Abstract
Background: Sarcopenia is typically defined as the loss of muscle mass, strength and low physical performance with aging. Ultrasound is a safe and easy method for evaluating muscle mass and quality by muscle thickness (MT) and pennation angle (PA), respectively. Although the positive correlations between MT and muscle mass and handgrip strength were observed, the relationship between MT, PA and physical performance remains unclear.
Purpose: This study aimed to investigate the correlation of aforementioned ultrasound parameters with muscle mass, muscle strength and physical performance and explore the utility of ultrasound in predicting sarcopenia.
Patients and methods: A total of 265 elderly Chinese community dwellers were included. MT of both forearm and lower leg as well as PA of gastrocnemius was assessed by ultrasound. Muscle mass was assessed by dual-energy X-ray absorptiometry. Muscle strength was measured by a Jamar hand dynamometer. Physical performance was assessed by the Short Physical Performance Battery (SPPB).
Results: Anterior radial MT in men and regional MTs except posterior fibula in women were negatively correlated with the age. No significant correlation was observed between PA and the age in both genders. Posterior tibial MT and posterior fibula MT were positively correlated with the relative appendicular skeletal muscle mass in men and women, respectively. Anterior ulnar MT was positively correlated with grip strength in both genders. Moreover, gastrocnemius medialis PA showed a positive association with gait speed and SPPB in women but not in men.
Conclusion: A combination of posterior fibula MT, anterior ulnar MT and gastrocnemius medialis PA measured by muscle ultrasound is helpful for the assessment of sarcopenia in Chinese elderly women. In addition, a combination of posterior tibial MT and anterior ulnar MT measured by muscle ultrasound is helpful for the assessment of sarcopenia in Chinese elderly men.
Keywords: B-mode ultrasound, muscle thickness, pennation angle, muscle mass, muscle strength, physical performance
Introduction
Sarcopenia is typically defined as the loss of muscle mass, strength and low physical performance with advancing age.1 The prevalence of sarcopenia is reported to be 5–13% in adults aged 60–70 years and up to 50% in those older than 80 years.2 The main consequences of sarcopenia include numerous physiological and psychosocial impacts, such as physical inactivity, loss of independent living and related depression/social isolation, frailty, falls, increased risks of chronic diseases such as diabetes, osteoarthritis, osteoporosis and coronary artery disease and increased mortality.3 Therefore, early recognition and intervention are important to mitigate deleterious outcomes in patients with sarcopenia.
Muscle mass depletion is an important characteristic of sarcopenia. Currently, muscle mass can be assessed by dual-energy X-ray absorptiometry (DXA), bioelectrical impedance (BIA), magnetic resonance imaging (MRI) and computed tomography (CT). Compared with the relatively high cost of CT and MRI, the radiation exposure of CT and DXA and the overestimate of muscle mass by BIA, B-mode muscle ultrasound shows several advantages such as simplicity, low cost, real-time, easy transport, and radiation-free. Therefore B-mode muscle ultrasound may be a potential method for assessing muscle mass and quality in every clinical setting.4 As we know, the age-related decline in muscle mass does not proceed in all anatomic regions at the same pace.5 Therefore, site-specific assessment of loss of muscle mass may be required for early and accurate detection, and the “regional” or “site-specific sarcopenia” has been detected by muscle ultrasound.6,7
However, defining sarcopenia only in terms of muscle mass has limited clinical value, while age-related decline of muscle strength and physical performance, which can reflect muscle quality and function, could be extremely important in evaluating sarcopenia. Compared with muscle mass, muscle strength declines much more rapidly with aging and may contribute to a high prevalence of falls, disability and mortality.8–10 Skeletal muscle strength is highly dependent on muscle mass, composition and architecture. Handgrip strength is the preferred method to assess muscle strength. In addition, the capacity to live independently is crucial to good quality of life, and low physical performance is the intimidation of an independent lifestyle at older age. Physical performance assessments such as the Short Physical Performance Battery (SPPB) can provide some perspective on the functional consequences of poor muscle quality. Low SPPB score has been shown to correlate with disability, decreased mobility and other clinical parameters including mortality.11 Nowadays, ultrasound is widely used to assess muscle mass and architecture which is related to muscle strength by muscle thickness (MT) and pennation angle (PA), respectively. Numerous studies have found a positive correlation between MT and handgrip strength, whereas the relationship between MT and physical performance remains uncertain.12–14 Up to now, only a few studies investigated the relationship between PA and muscle strength and no significant correlation was observed,15,16 while the association between PA and physical performance remains unclear.
