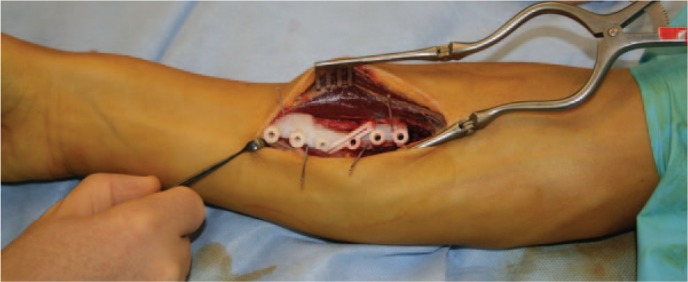
To reproduce the virtual osteotomy patient-specific guides are used.
Fig. 3d.
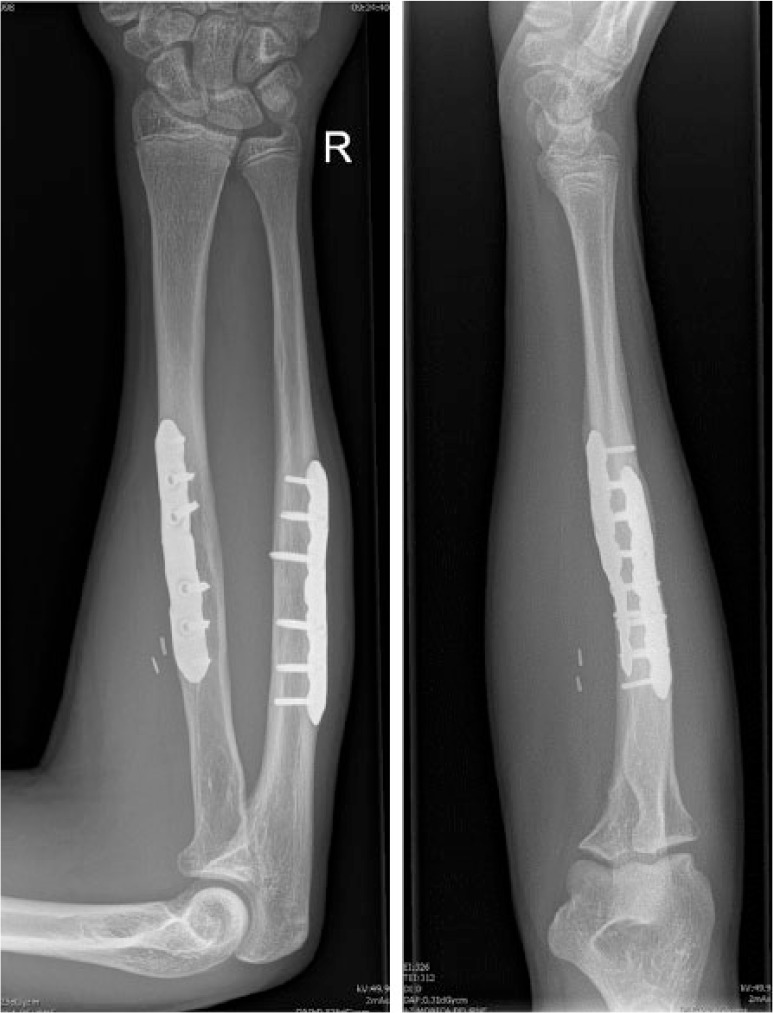
Post-operative radiographs showed a good anatomical reduction.
Case 4
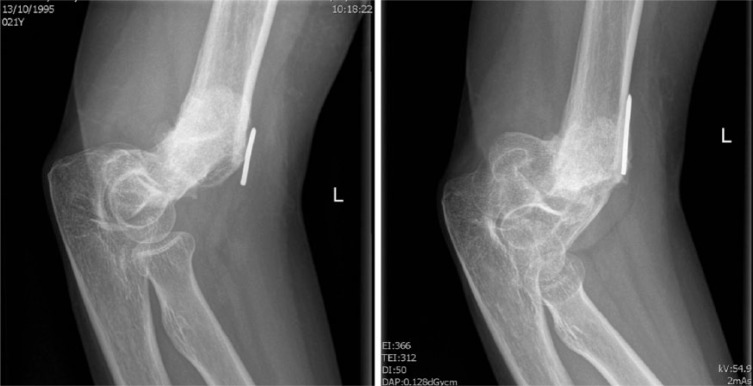
A 22-year-old woman had a complex distal humeral fracture, treated elsewhere with closed reduction and percutaneous fixation. After eight weeks the K-pins were removed, with subsequent collapse of the fracture. She developed a painful pseudarthrosis (Fig. 4a), with a complete loss of elbow function and VAS 9/10. A new attempt at reduction and internal fixation had to be made, and an additional informed consent for the use of 3D technology was obtained.
