Abstract
A total amount of 116 fungal strains, belonging to 30 genera, were acquired from the rhizosphere soil and plant of Galinsoga parviflora. A strain SYPF 7336, isolated from the rhizospheric soil, was identified as Seltsamia galinsogisoli sp. nov., by morphological and molecular analyses, which displayed high antibacterial activity. In order to study the secondary metabolites of Seltsamia galinsogisoli sp. nov., nine compounds were successfully seperated from the strain fermentation broth, including two new compounds and seven known compounds. Their structures were elucidated based on spectral analysis including 1D and 2D NMR. All the seperated compounds were evaluated for their antimicrobial activities. Compounds 2, 5 and 1 displayed antimicrobial activities against Staphylococcus aureus with MIC values of 25, 32 and 75 μg/mL, respectively. Moreover, morphological observation showed the coccoid cells of S. aureus to be swollen to a volume of 1.4 to 1.7-fold after treatment with compounds 1, 2 and 5, respectively. Molecular docking was carried out to investigate interactions of filamentous temperature-sensitive protein Z (FtsZ) with compounds 1, 2 and 5.
Subject terms: Pharmaceutics, Drug development
Introduction
Secondary metabolites coming from microorganism represent a large number of diverse components which have been treated as potential candidates for drugs1–5. Currently, due to the increase of antibiotic resistance, there is an urgent need for novel classes of lead compounds and novel mechanisms to confront the antibiotic crises6,7.
Microbial filamentous temperature-sensitive protein Z (FtsZ) is a novel target for drug discovery, which plays a key role in cell division8,9. The inhibitors of FtsZ prevent the cellular fission of bacteria, which lead to apoptosis of bacteria10–12. Therefore, morphological observation of microbial fission and molecular docking between the lead compound and FtsZ were accomplished to explore the possible mechanism13,14. Up to now, the discovered FtsZ inhibitors are divided into natural products (sanguinarine, berberine, totarol, curcumin and cinnamaldehyde) and synthetic small molecules (PC190723, UCM53, CCR-11)15–19. At present, PC190723 has been the candidate drugs to enter the clinical trials, which inspires scientists to make more effort to find potential FtsZ inhibitors as antibacterial agents20–22.
The endophytic fungi are known as a source of abundant secondary metabolites for functional bioactive substances. As we know, only a small amount of microbes have been studied so far, and a vast number of new taxa waiting for discovery, especially those separated from medicinal plants. This leads us to isolate and evaluate the potential pharmacological activity of bioactive compounds produced by endophytic fungi.
In the present study, the endophytic fungi diversity of the traditional Chinese herb, Galinsoga parviflora was surveyed and 116 fungal strains were isolated from the whole plant (stems, leaves and roots) which attributed to 30 genera. Forty-three percent of the strains revealed antimicrobial abilities against at least one kind of human pathogenic microorganisms. Strain SYPF 7336, presented the strongest antibacterial activity, could not be affiliated to any known taxon, so it was identified as a novel species of genus Seltsamia by phylogenetic analyses, given the name Seltsamia galinsogisoli sp. nov. The genus Seltsamia, (Cucurbitariaceae) was first proposed in 2018, and the only strain Seltsamia ulmi CBS 143002 was isolated on corticated Ulmus glabra in Norway23. Seltsamia ulmi produces pyriform, black ascomata and cylindrical asci. Asci contain 8 uni- to partly biseriately arranged ascospores and ascospores are fusoid to subclavate with 3 main septa. Due to none of the secondary metabolites of the genus Seltsamia having been recorded, the compounds and bioactivities of the novel species, strain SYPF 7336, were explored in the present study. Further, the molecular docking analysis was carried out for mechanism investigation.
Results
Species delineation and classification
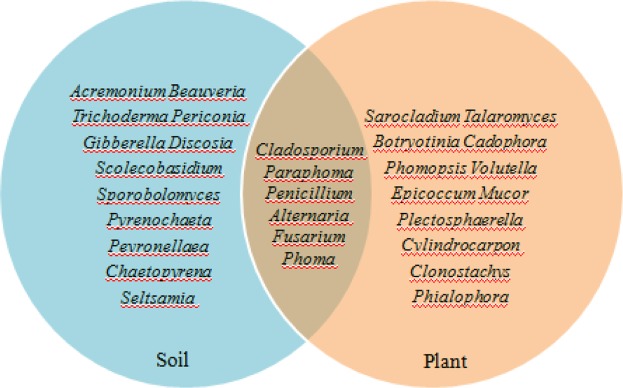
A total of 116 fungal strains were acquired from the soil and plant of Galinsoga parviflora, which were divided into 30 groups according to morphological characteristics and phylogenetic analyses. Twelve genera, Acremonium, Beauveria, Trichoderma, Periconia, Gibberella, Discosi, Scolecobasidium, Sporobolomyces, Pyrenochaeta, Peyronellaea, Chaetopyrena and Seltsamia, were found only in the soil. Twelve genera, Sarocladium, Talaromyces, Botryotinia, Cadophora, Phomopsis, Volutella, Epicoccum, Mucor, Plectosphaerella, Cylindrocarpon, Clonostachys, and Phialophora just survived in the plant. Six genera, Cladosporium, Paraphoma, Penicillium, Alternaria, Fusarium, and Phoma could simultaneously survive in the soil and plant of G. parviflora (Fig. 1).
Figure 1.
Genus distribution of endophytic fungi isolated from soil and plant of Galinsoga parviflora.
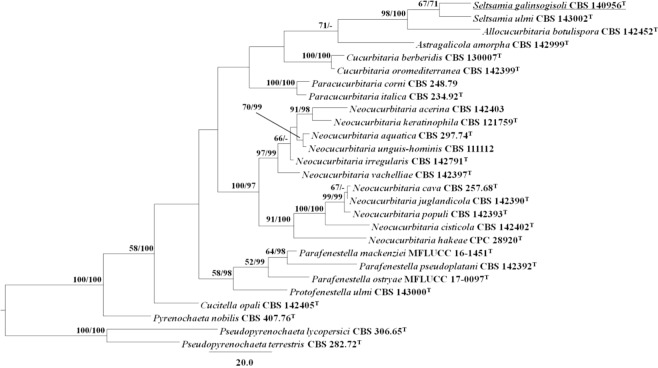
A strain SYPF 7336, isolated from the rhizosphere soil of G. parviflora, was most close to Seltsamia ulmi when using ITS sequence BLAST, but they did not match well in 13 different positions. So, the phylogenetic analyses were carried out based on two loci (ITS and LSU) to clarify the taxon status of strain SYPF 7336. In the MP analysis, 1938 characters were constant, 87 were parsimony-uninformative, and 152 were parsimony-informative. After phylogenetic analysis, the best MP tree was shown in Fig. 2 (TL = 576, CI = 0.575, RI = 0.630, RC = 0.362, HI = 0.425). In this tree, strain SYPF 7336 was placed in genus Seltsamia and formed a sister clade together with S. ulmi. The ITS and LSU sequences were deposited in GenBank with accession numbers KU759584 and KU759581, respectively (Table 1).