The aim of this study was to sonographically assess the regional MTs of both forearm and lower leg as well as PA of gastrocnemius in elderly Chinese community dwellers and to investigate the association between the ultrasound parameters with muscle mass, muscle strength and physical performance in order to explore the utility of ultrasound measurements in predicting sarcopenia.
Material and methods
Study participants
The study subjects were selected from 348 elder community dwellers who participated in the annual health screening program at Huaqiao Road Community Health Service Center in Jiangsu, China, from May to October 2017. The inclusion criteria were 60 years or older. After extensive description of the study, 316 people participated in our study. 21 patients were excluded who had diseases that may affect muscle metabolism such as inflammatory myopathy, Parkinson’s disease, stroke, myocardial infarction, significant liver disease, creatinine clearance of <30 mL/min or cancer. Mental and nutrition status of the study subjects was assessed by mini-mental status examination (MMSE) and mini-nutritional assessment (MNA), respectively, and 9 subjects with cognitive impairment or malnutrition were excluded. 2 subjects who were unable to walk independently or had severe painful hand or wrist problems were also excluded. Among the remaining 284 subjects, 19 subjects who failed to go through the assessment of sarcopenia were excluded. Finally, 97 men and 168 women were included in this study.
Muscle mass, grip strength and physical performance assessment
The DXA scanner (Hologic Inc., Bedford, MA, USA) was used to measure total lean mass (LM) and regional LM including android, gynoid, trunk, upper limb and lower limb. Limb lean tissue mass (appendicular) was used as a good proxy for muscle mass. Appendicular lean mass (aLM) was calculated as the sum of LM in the arms and legs. The relative appendicular skeletal muscle mass (RASM) was calculated as aLM divided by height squared (aLM/height2, kg/m2). The instruments exhibited stable long-term performance [coefficient of variation (CV) <0.5%] and satisfactory in vivo precision.
The grip strength of the dominant hand was measured three times by a hand dynamometer (Jamar®, Los Angeles, CA, USA). Three attempts with a 1-min interval between them were recorded, and the maximum value (in kg) was considered for further analysis.
Physical performance was assessed by SPPB including measurements of walking speed, standing balance and ability to rise from a chair as previously described.17 The assessments were performed by a single examiner in a silent room dedicated for clinical evaluations to avoid distractions during the procedures.
Ultrasound measurements
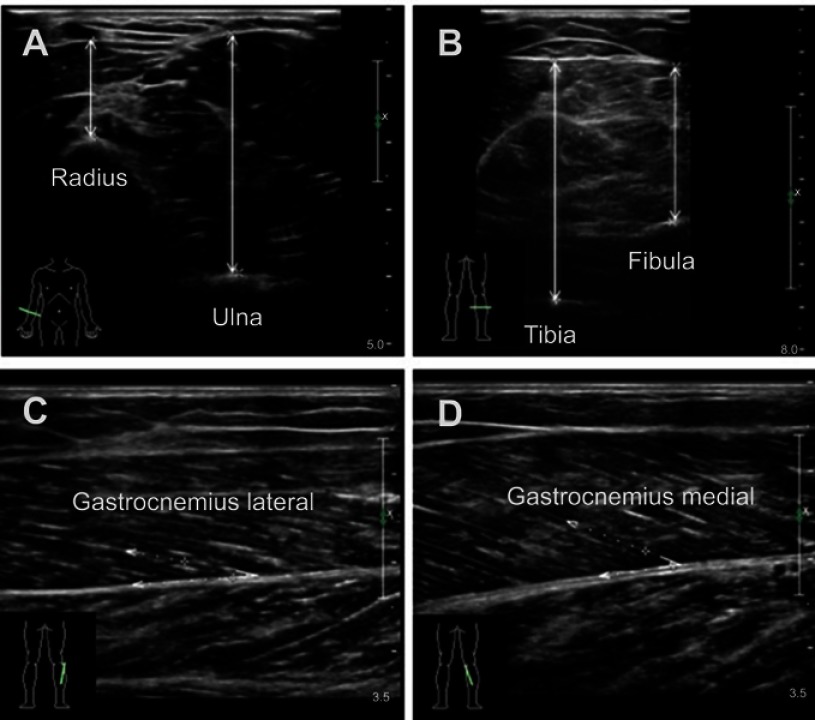