Fig. 4a.
Radiographs showing a non-union of a distal humerus fracture.
Three-dimensional images of the non-united and contralateral distal humerus were produced (Fig. 4b), and the mirrored version of the healthy contralateral side was superimposed on the non-united side. The bone fragments were reduced into their anatomical position, and plate fixation and screw orientation were virtually determined (Fig. 4c). To reproduce the planned reduction, patient-specific instruments and plates were printed and carefully positioned during surgery through a classical posterior approach of the distal humerus (Fig. 4d). A splint was provided for two weeks. After cast removal, the patient could start with passive and active range-of-motion exercises. Post-operative radiographs showed a good alignment of the elbow joint (Fig. 4e) and bony consolidation was acheived after approximately four months (Fig. 4f). Continued physiotherapy was necessary to regain a functional arc of motion of 30–100° of flexion and extension, with a pos-toperative VAS score of 2 at two years of follow-up.
Fig. 4b.
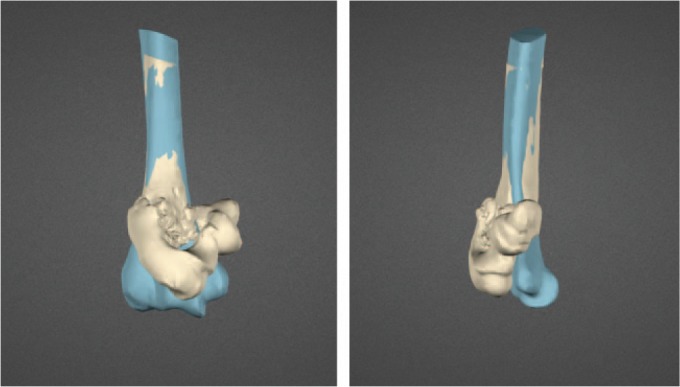
A mirrored version of the healthy contralateral side (blue) is superimposed on the non-united distal humerus.
Fig. 4c.
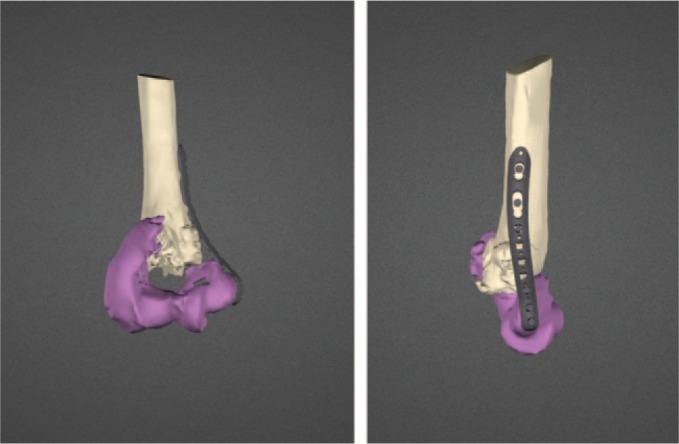
Virtual reduction of the fracture fragments and planned internal fixation.
Fig. 4d.