Figure 2.
Maximum Parsimony (MP) tree based on analysis of a combined dataset of ITS and LSU sequence data. MP bootstrap support values (MPB above 50%) and Bayesian posterior probabilities (BPP; above 70%) are given at the nodes (MPB/BPP). Drechmeria panacis sp. nov. is denoted by bold letters.
Table 1.
Strains used in the phylogenetic analyses and their corresponding GenBank accession numbers.
| Species | Culture accession No. | GenBank accession No. | |
|---|---|---|---|
| ITS | LSU | ||
| Allocucurbitaria botulispora | CBS 142452T | LT592932 | LN907416 |
| Astragalicola amorpha | CBS 142999T | MF795753 | MF795753 |
| Cucitella opali | CBS 142405T | MF795754 | MF795754 |
| Cucurbitaria berberidis | CBS 130007T | LT717673 | KC506793 |
| Cucurbitaria oromediterranea | CBS 142399T | MF795761 | MF795761 |
| Neocucurbitaria acerina | CBS 142403 | MF795768 | MF795768 |
| Neocucurbitaria aquatica | CBS 297.74T | LT623221 | EU754177 |
| Neocucurbitaria cava | CBS 257.68T | JF740260 | EU754199 |
| Neocucurbitaria cisticola | CBS 142402T | MF795772 | MF795772 |
| Neocucurbitaria hakeae | CPC 28920T | KY173436 | KY173526 |
| Neocucurbitaria irregularis | CBS 142791T | LT592916 | LN907372 |
| Neocucurbitaria juglandicola | CBS 142390T | MF795773 | MF795773 |
| Neocucurbitaria keratinophila | CBS 121759T | EU885415 | LT623215 |
| Neocucurbitaria populi | CBS 142393T | MF795774 | MF795774 |
| Neocucurbitaria unguis-hominis | CBS 111112 | LT623222 | GQ387623 |
| Neocucurbitaria vachelliae | CBS 142397T | MF795787 | MF795787 |
| Paracucurbitaria corni | CBS 248.79 | LT903672 | GQ387608 |
| Paracucurbitaria italica | CBS 234.92T | LT623219 | EU754176 |
| Parafenestella mackenziei | MFLUCC 16-145T | KY563071 | KY563074 |
| Parafenestella ostryae | MFLUCC 17-0097T | KY563072 | KY563075 |
| Parafenestella pseudoplatani | C BS 142392T | MF795788 | MF795788 |
| Protofenestella ulmi | CBS 143000T | MF795791 | MF795791 |
| Pseudopyrenochaeta lycopersici | CBS 306.65T | NR_103581 | EU754205 |
| Pseudopyrenochaeta terrestris | CBS 282.72T | LT623228 | LT623216 |
| Pyrenochaeta nobilis | CBS 407.76T | EU930011 | EU754206 |
| Seltsamia galinsogisoli a | CBS 140956 T | KU759584 | KU759581 |
| Seltsamia ulmi | CBS 143002T | MF795794 | MF795794 |
“a”New accession numbers produced in this study are bold.
Description of Seltsamia galinsogisoli Tianyuan Zhang & Yixuan Zhang, sp. nov. MB 820393
Seltsamia galinsogisoli (Ga.lin.so.gi’so’li. N.L. gen. n. galinsogisoli of soil of a G. parviflora, got from Huludao city, Liaoning Province, northeast of China).
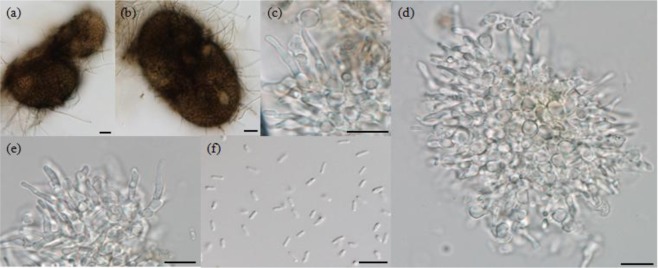
Vegetative hyphae hyaline, smooth walled. Pycnidia subglobose to globose, brown to dark brown, 70–125 × 45–96 μm. Surface roughened by colourless hyphal appendages (Fig. 3a,b). Conidiogenous cells phialidic, hyaline, smooth walled, 9–19.2 × 1.4–4.2 μm (Fig. 3c–e). Conidia 1-celled, hyaline, smooth, cylindrical, slightly curved, 2.5–4.3 × 0.8–1.2 μm (Fig. 3f).
Figure 3.
Morphological characters of Seltsamia galinsogisoli CBS 140956T. (a,b) Pycnidia. (c–e) Conidiogenous cells. (f) Conidia. Bars: a,b = 20 μm, c–f = 10 μm.
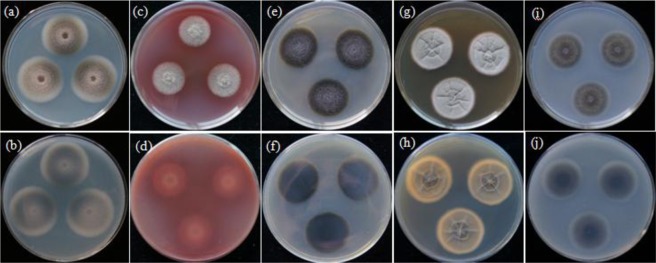
Colonies on PDA attaining 36.6 mm diameter after 11 d at 26 °C, surface floccose, grey to dark grey, reverse grey to dark grey (Fig. 4a,b). Colonies on PNA attaining 25.9 mm diameter after11 d at 26 °C, surface floccose, grey, reverse grey (Fig. 4c,d). Colonies on CMA attaining 30.2 mm diameter after 11 d at 26 °C, surface floccose, dark grey to dark green, reverse dark green to black (Fig. 4e,f). Colonies on MEA attaining 33.5 mm diameter after 11 d at 26 °C, surface velvety, grey with 5–8 radial and 1 annular groove, reverse grey with 5–8 radial and 1 annular cracks (Fig. 4g,h).Colonies on OA attaining 29.8 mm diameter after 11 d at 26 °C, surface floccose, grey to dark olive green, reverse dark olive green (Fig. 4i,j).
Figure 4.
Colony morphologies of Seltsamia galinsogisoli CBS 140956T (a–j). Colonies on PDA at 26 °C after 11 d (a, obverse; b, reverse), on PNA at 26 °C after 11 d (c, obverse; d, reverse), on CMA at 26 °C after 11 d (e, obverse; f, reverse), on MEA at 26 °C after 24 d (g, obverse; h, reverse) and on OA at 26 °C after 11 d (i, obverse; j, reverse).