A B-mode ultrasound (Philips iU Elite, Bothell, WA, USA) with a linear transducer (5–12 MHz) was used to evaluate MT and PA. Cross-sectional images of the forearm MT were obtained on the anterior of the right forearm (at 30% proximal between the styloid process and the head of the radius) when the participants were in the supine position with their elbow extended, relaxed and their forearm supinated. Cross-sectional images of the lower limb MT were obtained on the posterior of the right shank (at 30% proximal between the lateral malleolus of the fibula and the lateral condyle of the tibia) when the participants were in the prone position with their legs extended and their feet hanging on the side of the examination table. MT was defined as the distance between subcutaneous adipose tissue–muscle interface and muscle–bone interface of radius, ulna, tibia and fibula (Figure 1A and B). PA (the angle of insertion of muscle fascicles into the deep aponeurosis) was measured on the longitudinal view by rotating the probe parallel to either the lateral or medial head of sural muscle on the position where we measured MT of the lower limb (Figure 1C and D). The probe was coated with water-soluble transmission gel to provide acoustic contact and reduce pressure. The pressure was reduced while scanning by suspending the linear probe on the skin surface and keeping perpendicular to the bed to achieve a clear image. To obtain precise measurements of muscle parameters, all measurements were performed by the same sonographer with 5 years of experience. All data were measured three times, and the average value was used for further analysis.
Figure 1.
Typical ultrasound images. Transversal ultrasound images of anterior radial muscle and anterior ulnar muscle (A), posterior tibial muscle and posterior fibula muscle (B). Maximal muscle thickness was measured separately between the upper fascia to radius and ulna leading edge or to tibia and fibula trailing edge at the widest distance. Pennation angles were measured between muscle fiber and the deep fascia of the muscle (C and D).
Statistical analysis
Descriptive data were shown as the mean ± SD. Differences in basic characteristics by gender were compared using Student’s t-test for continuous variables. The association between the age and other basic characteristics was determined using Pearson’s correlation analysis. The association between MT, PA and regional LM, grip strength, gait speed or SPPB was determined using Pearson’s correlation analysis after adjustment for the age. Multiple linear regression models were used for RASM, grip strength, gait speed and SPPB analyses. All statistical analyses were performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA), and p<0.05 was considered a significant difference.
Results
Comparison of general characteristics between men and women
The study population consisted of 97 men (mean age: 69.33±6.77 years) and 168 women (mean age: 67.79±6.17 years). The general characteristics, MT and PA assessed by ultrasound, muscle mass, grip strength, gait speed and SPPB of the study population, are shown in Table 1. There were no significant differences in the age, body mass index (BMI), PA, gait speed and SPPB between men and women. As expected, men had greater MT, muscle mass and grip strength than women. Due to the differences between men and women, we performed further analyses separately based on gender.
Table 1.