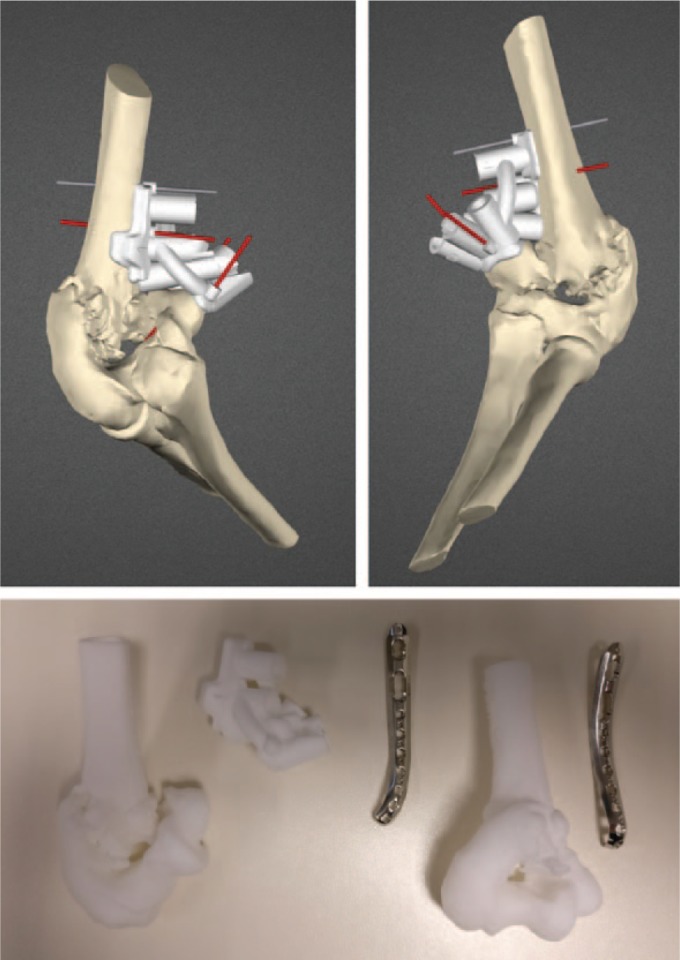
To reproduce the planned reduction, bone models and patient-specific instruments are three-dimensionally printed.
Fig. 4e.
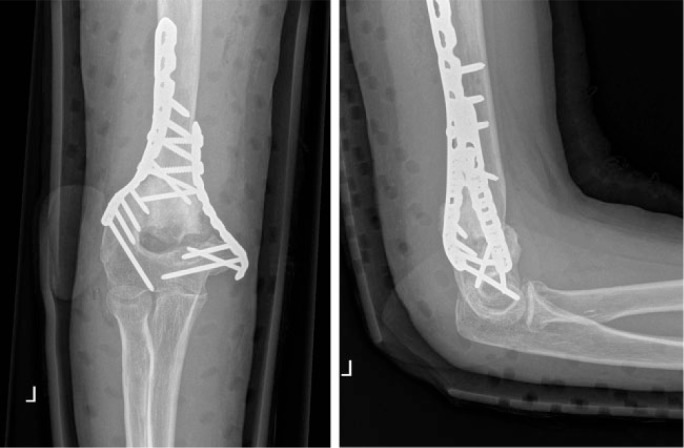
Post-operative radiographs showed a good anatomical reduction.
Fig. 4f.
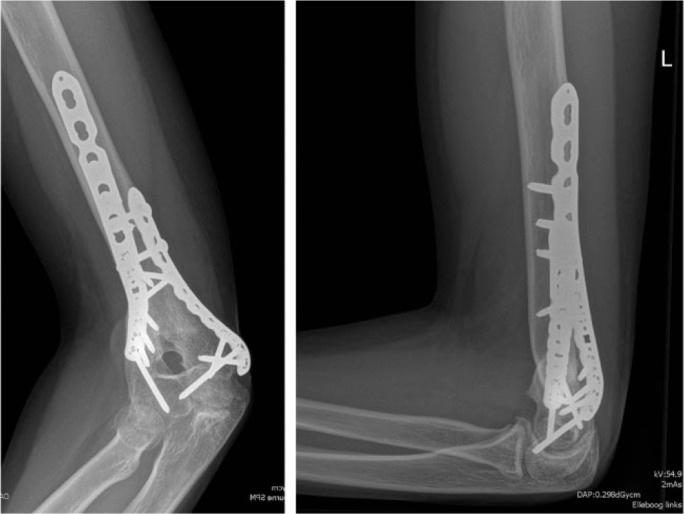
Complete consolidation was obtained after four months.