Type specimen
China, Liaoning province, Huludao city, 40°82′26.5″N, 119°78′52.0″E, Sep 2014, from the rhizosphere of G. parviflora. Ex-type culture CBS 140956 = CGMCC 3.17981 = SYPF 7336.
Identification of the compounds
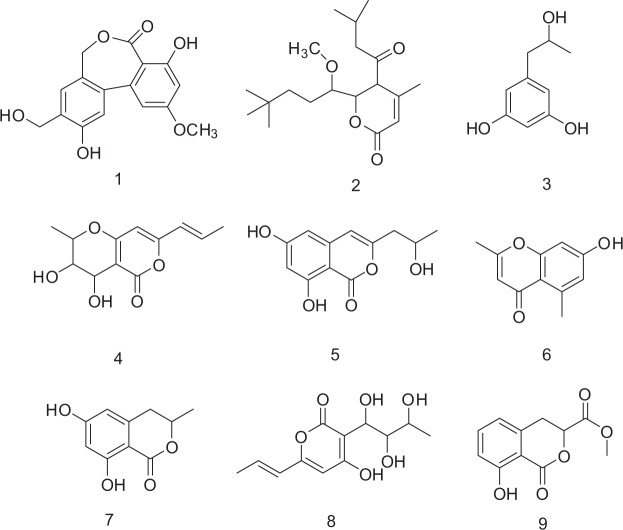
Seltsamiayu (1) (Fig. 5) was isolated as white flakes. The absorption bands at 3448 (strong wide wave), 1636.6 cm−1 in the IR spectrum suggested the presence of hydroxyl carboxylic acid carbonyl, and carbonyl functionalities (Figs S1–S6). The molecular formula of compound 1 was determined as C16H14O6 on the basis of its ion [M + Na]+ at m/z 325.2738 obtained by HRESI/MS.
Figure 5.
Chemical structures of compounds 1–9.
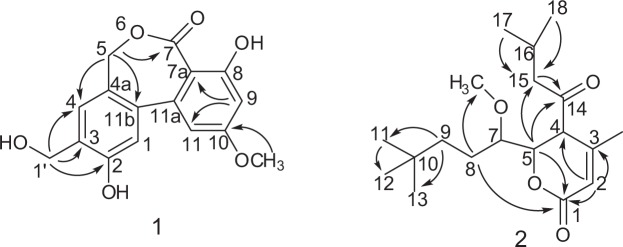
The 1D NMR spectra of compound 1 (Table 2) indicated the presence of two benzene rings [δC 162.2, 159.9, 146.6, 145.9, 140.1, 129.8, 126.7, 115.6, 115.5, 109.5, 105.0, 100.8; δH 7.04 s, 6.90 s, 6.51 d (J = 2.2 Hz),6.50 d (J = 2.2 Hz)] and one methoxy [δC 55.4,δH 3.8 s]. The spectra of 1 were in part very similar to those of alter lactone except for the absence of a methylene group24. The HMBC spectra indicated the presence of long-range correlations (Fig. 6) from H-1′ with C-2, C-3, and C-4, from H-5 with C-4a, C-7 and C-11b, from H-9 with C-7a and C-11, combined with the HSQC and HRESIMS data, the structure of compound 1 was verified as shown in Fig. 5 and it was given the common name Pyrenochaetayu.
Table 2.
1H (600 MHz, DMSO-d4) and 13C NMR (150 MHz, DMSO-d4) data of compounds 1 and 2.
| Compd. No | 1 | Compd. No. | 2 | ||
|---|---|---|---|---|---|
| δC, type | δH (J, Hz) | δC, type | δH (J, Hz) | ||
| 1 | 115.5 | 7.04 (s, 1H) | 1 | 174.3, C | — |
| 2 | 146.6 | — | 2 | 116.3, CH | 5.85 (s, 1H) |
| 3 | 145.9 | — | 3 | 151.2, C | — |
| 4 | 115.6 | 6.90 (s,1H) | 4 | 103.5, C | — |
| 4a | 140.1 | — | 5 | 87.7, CH | 4.83 (m,1H) |
| 5 | 67.8 |
4.86 (d, 1H, J = 11.0) 4.85 (d, 1H, J = 11.0) |
6 | O | — |
| 7 | 168.7 | — | 7 | 51.9, CH | 3.6 (m, 1H) |
| 7a | 109.5 | — | 7-OCH3 | 58.9, CH3 | 3.68 (m, 3H) |
| 8 | 159.9 | — | 8 | 46.4, CH2 | 4.05 (m, 2H) |
| 9 | 100.8 | 6.51 (d, 1H, J = 2.2) | 9 | 32.4, CH2 | 2.66 (m, 2H) |
| 10 | 162.2 | — | 10 | 29.1, C(丟) | — |
| 11 | 105.0 | 6.50 (d, 1H, J = 2.2) | 12-CH3 | 20.7, CH3 | 1.59 (m, 3H, J = 7.3) |
| 11a | 126.7 | — | 11-CH3 | 8.2, CH3 | 0.92 (t, 3H, J = 7.3) |
| 11b | 129.8 | — | 13-CH3 | 13.4, CH3 | 0.97 (t, 3H, J = 7.3) |
| 10-OCH3 | 55.4 | 3.8 (s, 3H) | 14 | 193.0, C | — |
| 3-CH2-OH | 48.6 | 4.83 (dd, 2H, J = 11.15) | 15 | 23.5, CH2 |
1.95 (m,1H) 1.75 (m,1H) |
| 2-OH | — | 9.39 | 16 | 45.6, CH | 2.92 (brs, 1H) |
| 3-CH2-OH | — | 9.46 | 17 | 22.2, CH3 | 0.849 (m, 3H) |
| 8-OH | — | 10.2 | 18 | 22.2, CH3 | 0.877 (m, 3H) |
Figure 6.
Selected HMBC correlations of compounds 1 and 2.