Comparison of anthropometrics, MT, PA, muscle mass, grip strength, gait speed and SPPB of the subjects by gender
| Characteristic | Male (n=97) | Female (n=168) | p |
|---|---|---|---|
| Age (years) | 69.33±6.77 | 67.79±6.17 | 0.063 |
| Height (cm) | 168.38±5.85 | 155.58±5.97 | <0.001 |
| Weight (kg) | 69.06±9.25 | 58.39±8.52 | <0.001 |
| BMI (kg/m2) | 24.34±2.82 | 24.09±3.04 | 0.515 |
| Waist circumference (cm) | 90.22±8.82 | 84.70±8.97 | <0.001 |
| Hip circumference (cm) | 96.55±6.25 | 95.18±6.02 | 0.08 |
| Waist-to-hip ratio | 0.93±0.06 | 0.89±0.072 | <0.001 |
| MT (muscle thickness) (mm) | |||
| Anterior radial | 19.44±2.77 | 15.68±2.24 | <0.001 |
| Anterior ulnar | 39.24±3.1 | 33.18±3.17 | <0.001 |
| Posterior tibial | 57.96±6.2 | 52.96±5.83 | <0.001 |
| Posterior fibula | 35.14±7.26 | 31.51±5.91 | <0.001 |
| PA (pennation angle) (°) | |||
| Gastrocnemius lateralis | 18.95±3.99 | 18.91±4.47 | 0.946 |
| Gastrocnemius medialis | 24.12±4.65 | 24.83±5.58 | 0.292 |
| Muscle mass | |||
| LM (kg) | 46.5±5.7 | 33.38±4.34 | <0.001 |
| Android LM (kg) | 3.38±0.5 | 2.37±0.39 | <0.001 |
| Gynoid LM (kg) | 6.99±0.84 | 5.0±0.73 | <0.001 |
| Trunk LM (kg) | 22.36±3.09 | 16.6±2.26 | <0.001 |
| Upper limb LM (kg) | 5.53±0.89 | 3.35±0.57 | <0.001 |
| Lower limb LM (kg) | 14.62±1.99 | 10.11±1.5 | <0.001 |
| aLM (kg) | 20.15±2.75 | 13.45±1.99 | <0.001 |
| RASM (kg/m2) | 7.09±0.8 | 5.55±0.73 | <0.001 |
| Grip strength (kg) | 38.79±7.93 | 24.59±4.35 | <0.001 |
| Gait speed (m/s) | 1.28±0.27 | 1.27±0.26 | 0.575 |
| SPPB | 11.43±1.15 | 11.31±1.59 | 0.525 |
Notes: Variables are expressed as mean ± SD. p-values were determined using Student’s t test for continuous variables.
Abbreviations: MT, muscle thickness; PA, pennation angle; BMI, body mass index; LM, total lean mass; aLM, appendicular lean mass; RASM, relative appendicular skeletal muscle mass; SPPB, Short Physical Performance Battery.
Effects of the age on general characteristics, MT, PA, muscle mass, grip strength and physical performance
To investigate the effects of the age on BMI, waist-to-hip ratio, MT, PA, muscle mass and function, we calculated Pearson correlation coefficients. As shown in Table 2, anterior radial MT, total LM, trunk LM, upper limb LM and aLM were negatively correlated with the age in men. Anterior radial MT, anterior ulnar MT, posterior tibial MT, total and all evaluated regional MTs were negatively correlated with the age in women. No significant correlation was observed between PA and the age both in men and in women. Notably, muscle function, either grip strength, gait speed and SPPB were all negatively associated with the age in both genders.
Table 2.
Effects of age on general characteristics, MT, PA, muscle mass, grip strength, gait speed and SPPB
| Characteristic | Correlation with age | |
|---|---|---|
| Male | Female | |
| BMI | −0.043 | −0.109 |
| Waist-to-hip ratio | 0.078 | 0.220** |
| Anterior radial muscle thickness | −0.236* | −0.211** |
| Anterior ulnar muscle thickness | −0.148 | −0.234** |
| Posterior tibial muscle thickness | −0.103 | −0.209** |
| Posterior fibula muscle thickness | −0.038 | −0.097 |
| PA (gastrocnemius lateralis) | 0.112 | −0.017 |
| PA (gastrocnemius medialis) | −0.069 | −0.055 |
| LM | −0.249* | −0.268*** |
| Android LM | −0.074 | −0.243** |
| Gynoid LM | −0.168 | −0.295*** |
| Trunk LM | −0.243* | −0.254*** |
| Upper limb LM | −0.305** | −0.205** |
| Lower limb LM | −0.193 | −0.233** |
| aLM | −0.239* | −0.234** |
| RASM | −0.136 | −0.059 |
| Grip strength | −0.363*** | −0.320*** |
| Gait speed | −0.239* | −0.357*** |
| SPPB | −0.488*** | −0.189* |
Notes: *p<0.05; **p<0.01; ***p<0.001.