Discussion
Our most important finding with this new technology is that it facilitates restoration of normal anatomy, especially in complex deformities of the upper limb. It allows an accurate, reproducible and safe correction, and it reduces operation time, blood loss volume and radiation exposure during surgery.8,10,11–13
The incidence of upper limb injuries caused by high-energy trauma has increased, leading to a rise in number of complex fracture patterns.12,13 Improper initial treatment of upper limb fractures can lead to non-union or malunion, the most common serious complications, which can lead to pain, continuous swelling, decreased range of motion, reduced strength, instability of the adjacent joint, cosmetic deformity and nerve palsy.2,18–22 Although the final indication to perform corrective surgery is based on these clinical symptoms, improved visualization of the deformity will allow better planning of surgical correction. There is very little literature available that directly compares the results of 3D-assisted techniques with more conventional planning techniques. However, there is evidence that especially complex deformities of the upper limb will benefit from the use of 3D technology and the use of patient-specific guides.12,14,20
Approximately 5–10% of scaphoid fractures treated conservatively with cast immobilization develop non-union.26 Additionally, scaphoid non-unions will also occur in an unknown number of unrecognized scaphoid fractures. Treatment of a scaphoid non-union is necessary as it leads to significant degenerative changes in the wrist.26 The use of 3D technology in the presented case of scaphoid non-union was made to evaluate the important rotational deformities. It also allowed us to measure the residual bony defect and harvest an autologous bone graft accordingly. Schmidle et al showed in an anatomical study that in scaphoid fractures the best reference values to assess malrotation were with a 3D CT model of the opposite side, which may influence anatomical reconstruction of scaphoid fractures.28
Approximately 5% of distal radius fractures1 lead to malunion for which surgical reconstruction may be indicated. These are often complex three-dimensional deformities, with joint involvement, and precise planning and surgical reconstruction of normal anatomy can be problematic. When conventional techniques are used to correct distal radius malunions, the radiological parameters are only corrected precisely in 40% of cases.18 Large randomized trials are missing, but there is growing evidence that the use of 3D technology leads to a better clinical and radiographic outcome following corrective surgery for malunion14,23. Buijze et al compared both techniques in a randomized trial for distal radius correction and confirmed a significantly better radiographic outcome in favour of the 3D-planned and guided group.24 There was also a trend towards better PROMs, but these parameters did not reach statistical significance, which could be attributed to a lack of power of the study. We recently prospectively reviewed 30 patients in our unit, treated with a 3D corrective osteotomy of the distal radius, and found an overall improvement of wrist function, grip strength and pain. Furthermore, the patient-rated DASH score improved significantly. Radiological parameters were corrected in 73%, and there were no outliers (> 10% difference between planned and final radiographic correction).25
In patients with intra-articular deformities of the distal radius, 3D technology may help limit the need for salvage procedures as it can reliably restore articular congruency in these complex cases.22
Approximately 4–35% of paediatric forearm fractures15,16 treated conservatively lead to malunion, which may cause pain or instability in the DRUJ as well as a rotational impairment. Malunions of the forearm are often complex multiplanar deformities, and precise restoration of the anatomy of both bones is needed to restore forearm rotation. Bauer et al reviewed children with limited forearm rotation and DRUJ pain or instability. With the use of 3D technology the mean maximum angulation at the deformity of the radius and ulna improved significantly, and the main arc of pronation and supination improved from 101° to 132°.29 Byrne et al reported on a series of forearm corrections using 3D-printed titanium plates, and obtained precise radiographic corrections and good clinical results.21 They also found significant improvement in pain, grip strength and forearm supination. Miyake et al30 and Murase et al31 showed similar results in terms of accuracy.
Malunion of supracondylar fractures occurs mainly in children and arises in 4–58% of cases 17. Robinson et al showed a non-union rate of 4.5% in complex distal humeral fractures (type C according to the AO classification) after surgery, and 37.5% after non-operative treatment.27 Yang et al showed a higher elbow function score using 3D-printing technology for the treatment of complex elbow fractures compared with conventional techniques.12 Furthermore, patients gave high satisfaction scores when 3D-printed models were used to explain the patient’s condition, improving patient compliance with the surgeon’s recommendations.12
In addition to a more precise visualization of the deformities and virtual planning of the reconstruction, computer planning software also allows pre-operative selection of the most appropriate fixation plate, and positioning of the plate and screws at the correct location. This may decrease the risk of soft tissue complications, caused by mal-positioning of hardware. In most cases, standard fixation plates can be used, and when small adjustments in shape are necessary, the plates can be pre-bent on anatomical models before the procedure. In cases with more complex anatomy, the use of printed patient-specific fixation plates may be preferred. It allows the design and fabrication of a plate that fits perfectly on the anatomy of the bone which facilitates the final correction of the deformity. Specific features, such as plate shape, thickness and screw position can be adjusted as needed in each specific case.
One of the major disadvantages of the 3D technique is the additional cost, which depends largely on the application that is needed. Although most radiology departments can provide 3D rendering, 3D evaluation and planning require specific computer software. The working hours of a dedicated clinical engineer need to be included, as well as the cost of 3D printing of the models and instruments. Affordable 3D printers are available that can print in medical grade resins, but for printing in polyamide or metals such as titanium, complex and expensive equipment is needed. Whereas some units that specialize in the use of 3D technology will have the ability to set up an in-hospital 3D-printing lab, others will need to co-operate with commercial companies that offer these services and will charge for it.14 Three-dimensional printing, even in a hospital setting, is time-consuming and usually done overnight. For these reasons, the use of 3D technology is not yet adapted for use in real emergency settings.9,11,13 In our unit, we often use 3D technology for fractures as a diagnostic tool, which is different from the technology used for planning malunion correction.