Galinsogisoliyu (2) was seperated as a brown powder, + 3.7 (c 0.25, MeOH). The absorption bands at 3443 (strong wide wave),1703 and 1642 cm−1 in IR spectrum suggested the presence of hydroxyl carboxylic acid carbonyl, and carbonyl functionalities (Figs S7–12). The HRESIMS indicated the presence of an ion peak at m/z 347.4509 [M + Na]+ (calcd. for C19H32O4Na, 347.4504), indicating the molecular formula of C19H32O4. The 1H NMR spectrum of 2 (Table 2) indicated the presence of five methyl groups at δH 0.92(t, 3H), 0.97 (t, 3H), 0.849 (m, 3H), 0.877 (m, 3H), 1.59 (m, 3H), one methoxyl groups at δH 3.68 (3H, m) [Henrick et al. 1975, Watanabe et al. 2000]. It also showed four methylene protons at δH 4.05 (2H, m, H-8), 2.66 (2H, m, H-9), 1.95 (m, 1H, H-15a) and 1.75 (m, 1H, H-15b), five methine protons at 5.85 (1H, s, H-2), 4.83 (1H, m, H-5), 3.6 (1H, m, H-7), 3.6 (1H, m, H-7), 2.92 (1H, brs, H-16); The 13C NMR spectra (Table 2) indicated the presence of two carbonyl carbons (δC 174.3 and 193.0), a pair of olefinic carbons (δC 174.3 and 193.0), five methyl carbons (δC 22.2*2, 20.7, 13.4 and 8.2), one oxygenated methylene proton carbon (δC 58.9), three methylene carbons (δC 46.4, 32.4 and 23.5). The 1H and 13C NMR spectra of 2 were similar to those of 4-methyl-5,6-dihydro-2H-pyran-2-one except for the absence of two side chains25,26. The planar structure of 2 (Fig. 6) was established by the 2D NMR data. The 2D NMR spectra of compound 2 (Fig. 6) indicated the presence of long-range correlations from H-2 with C-1, C-3 and C-4, H-5 with C-1, C-14 and C-15, H-9 with C-11 and C-13 and H-15 with C-5 and C-14. According to the above evidence, the structure of 2 was verified as shown in Fig. 5 and it was given the common name Galinsogisoliyu.
Additionally, the discovery metabolites of Seltsamia galinsogisoli sp.nov. resulted the isolation of seven known compounds (3–9), including 1,3-Benzenediol,5-(2-hydroxypropyl) (3)27, 3,4-Dihydroxy-2-methyl-7-[prop-1-enyl]-3,4-dihydro-2H-pyrano[4,3-b]pyran-5-one (4)28–30, 1H-2-Benzopyran-1-one,6,8-dihydroxy-3-(2-hydroxypropyl) (5)31,32, 2,5-dimethyl-7-hydroxyl chromone (6)33, 3-methyl-6,8-dihydroxyisocoumarin (7)34, Curvulapyrone (8)34, and 3,4-dihydro-8-hydroxyisocoumarin-3-carboxylic methyl ether (9)35.
The antimicrobial results of compounds 1–9
All the seperated metabolites were tested for antimicrobial effects against five common pathogenic bacteria, S. aureus, B. subtilis, P. aeruginosa, K. pneumonia and Bacillus cereus. The results were shown in Table 3.
Table 3.
Antibacterial effects of compounds 1–9.
| Compd. | Staphylococcus aureus | Bacillus subtillis | Pseudomonas aeruginosa | Klebsiella pneumonia | Bacillus cereus |
|---|---|---|---|---|---|
| 1 | +++ | ++ | ++ | + | + |
| 2 | +++ | + | + | + | + |
| 3 | ++ | +++ | − | ++ | ++ |
| 4 | + | +++ | + | + | ++ |
| 5 | +++ | +++ | ++ | + | ++ |
| 6 | ++ | + | + | + | ++ |
| 7 | ++ | ++ | ++ | + | ++ |
| 8 | ++ | +++ | − | + | ++ |
| 9 | + | − | + | + | + |
| Ampicillina | ++++ | ++++ | ++++ | ++++ | ++++ |
aAs positive control. “+”: antibacterial rate 0–30%, “+”: 30–60%, “+++”, 60–80%, “++++”, 80–100%, “−” no antibacterial activities.
Compounds 2, 5 and 1 showed antimicrobial activities against S. aureus with MIC values of 25, 32 and 75 μg/mL, respectively. Compounds 3–4, 6–7 and 8–9 showed weak antimicrobial effects.
Morphological observation and molecular docking
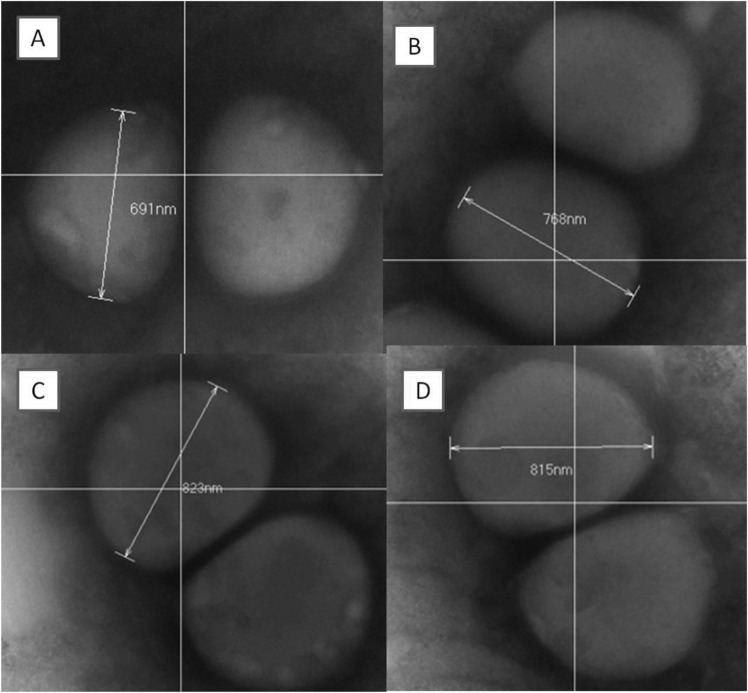
The cells of S. aureus treated with compounds were observed carefully (Fig. 7). Interestingly, the coccoid cells of S. aureus were swelled to larger volume after treatment with compound 1 (1.4 fold), 2 (1.7 fold) and 5 (1.6 fold), respectively. In order to explain the possible mechanism, FtsZ, key protein of cell division35, was explored for molecular docking study.
Figure 7.
Electron micrographs of S. aureus in the absence (A) or presence of compounds 1 (B), 2 (C) and 5 (D). (A) Untreated control cells of S. aureus with average diameter length of 691 nm. (B) cells of S. aureus with average diameter length of 768 nm. (C) Cells of S. aureus with average diameter length of 823 nm. (D) Cells of S. aureus with average diameter length of 815 nm.