Abbreviations: BMI, body mass index; PA, pennation angle; LM, total lean mass; aLM, appendicular lean mass; RASM, relative appendicular skeletal muscle mass; SPPB, short physical performance battery.
Correlation between MT, PA and regional LMs, RASM, grip strength or physical performance
To investigate the correlation between regional MT, PA and regional LMs, RASM, grip strength, gait speed or SPPB, we performed partial correlation analysis adjusted for the age. As shown in Table 3, all evaluated regional MTs were positively associated with both absolute LM including upper limb LM, lower limb LM, aLM and relative muscle index RASM in both genders. For the association between MT and muscle function, forearm MT was positively associated with grip strength in men, while both forearm and lower leg MTs were positively associated with grip strength in women. In addition, anterior ulnar MT was positively associated with gait speed only in women. Gastrocnemius medialis PA was positively associated with gait speed and SPPB in women, and no significant correlation was observed between PA and muscle mass or function in men.
Table 3.
Partial correlation between MT, PA and regional LMs, RASM, grip strength, gait speed or SPPB
| Upper limb LM | Lower limb LM | aLM | RASM | Grip strength | Gait speed | SPPB | |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| Muscle thickness | |||||||
| Anterior radial | 0.211* | 0.247* | 0.248* | 0.331** | 0.259* | 0.058 | 0.016 |
| Anterior ulnar | 0.522*** | 0.425*** | 0.477*** | 0.435*** | 0.494*** | −0.033 | −0.017 |
| Posterior tibial | 0.390*** | 0.566*** | 0.539*** | 0.570*** | 0.071 | −0.022 | −0.029 |
| Posterior fibula | 0.285** | 0.391*** | 0.377*** | 0.483*** | 0.013 | 0.034 | −0.018 |
| PA | |||||||
| Gastrocnemius lateralis | −0.013 | −0.110 | −0.084 | −0.084 | −0.025 | −0.067 | −0.011 |
| Gastrocnemius medialis | −0.083 | −0.120 | −0.115 | −0.042 | −0.001 | −0.087 | −0.072 |
| Female | |||||||
| Muscle thickness | |||||||
| Anterior radial | 0.398*** | 0.267*** | 0.316*** | 0.283*** | 0.165* | 0.042 | 0.027 |
| Anterior ulnar | 0.443*** | 0.408*** | 0.435*** | 0.325** | 0.323*** | 0.180* | 0.002 |
| Posterior tibial | 0.483*** | 0.524*** | 0.534*** | 0.468*** | 0.236** | 0.082 | 0.026 |
| Posterior fibula | 0.452*** | 0.506*** | 0.511*** | 0.498*** | 0.246** | 0.126 | −0.031 |
| PA | |||||||
| Gastrocnemius lateralis | −0.044 | 0.010 | −0.005 | −0.027 | 0.063 | 0.089 | 0.118 |
| Gastrocnemius medialis | −0.031 | 0.010 | −0.001 | −0.029 | 0.089 | 0.198* | 0.170* |
Notes: R correlation coefficient, *p<0.05; **p<0.01; ***p<0.001, Adjustment for age.
Abbreviations: LM, total lean mass; aLM, appendicular lean mass; RASM, relative appendicular skeletal muscle mass; SPPB, short physical performance battery; PA, pennation angle.