Other drawbacks include the exposure to radiation during CT-scanning, the inability to differentiate bone details smaller than 0.8 mm,9 and the lack of available information on soft tissues and neurovascular structures.
Conclusions
Three-dimensional technology can assist surgeons at different stages in the treatment of complex upper limb problems. It allows 3D visualization of pathology, 3D printing of anatomical models, virtual planning of surgical procedures and fabrication of patient-specific instruments and implants. There is growing evidence of the clinical benefits of this technology. Further technical developments and clinical investigations are necessary to better define the added value and cost/benefit relationship of 3D in the treatment of complex fractures, non-unions and malunions.
Footnotes
ICMJE Conflict of interest statement: FV reports consultancy and payment received for lectures including service on speakers bureaus for Zimmer Biomet; patents (planned, pending or issued) with and royalties received from Materialise; and travel/accommodations/meeting expenses unrelated to activities listed from Sobi.
RV reports personal fees from Wright Medical and personal fees from Acumed, outside the submitted work.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Cooney WP, Dobyns JH, Linscheid RL. Complications of Colles’ fractures. J Bone Joint Surg Br 1980;62:613–619. [PubMed] [Google Scholar]
- 2. Fernandez DL. Correction of post-traumatic wrist deformity in adults by osteotomy, bone-grafting, and internal fixation. J Bone Joint Surg Am 1982;64:1164–1178. [PubMed] [Google Scholar]
- 3. Villar RN, Marsh D, Rushton N, Greatorex RA. Three years after Colles’ fracture: a prospective review. J Bone Joint Surg Br 1987;69:635–638. [DOI] [PubMed] [Google Scholar]
- 4. Fernandez DL. Reconstructive procedures for malunion and traumatic arthritis. Orthop Clin North Am 1993;24:341–363. [PubMed] [Google Scholar]
- 5. Matthews LS, Kaufer H, Garver DF, Sonstegard DA. The effect on supination-pronation of angular malalignment of fractures of both bones of the forearm. J Bone Joint Surg Am 1982;64:14–17. [PubMed] [Google Scholar]
- 6. Sarmiento A, Ebramzadeh E, Brys D, Tarr R. Angular deformities and forearm function. J Orthop Res 1992;10:121–133. [DOI] [PubMed] [Google Scholar]
- 7. Gupta R, Khanchandani P. Intercondylar fractures of the distal humerus in adults: a critical analysis of 55 cases. Injury 2002;33:511–515. [DOI] [PubMed] [Google Scholar]
- 8. Zheng W, Su J, Cai L, et al. Application of 3D-printing technology in the treatment of humeral intercondylar fractures. Orthop Traumatol Surg Res 2018;104:83–88. [DOI] [PubMed] [Google Scholar]
- 9. Zhang YZ, Lu S, Chen B, Zhao JM, Liu R, Pei GX. Application of computer-aided design osteotomy template for treatment of cubitus varus deformity in teenagers: a pilot study. J Shoulder Elbow Surg 2011;20:51–56. [DOI] [PubMed] [Google Scholar]
- 10. Bizzotto N, Tami I, Tami A, et al. 3D printed models of distal radius fractures. Injury 2016;47:976–978. [DOI] [PubMed] [Google Scholar]
- 11. Chen C, Cai L, Zhang C, Wang J, Guo X, Zhou Y. Treatment of die-punch fractures with 3D printing technology. J Invest Surg 2018;31:385–392. [DOI] [PubMed] [Google Scholar]
- 12. Yang L, Grottkau B, He Z, Ye C. Three dimensional printing technology and materials for treatment of elbow fractures. Int Orthop 2017;41:2381–2387. [DOI] [PubMed] [Google Scholar]
- 13. Shuang F, Hu W, Shao Y, Li H, Zou H. Treatment of intercondylar humeral fractures with 3D-printed osteosynthesis plates. Medicine (Baltimore) 2016;95:e2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Muinck Keizer RJO, Lechner KM, Mulders MAM, Schep NWL, Eygendaal D, Goslings JC. Three-dimensional virtual planning of corrective osteotomies of distal radius malunions: a systematic review and meta-analysis. Strateg Trauma Limb Reconstr 2017;12:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuller DJ, McCullough CJ. Malunited fractures of the forearm in children. J Bone Joint Surg Br 1982;64:364–367. [DOI] [PubMed] [Google Scholar]