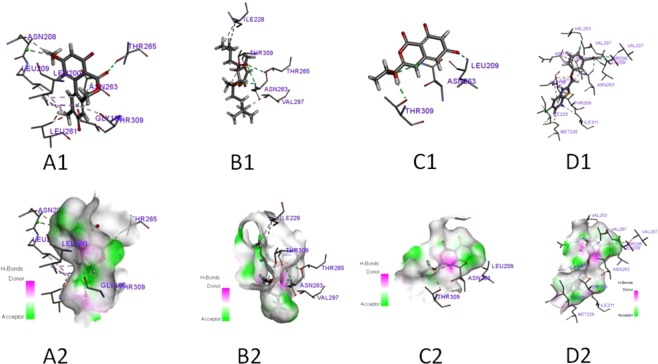
The docking simulation of active compounds 1, 2 and 5 to FtsZ from S. aureus (PDB:ID 3VOB) (Fig. 8) resulted in the binding energies of −109, −125, and −113 kcal/mol, respectively (Table 4). Thus, compound 2 had the best binding energies with FtsZ. Furthermore, the binding patterns were also different. Compound 2 displayed four hydrogen bonds and one more hydrophobic bond to relevant residues comparing with compound 5 (Table 4). Compound 1 showed two hydrogen bonds and five hydrophobic bonds to relevant residues. However, an unfavorable bump LEU261 was observed for compound 1 (Table 4). Five hydrophobic bonds for compound 1 were observed and considered to make major contribution to the combinations. The interactions between FtsZ with compounds 1–2 and 5 are displayed in Table 4.
Figure 8.
In silico docking simulation of compounds 1, 2, 5 and ligand to FtsZ of S. aureus. (A1–D1) H-bond interactions between 1, 2, 5 and ligand to FtsZ of S. aureus. (A2–D2) Hydrophobic interaction between 1, 2, 5 and ligand to FtsZ of S. aureus in a 3D docking model.
Table 4.
Binding residues involved in the formation of hydrophobic bonds and hydrogen bonds with compounds 1, 2, 5 and ligand.
| Comd. | Docking score (Kcal/mol) |
Unfavorable Bump | Residues | |
|---|---|---|---|---|
| Conventional hydrogen bond | Hydrophobic bond | |||
| 1 | −109 | LEU261 | LEU209, LEU200 | ASN208, THR309, ASN263, GLY196,THR265 |
| 2 | −125 | — | ILE228*2, THR309, VAL297 | ASN263*2,THR309, THR265 |
| 5 | −113 | — | — | THR309, ASN263, LEU209 |
| ligand | −167 | — | LEU209, ASN 263, THR 296, VAL 207, GLY 196, THR 309, VAL 203 | ILE 228, ILE311, ILE 197, LEU200, ASP 199, VAL 297, MET 226 |
“—” No bond connected “*” Multiple bond connected.
Discussion
All the endophytic fungi isolated from the rhizosphere of G. parviflora were fermented and the crude extracts of each strain were tested for the microbial activities (Table S1). Forty-three percent of the strains showed antimicrobial activities against at least one kind of human pathogenic microorganisms. These results provide references for further study of the strains.
Among the stains, SYPF 7336, showed the best antibacterial activity. The strain SYPF 7336 was carefully studied and identified as Seltsamia galinsogisoli sp. nov. by morphology and molecular analyses, and only pycnidia was observed whereas no perfect stage available. This is the first-found of pycnidia in the genus Seltsamia for only perfect stage was recorded whereas no asexual information in the publication in S. ulmi, which was isolated from Hapalocystis bicaudata on corticated Ulmus glabra in Norway in 201823. Though differences of the reproductive body between the two species could not be compared, they are remarkably different species based on the phylogenetic analyses (Fig. 2). Moreover, the difference between the two species are that Seltsamia galinsogisoli sp. nov. produces dark grey colonies with annular and radical grooves on MEA23. Seltsamia is a newly introduced genus, the finding of Seltsamia galinsogisoli sp. nov. expands the host range of this genus.
Another aim of this study is to isolate antimicrobial secondary metabolites secreted by the novel strain, Seltsamia galinsogisoli sp. nov. It is the first time to report secondary metabolites from the genus Seltsamia, family Cucurbitariaceae. Two new compounds (1–2) and seven known compounds (3–9) (Fig. 5) were purified, identified and tested for their antimicrobial abilities against S. aureus, B. subtilis, P. aeruginosa, K. pneumonia, and E. coli. As results, compounds 2, 5 and 1 displayed well antibacterial activities toward S. aureus with MIC values of 25, 32 and 75 μg/mL, respectively. These results from the present work provide further information about the diversity and activities of compounds in the genus Seltsamai.
FtsZ is a pop target for drug discovery in recent years. The gene of FtsZ has the ability of high conservation and presented almost in all bacteria9,36. In bacterial cytokinesis, FtsZ protein is the earliest known step to build a contractile ring on the inner surface of the cytoplasmic membrane9,36. The inhibitors of FtsZ might prevent the cellular fission of bacteria, which lead to apoptosis of bacteria. Therefore, morphological observation and molecular docking were carried out to search the possible interactions between the active compounds and FtsZ.
Thus, the cells of S. aureus treated with active compounds 1, 2 and 5 were observed to further study the possible antibacterial mechanism. Interestingly, As Fig. 7 showed, the coccoid cells of S. aureus were swollen to 1.4 to 1.7-fold volume after treatment with compound 1 (1.4 fold), 2 (1.7 fold) and 5 (1.6 fold), respectively. In order to explain this interesting appearance, FtsZ, the key protein of cell division8,9, was explored to illustrate the mechanism of cells that became swollen. Thus, a molecular docking study was carried out to verify the deduction.
The docking results are shown in Fig. 8 and Table 4. The FtsZ from S. aureus (PDB:ID 3VOB) displayed the docking score of the ligand (−167 kcal/mol) was the lowest. Compound 5 (−113 kcal/mol) displayed three hydrophobic bonds with THR309, ASN263, LEU209 residues but no hydrogen bonds with neighbouring amino acid residues. Compound 2 (−125 kcal/mol) formed four hydrogen bonds with the ILE228*2, THR309, VAL297 residues and four hydrophobic bonds with the ASN263*2,THR309 and THR265 residues. Although compound 1 formed two hydrogen bonds and five hydrophobic bonds with the residues, one unfavorable interactions between compound 1 and the active region or intramolecular of FtsZ were existed. Thus, compound 1 showed a weak ability to combine with the docking score of −109 kcal/mol. The docking scores −125 kcal/mol (2) −113 kcal/mol (5) and -109 kcal/mol (1), indicated that compounds 2 and 5 might form lower potential energies and more stable binding sites with the target protein FtsZ compared to coumpound 1 which validated the observed antimicrobial activities. Based on the antimicrobial activities, phenotypic consequences and docking studies, compounds 2 and 5 were identified as promising antimicrobial lead molecules.