Multiple linear regression analysis of MT and PA on RASM, grip strength or physical performance
Given the significant relationship between the age, MT, PA and RASM, grip strength, gait speed or SPPB, we further performed multiple linear regression analysis. In the model adjusted for the age and BMI (Table 4), posterior tibial MT was positively associated with RASM in men (β=0.211, p=0.03), and posterior fibula MT was positively associated with RASM in women (β=0.272, p=0.001). Anterior ulnar MT was positively associated with grip strength in both genders. The positive correlation between gastrocnemius medialis PA and gait speed and SPPB still persisted in women (β0.187, p=0.015, for gait speed; β=0.179, p=0.031, for SPPB).
Table 4.
Multiple linear regression analysis of MT and PA on RASM, grip strength, gait speed or SPPB
| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| RASM | ||||||
| Anterior radial muscle thickness | -0.042 | -0.498 | 0.620 | 0.042 | 0.575 | 0.566 |
| Anterior ulnar muscle thickness | 0.164 | 1.952 | 0.054 | 0.066 | 0.856 | 0.394 |
| Posterior tibial muscle thickness | 0.211 | 2.205 | 0.030 | 0.083 | 0.903 | 0.368 |
| Posterior fibula muscle thickness | 0.018 | 0.196 | 0.845 | 0.272 | 3.349 | 0.001 |
| PA (Gastrocnemius lateralis) | -0.121 | -1.553 | 0.124 | 0.005 | 0.076 | 0.939 |
| PA (Gastrocnemius medialis) | -0.145 | -1.790 | 0.077 | -0.107 | -1.624 | 0.106 |
| Grip strength | ||||||
| Anterior radial muscle thickness | 0.051 | 0.471 | 0.639 | -0.035 | -0.405 | 0.686 |
| Anterior ulnar muscle thickness | 0.537 | 4.965 | 0.001 | 0.283 | 3.167 | 0.002 |
| Posterior tibial muscle thickness | -0.025 | -0.204 | 0.838 | 0.015 | 0.139 | 0.890 |
| Posterior fibula muscle thickness | -0.030 | -0.251 | 0.803 | 0.148 | 1.574 | 0.118 |
| PA (Gastrocnemius lateralis) | 0.038 | 0.381 | 0.704 | 0.069 | 0.878 | 0.381 |
| PA (Gastrocnemius medialis) | -0.124 | -1.187 | 0.239 | 0.046 | 0.597 | 0.552 |
| Gait speed | ||||||
| Anterior radial muscle thickness | 0.099 | 0.760 | 0.449 | -0.081 | -0.959 | 0.339 |
| Anterior ulnar muscle thickness | -0.095 | -0.723 | 0.472 | 0.213 | 2.391 | 0.018 |
| Posterior tibial muscle thickness | -0.139 | -0.935 | 0.352 | 0.006 | 0.059 | 0.953 |
| Posterior fibula muscle thickness | 0.060 | 0.413 | 0.681 | 0.140 | 1.491 | 0.138 |
| PA (Gastrocnemius lateralis) | -0.045 | -0.374 | 0.709 | 0.045 | 0.572 | 0.568 |
| PA (Gastrocnemius medialis) | -0.099 | -0.785 | 0.435 | 0.187 | 2.449 | 0.015 |
| SPPB | ||||||
| Anterior radial muscle thickness | 0.059 | 0.492 | 0.624 | 0.020 | 0.215 | 0.830 |
| Anterior ulnar muscle thickness | 0.012 | 0.097 | 0.923 | 0.004 | 0.043 | 0.965 |
| Posterior tibial muscle thickness | -0.028 | -0.209 | 0.835 | 0.133 | 1.169 | 0.244 |
| Posterior fibula muscle thickness | 0.034 | 0.259 | 0.796 | -0.058 | -0.570 | 0.570 |
| PA (Gastrocnemius lateralis) | 0.044 | 0.396 | 0.693 | 0.034 | 0.401 | 0.689 |
| PA (Gastrocnemius medialis) | -0.081 | -0.701 | 0.485 | 0.179 | 2.171 | 0.031 |
Notes: Adjustment for age and BMI, β standardized coefficient.
Abbreviations: RASM, relative appendicular skeletal muscle mass; PA, pennation angle; SPPB, short physical performance battery.