- 16. Schmittenbecher PP. State-of-the-art treatment of forearm shaft fractures. Injury 2005;36:A25–A34. [DOI] [PubMed] [Google Scholar]
- 17. Piggot J, Graham HK, McCoy GF. Supracondylar fractures of the humerus in children: treatment by straight lateral traction. J Bone Joint Surg Br 1986;68:577–583. [DOI] [PubMed] [Google Scholar]
- 18. von Campe A, Nagy L, Arbab D, Dumont CE. Corrective osteotomies in malunions of the distal radius: do we get what we planned? Clin Orthop Relat Res 2006;450:179–185. [DOI] [PubMed] [Google Scholar]
- 19. Leong NL, Buijze GA, Fu EC, Stockmans F, Jupiter JB; Distal Radius Malunion (DiRaM) collaborative group. Computer-assisted versus non-computer-assisted preoperative planning of corrective osteotomy for extra-articular distal radius malunions: a randomized controlled trial. BMC Musculoskelet Disord 2010;11:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stockmans F, Dezillie M, Vanhaecke J. Accuracy of 3D virtual planning of corrective osteotomies of the distal radius. J Wrist Surg 2013;2:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Byrne AM, Impelmans B, Bertrand V, Van Haver A, Verstreken F. Corrective osteotomy for malunited diaphyseal forearm fractures using preoperative 3-dimensional planning and patient-specific surgical guides and implants. J Hand Surg Am 2017;42:836.e1–836.e12. [DOI] [PubMed] [Google Scholar]
- 22. Schweizer A, Fürnstahl P, Nagy L. Three-dimensional correction of distal radius intra-articular malunions using patient-specific drill guides. J Hand Surg Am 2013;38:2339–2347. [DOI] [PubMed] [Google Scholar]
- 23. Miyake J, Murase T, Yamanaka Y, Moritomo H, Sugamoto K, Yoshikawa H. Comparison of three dimensional and radiographic measurements in the analysis of distal radius malunion. J Hand Surg Eur Vol 2013;38:133–143. [DOI] [PubMed] [Google Scholar]
- 24. Buijze GA, Leong NL, Stockmans F, et al. Three-dimensional compared with two-dimensional preoperative planning of corrective osteotomy for extra-articular distal radial malunion: a multicenter randomized controlled trial. J Bone Joint Surg Am 2018;100:1191–1202. [DOI] [PubMed] [Google Scholar]
- 25. Michielsen M, Van Haver A, Bertrand V, Vanhees M, Verstreken F. Corrective osteotomy of distal radius malunions using three-dimensional computer simulation and patient-specific guides to achieve anatomic reduction. Eur J Orthop Surg Traumatol 2018;28:1531–1535. [DOI] [PubMed] [Google Scholar]
- 26. Prosser GH, Isbister ES. The presentation of scaphoid non-union. Injury 2003;34:65–67. [DOI] [PubMed] [Google Scholar]
- 27. Robinson CM, Hill RMF, Jacobs N, Dall G, Court-Brown CM. Adult distal humeral metaphyseal fractures: epidemiology and results of treatment. J Orthop Trauma 2003;17:38–47. [DOI] [PubMed] [Google Scholar]
- 28. Schmidle G, Rieger M, Klauser AS, et al. Intraosseous rotation of the scaphoid: assessment by using a 3D CT model—an anatomic study. Eur Radiol 2014;24:1357–1365. [DOI] [PubMed] [Google Scholar]
- 29. Bauer AS, Storelli DAR, Sibbel SE, McCarroll HR, Lattanza LL. Preoperative computer simulation and patient-specific guides are safe and effective to correct forearm deformity in children. J Pediatr Orthop 2017;37:504–510. [DOI] [PubMed] [Google Scholar]
- 30. Miyake J, Murase T, Oka K, Moritomo H, Sugamoto K, Yoshikawa H. Computer-assisted corrective osteotomy for malunited diaphyseal forearm fractures. J Bone Joint Surg Am 2012;94:e150. [DOI] [PubMed] [Google Scholar]
- 31. Murase T, Oka K, Moritomo H, Goto A, Yoshikawa H, Sugamoto K. Three-dimensional corrective osteotomy of malunited fractures of the upper extremity with use of a computer simulation system. J Bone Joint Surg Am 2008;90:2375–2389. [DOI] [PubMed] [Google Scholar]