Methods
General experimental procedures
Optical rotations were recorded using a P-2000 Digital Polarimeter (JASCO, United Kingdom)37. IR spectra were measured on an Equinox55 spectrophotometer in KBr discs (Bruker Optik BmbH, Ettlingen, Germany). The 1D- and 2D-NMR spectra were recorded at 600 for 1H and 150 MHz for 13C (Bruker, Rheinstetten, Germany). HR-ESI-MS data were acquired on a Bruker Customer micrOTOF-Q 125 mass spectrometer (MA, Germany). Solvents were purchased from Tianjin Kemiou Chemical Reagent Company (Tianjin, China), MeOH and CH3CN for HPLC analysis were chromatographic grades (Merck, Darmstadt, Germany). Silica gel (200–300 mesh, Qingdao Marine Chemistry Ltd, Qingdao, China) were used for column chromatography.
Sampling, fungal isolation, morphological study
Samples of soil and plant were collected from the field of a traditional Chinese medical herb G. parviflora in Huludao city, Liaoning province, northeast of China (40°82′26.5″N, 119°78′52.0″E). The samples were conducted as described previously38,39. All plates were incubated at 26 °C and examined daily. Single colonies were picked and transferred to freshly prepared PDA plates. The single spore-origin strain was stored at 4 °C and/or conidia suspension in 20% glycerol for further study.
The colony morphology of the isolate was studied on PDA, pine-needle agar (PNA), corn meal agar (CMA), malt extract agar (MEA) and oatmeal agar (OA) plates and incubated at 26 °C37. Mycelium structure was observed under an optical microscope Olympus BX53 (Olympus, Tokyo, Japan).
DNA isolation, PCR and sequencing
The mycelia grown in PDB at 26 °C for 7 days were prepared for DNA isolation. Total genomic DNA was extracted as described previously40,41. The internal transcribed spacer (ITS) region was amplified with primers ITS4 and ITS542. The partial 28S ribosomal RNA (LSU) gene region was amplified with primers LROR and LR743,44. The thermocycling conditions for amplifications had an initial denaturing step of 94 °C for 5 min, 32 cycles of 94 °C for 60 s, 55 °C for 30 s, 72 °C for 90 s, followed by a final elongation step at 72 °C for 7 min. A G1000 Thermal Cycler (BIOER, Hangzhou, China) was used for PCR amplification. Amplicons were verified with 1% agarose electrophoresis gel, and the expected bands were excised and purified with an AxyPrepTM gel purification kit (Axygen, Hangzhou, China). These fragments were cloned into the pEASY-T5 zero cloning kit (Transgen Biotech, Beijing, China) and followed by sequencing (Sangon Biotech, Shanghai, China). The nucleotide sequences of the genes have been deposited in GenBank (Table 1).
Phylogenetic analyses
All the sequences (Table 1) were aligned by Clustal X (Larkin, Blackshields et al. 2007) and Mega 7.045,46. Datasets were analyzed using both maximum parsimony (MP) and Bayesian tree inference (BI). MP analyses were performed using PAUP* 4.0b10, a heuristic search option was chosen with random addition of sequences as 1,000 replications; gaps were treated as missing data47. BI analyses were run with MrBayes 3.2.4 using the GTR substitution model with gamma-distributed rate variation across sites and a proportion of invariable sites48. Two sets of four chains were executed until the standard deviation of split frequencies reached 0.01. Sample frequency was set at 100 and 25% of trees removed as burn-in.
Fermentation and extraction
This assay was performed according to our previous method37. Briefy, sterile water (53 ml) and rice (40 g) were mixed to an Erlenmeyer flask (250 ml), which were autoclaved at 121 °C for 30 min. The strain of SYPF 7336 (1 ml) was inoculated in each Erlenmeyer flask (250 ml × 120), which were cultivated at 28 °C for 30 days. The fermented material was extracted using ethyl acetate (12 L × 3) to give the crude extract (127 g). Then it was dissolved in 90% MeOH–H2O (1 L), and extracted by hexane (1 L × 3) to obtain the residue (62 g).
Isolation of secondary metabolites
Silica gel chromatography was used to separate the extract (41.44 g) eluting with CH2Cl2/CH3OH (v/v 80:1–3:1), and yielding four fractions (A-D). Fraction B (10.2 g) was separated by ODS eluting with MeOH-H2O (v/v 10:90 to 90:10) to give another eight fractions (B1-B7). Fraction B1 (1.6 g) was further subjected to semipreparative HPLC [Agilent 1100 instrument; YMC 5 µm C18 column (250 mm × 10 mm)], eluted with CH3CN-H2O (v/v 18:82, 3.5 ml/min) to yield compounds 1 (10 mg), 3 (12 mg) and 8 (10 mg). Subfraction B 3–4 (0.8 g) was further purified by semipreparative HPLC (CH3CN-H2O, v/v 20:80, 3.5 ml/min) to produce compounds 2 (10 mg), 4 (13 mg) and 9 (11 mg). Similarly, subfraction B7 (1.2 g) was subjected to semipreparative HPLC, eluted with CH3CN-H2O (v/v 22:78, 3.5 ml/min) to afford compounds 5 (13.6 mg), 6 (11.2 mg) and 7 (17 mg).
Compound 1: white flakes; 1H (600 MHz, DMSO-d6) and 13C NMR (150 MHz, DMSO-d6) data, see Table 2; HRESIMS m/z 325.2738 [M + Na]+ (calcd for C16H14O6 Na, 325.2733).
Compound 2: yellowish oil; − 3.0 (c 0.25, MeOH). 1H (600 MHz, DMSO-d6) and 13C NMR (150 MHz, DMSO-d6) data, see Table 2; HRESIMS m/z 307.2551 [M + Na]+ (calcd for C13H16O7Na, 307.2556).
Antimicrobial assay
Bacterial strains used in our anti-bacterial studies are from National Center for Medical Culture Collections (CMCC), and the CMCC numbers are listed as below: B. subtilis (CMCC63501), S. aureus (CMCC26003), P. aeruginosa (CMCC10104), K. pneumonia (CMCC46117), and E. coli (CMCC44102)49,50. The antimicrobial assay was performed according to the procedure of Zhu et al.50. Briefly, DMSO was used to dissolved the positive control and compounds 1–9 at an initial concentration of 10 mg/ml, respectively. Luria-Bertani (LB) media was used for the five pathogenic bacteria for 24 h at 37 °C until the absorbance OD600 = 0.2. The bacterial solution was diluted with LB medium (4%). 2 μl sample solution and 198 μl bacterial culture were mixed into 96-well plate. The 96-well plate was determined by a microplate reader (OD600) after 24 h of cultivation at 37 °C. Growth inhibition (%) was calculated as [1 − (OD600 of treatment/OD600 of control)] × 100. Every experiment was performed in triplicate to validate the biological activities.
Minimal inhibitory concentration (MIC) of compounds 1, 2 and 5 were assessed against bacteria S. aureus (CMCC26003). The MIC values of the isolated compounds against human pathogenic bacteria were determined by the modified CLSI M38-A method49,50. Briefly, compounds 1, 2 and 5 were dissolved using DMSO with the final concentrations of 100, 50, 25, 12.5, and 6.25 ug/mL, respectivly for the MIC determination. The dosage-response curve was drawed according to different concentrations of compound to S. aureus cells growth inhibition rates. The MIC values were calculated from the dosage-response curves. DMSO and Ampicillin were used as the negative control and positive control, respectively.
Molecular docking
This assay was performed according to our previous method37. Briefy, the crystal structure of protein obtained from RCSB Protein Data Bank (PDB Code 3VOB) were used for docking51. The 3D structures of the compounds 1–2 and 5 were prepared and Gasteiger-Hückel charges were added using Sybyl software (Tripos, America). The ligand, guanosine-5′-diphosphate, was subjected to energy minimization with Tripos force filed parameters51. Blind docking was carried out using Molegro Virtual Docker 4.0 (Molegro ApS, Aarhus, Denmark) program. The 3D docking grid was sufficiently large to cover the protein.
Morphological observation of bacterial fission
To identify whether there are changes in morphology of S. aureus after treated with compounds 1–2 and 5, observations under a transmission electron microscope (TEM, HT7700, Japan) were performed. S. aureus cells were grown at 37 °C on an agar plate, then diluted by LB broth to an OD600 of 0.2, and 25 μg/ml compounds 1–2 and 5 were added to the suspensions. Samples of treated cells and controls were further cultivated at 37 °C for 3 h. Then, the bacterial suspensions were dyed with 2% phosphotungstic acid (v/v = 1:1 pH 6.5) for 3–5 min, and transmission scan was performed as previously described20.
Supplementary information
Acknowledgements
This research program is financially supported by the National Natural Science Foundation of China (Nos 81703397, 31370125, 31770545), the Science and Technique Programs of Yunnan Province (2016ZF001-001, 2017IB038, 2015IC017), the National Science and Technology Major Project (2018ZX09735001-002-002) and Liaoning Outstanding Science and Technology Talent (LR2015065).
Author Contributions
T.-Y.Z., Y.-Y.W. and Y.-X.Z. designed and coordinated the research. Y.-Y.W. and H.-J.Y. performed the isolation, purification and structure elucidation of the compounds. T.-Y.Z., M.-Y.Z., J.C. and H.-J.Y. supplied the strain of Seltsamia galinsogisoli sp. nov. and finished the morphometric identification and fermentation. M.-Y.Z. and J.C. carried out the antimicrobial activity. Y.-Y.W. and M.-Y.Z. completed the molecular docking and morphological observation of bacterial fission. T.-Y.Z. and Y.-Y.W. wrote the manuscript. Blessings Dube edited the manuscript. All authors read, revised and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tian-Yuan Zhang and Ying-Ying Wu contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44810-2.
References
- 1.Katz L, Baltz RH. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016;43:155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 2.Berdy J. Bioactive Microbial Metabolites: A Personal View. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 3.Xie J, et al. New and bioactive natural products from an endophyte of Panax notoginseng. Rsc Advances. 2017;7(60):38100–38109. doi: 10.1039/C7RA07060H. [DOI] [Google Scholar]
- 4.Tian XR, et al. Review of Bioactive Secondary Metabolites From Marine Bryozoans in the Progress of New Drugs Discovery. Future. Med. Chem. 2018;10:1497–1514. doi: 10.4155/fmc-2018-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao JC, et al. Indole diterpenoids from the endophytic fungus Drechmeria sp. as natural antimicrobial agents. Phytochemistry. 2018;148:21–28. doi: 10.1016/j.phytochem.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Organization WH. Antibiotic-Resistant Gonorrhoea On the Rise, New Drugs Needed. Saudi. Med. J. 2017;38:878–879. [Google Scholar]
- 7.Dickey SW, Gyc C, Otto M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug. Discov. 2017;16:457–471. doi: 10.1038/nrd.2017.23. [DOI] [PubMed] [Google Scholar]
- 8.Lock RL, Harry EJ. Cell-Division Inhibitors: New Insights for Future Antibiotics. Nat. Rev. Drug. Discov. 2008;7:324–338. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 9.Boberek JM, et al. Filamentous Temperature-Sensitive Mutant Z (FtsZ) Protein as an Antibacterial Target. Adva. Mol. Cell. Micr. 2012;22:135–146. [Google Scholar]
- 10.Margolin W. FtsZ and the Division of Prokaryotic Cells and Organelles. Nat. rev. mol. cell Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addinall SG, Holland B. The Tubulin Ancester, FtsZ, Draughtsman, Designer and Driving Force for Bacterial Cytokinesis. J. Mol. Biol. 2002;318:219–236. doi: 10.1016/S0022-2836(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 12.Bi EF, Lutkenhaus J. FtsZ Ring Structure Associated with Division in Escherichia Coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, et al. Structural Reorganization of the Bacterial Cell-Division Protein FtsZ From Staphylococcus Aureus. Acta Crystallographica. 2012;68:1175–1188. doi: 10.1107/S0907444912022640. [DOI] [PubMed] [Google Scholar]
- 14.Haydon DJ, et al. An Inhibitor of FtsZ with Potent and Selective Anti-Staphylococcal Activity. Science. 2008;321:1673–1675. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- 15.Singh P, Jindal B, Surolia A, Panda D. A Rhodanine Derivative CCR-11 Inhibits Bacterial Proliferation by Inhibiting the Assembly and GTPase Activity of FtsZ. Biochemistry. 2012;51:5434–5442. doi: 10.1021/bi201813u. [DOI] [PubMed] [Google Scholar]
- 16.Beuria TK, Santra MK, Panda D. Sanguinarine Blocks Cytokinesis in Bacteria by Inhibiting FtsZ Assembly and Bundling. Biochemistry. 2005;44:16584–16593. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 17.Domadia PN, et al. Berberine Targets Assembly of Escherichia Coli Cell Division Protein FtsZ. Biochemistry. 2008;47:3225–3234. doi: 10.1021/bi7018546. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal R, et al. Totarol Inhibits Bacterial Cytokinesis by Perturbing the Assembly Dynamics of FtsZ†. Biochemistry. 2007;46:4211–4220. doi: 10.1021/bi602573e. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues KF, Hesse M, Werner C. Antimicrobial Activities of Secondary Metabolites Produced by Endophytic Fungi From Spondias Mombin. J. Basic. Microbiol. 2015;40:261–267. doi: 10.1002/1521-4028(200008)40:4<261::AID-JOBM261>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Ballu S, Itteboina R, Sivan SK, Manga V. Structural Insights of Staphylococcus Aureus FtsZ Inhibitors through Molecular Docking, 3D-QSAR and Molecular Dynamics Simulations. J. Rece. Sign. Trans. Res. 2018;38:61. doi: 10.1080/10799893.2018.1426607. [DOI] [PubMed] [Google Scholar]
- 21.Lian ZM, Sun J, Zhu HL. Design, Synthesis and Antibacterial Activity of Isatin Derivatives as FtsZ Inhibitors. J Mol Struct. 2016;1117:8–16. doi: 10.1016/j.molstruc.2016.03.036. [DOI] [Google Scholar]
- 22.Ma S, Ma S. The Development of FtsZ Inhibitors as Potential Antibacterial Agents. Chem. Med. Chem. 2012;7:1161–1172. doi: 10.1002/cmdc.201200156. [DOI] [PubMed] [Google Scholar]
- 23.Jaklitsch WM, et al. A Preliminary Account of theCucurbitariaceae. Stud. Mycol. 2018;90:71–118. doi: 10.1016/j.simyco.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aly AH, et al. Cytotoxic Metabolites From the Fungal Endophyte Alternaria Sp. And their Subsequent Detection in its Host Plant Polygonum Senegalense. Plant Med. 2007;71:972–980. doi: 10.1021/np070447m. [DOI] [PubMed] [Google Scholar]
- 25.Ball-Jones NR, Fahnhorst GW, Hoye TR. Poly(Isoprenecarboxylates) From Glucose Via Anhydromevalonolactone. ACS. Macro. Lett. 2016;5:1128–1131. doi: 10.1021/acsmacrolett.6b00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Deciphering the Chemical Origin of the Semen-Like Floral Scents in Three Angiosperm Plants. Phytochemistry. 2018;145:137–145. doi: 10.1016/j.phytochem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Shigemori H, Tenma M, Kengo Shimazaki A, Kobayashi J. Three New Metabolites From the Marine Yeast Aureobasidium Pullulans. J. Nat. Prod. 1998;61:696–698. doi: 10.1021/np980011u. [DOI] [PubMed] [Google Scholar]
- 28.And HS, Canning AM. Novel Radicinol Derivatives From Long-Term Cultures of Alternaria Chrysanthemi. J. Nat. Prod. 1999;62:1568–1569. doi: 10.1021/np990154w. [DOI] [PubMed] [Google Scholar]
- 29.Michele Solfrizzo, et al. Radicinols and Radicinin Phytotoxins Produced by Alternaria Radicina On Carrots. J. Agri. Food Chem. 2004;52:3655–3660. doi: 10.1021/jf035254t. [DOI] [PubMed] [Google Scholar]
- 30.Hosoe T, Gloer JB, Raja H, Shearer CA. Radicinol Analogs From the Freshwater Aquatic Fungus Xylomyces Chlamydosporus. Jsm. Mycotoxins. 2010;60:1–6. doi: 10.2520/myco.60.1. [DOI] [Google Scholar]
- 31.Harris JP, Mantle PG. Biosynthesis of Diaporthin and Orthosporin by Aspergillus Ochraceus. Phytochemistry. 2001;57:165–169. doi: 10.1016/S0031-9422(01)00004-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Liu D, Proksch P, Yu S, Lin W. Isocoumarin Derivatives From the Sponge-Associated Fungus Peyronellaea Glomerata with Antioxidant Activities. Chem. Biodivers. 2016;13:1186–1193. doi: 10.1002/cbdv.201600012. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JW, et al. Studies On the Chemical Constituents of Toricellia Angulata Var. Intermedia. Zhong Yao Cai. 2010;33:1725–1727. [PubMed] [Google Scholar]
- 34.Trisuwan K, et al. Modiolide and pyrone derivatives from the sea fan-derived fungus Curvularia sp. PSU-F22. Archives of Pharmacal Research. 2011;34:709–714. doi: 10.1007/s12272-011-0502-8. [DOI] [PubMed] [Google Scholar]
- 35.Fang ZF, et al. A New Isocoumarin From Metabolites of the Endophytic Fungus Alternaria tenuissima (Nees & T.Nees:Fr.) Wiltshire. Chin. Chem. Lett. 2012;23:317–320. doi: 10.1016/j.cclet.2011.11.021. [DOI] [Google Scholar]
- 36.Lock RL, Harry EJ. Cell division inhibitors: new insights for future antibiotics. Nat. Rev. Drug Discov. 2008;7:324–338. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 37.Wu YY, et al. An endophytic Fungi of, Ginkgo biloba, L. produces antimicrobial metabolites as potential inhibitors of FtsZ of, Staphylococcus aureus. Fitoterapia. 2018;128:265–271. doi: 10.1016/j.fitote.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Zhang TY, et al. Verruconis panacis sp. nov., an endophyte isolated from Panax notoginseng. Inte. J. of Syst. Evolut. Microbio. 2018;8:2499–2503. doi: 10.1099/ijsem.0.002862. [DOI] [PubMed] [Google Scholar]
- 39.Zhang T-Y, et al. Absidia panacisoli sp. nov., isolated from rhizosphere of Panax notoginseng. Inte. J. of Syst. Evolut. Microbio. 2018;5:2468–2472. doi: 10.1099/ijsem.0.002857. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, et al. Pseudochaetosphaeronema Ginkgonis Sp. Nov., An Endophyte Isolated From Ginkgo Biloba. Inte. J. of Syst. Evolut. Microbio. 2016;66:4377–4381. doi: 10.1099/ijsem.0.001359. [DOI] [PubMed] [Google Scholar]
- 41.Yu Y, et al. Drechmeria panacis sp. nov., an endophyte isolated from Panax notoginseng. Inte. J. of Syst. Evolut. Microbio. 2018;10:3255–3259. doi: 10.1099/ijsem.0.002971. [DOI] [PubMed] [Google Scholar]
- 42.White TJ, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. 1990;18:315–322. [Google Scholar]
- 43.Rehner SA, Samuels GJ. Molecular Systematics of the Hypocreales: A Teleomorph Gene Phylogeny and the Status of their Anamorphs. Cana. J. Botany. 1995;73:816–823. doi: 10.1139/b95-327. [DOI] [Google Scholar]
- 44.Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J.Bacteriol. 1990;8:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin M, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mole. Bio. Evol. 2016;33:1870. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swofford DL. Phylogenetic Analysis Using Parsimony. Mac Version. 2002;18:233–234. [Google Scholar]
- 48.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biolo. 2012;3:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Espinel-Ingroff A, et al. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 2005;43:5243–5246. doi: 10.1128/JCM.43.10.5243-5246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu H, et al. α-Pyrones, secondary metabolites from fungus Cephalotrichum microsporum and their bioactivities. Bioorganic Chemistry. 2018;83:129–134. doi: 10.1016/j.bioorg.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 51.Feng Q, et al. Four new hybrid polyketide-terpenoid metabolites from the Penicillium sp. SYPF7381 in the rhizosphere soil of Pulsatilla chinensis. Fitoterapia. 2018;125:249–257. doi: 10.1016/j.fitote.2018.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.