Discussion
In this study, we made the following findings: only site-specific MT but not PA was negatively correlated with the age; posterior tibial MT and posterior fibula MT were positively associated with RASM in men and women, respectively; anterior ulnar MT was positively associated with grip strength in both genders; and gastrocnemius medialis PA was positively associated with both gait speed and SPPB only in women.
Loss of skeletal muscle mass has been regarded as the key element for diagnosing sarcopenia; thus, a precise quantitative estimate of muscle mass is fundamental for diagnosing sarcopenia in older individuals.1 In the present study, the sites for measuring MT were forearm (anterior radius, anterior ulna) and lower leg (posterior tibia, posterior fibula), which represented the upper and lower limbs, respectively. These sites were easy to measure, were closely related to the limb functio, and have been examined in previous studies.14,18,19 Our data demonstrated that only anterior radial MT was negatively correlated with the age in both men and women, consistent with a previous study.20 Previous studies have highlighted the importance of muscle quality rather than muscle mass alone in evaluating muscle performance and sarcopenia.21–23 Muscle quality refers to the muscle’s functional capacity, including force, contraction ability, metabolism and other functions. PA is one of the most important architectural parameters which can determine the maximum force of contraction.24 Previous studies demonstrated that the elderly have reduced gastrocnemius medialis PA compared with young individuals.25,26 Human gastrocnemius medialis PA increased monotonically since birth, reached a stable value after the adolescent growth spurt and did not change until 70 years old.27 The age of our study population was between 60 and 86 years, and age-associated reduced PA was not observed in our study.
Multiple studies demonstrated that ultrasonic measurement of regional MT has a high concordance with DXA-predicted estimates of muscle mass,18,28,29 CT-derived muscle volume30,31 and MRI-based muscle mass,32–34 suggesting that muscle ultrasound can provide qualitative information on skeletal muscle. Consistently, our partial correlation analysis found that regional MTs of forearm and posterior leg were significantly correlated to DXA-measured absolute LM and relative muscle index RASM in both genders. Even in multiple linear regression model, MT of the posterior leg, mainly containing gastrocnemius and soleus, was positively associated with RASM. Compared to previous studies reporting that forearm MT was related with aLM or total LM,14,27 our results indicated that ultrasound MT measurement of the posterior leg is more useful to predict muscle mass in the elderly. As we know, the age-related loss of MT has been shown to be greater in the abdomen and thigh anterior than in other body parts.18 However, because MTs of these sites were not evaluated in the present study, we cannot make a conclusion regarding the site at which MT value may be a representative predictor of sarcopenia in Chinese elderly.
The European Working Group on Sarcopenia in Older People (EWGSOP) recommended that handgrip strength is the most useful criteria of age-related change in muscle strength in older individuals and is related to lower-extremity muscle strength.1 Numerous studies have revealed a significant relationship between forearm MT and handgrip strength in both young and elderly volunteers.14,35 Coincidentally, our results confirmed that anterior ulnar MT was significantly correlated with handgrip strength in both genders; thus it may be a more useful parameter for predicting handgrip strength in Chinese elderly. Why the correlation was only observed for anterior ulnar but not anterior radial MT? The reason is unknown, but may be related to the composition of anterior ulnar muscles. Anterior ulnar MT included mainly two major flexor muscles (flexor digitorum profundus and flexor digitorum superficialis), which produce flexion movement for the middle phalanges of the fingers, and may be an important contributor for measuring handgrip strength by a dynamometer.14 Additionally, we found no significant correlation between PA and handgrip strength, consistent with previous studies.15,16
Although the parameters of physical performance, such as gait speed and SPPB, have been emphasized in the most recent definitions of sarcopenia, the role of skeletal muscle ultrasound for evaluating physical performance in elderly individuals remains speculative. Age-related loss of muscle mass has been considered as a factor affecting physical performance,36 whereas the relationship between muscle mass and physical performance remains controversial.12–14,37 Different age, muscle status and muscle assessment site may lead to an inconsistent conclusion. In our multiple linear regression model, only anterior ulnar MT was positively correlated to gait speed in women, while no relationship was observed between other regional MTs and gait speed and SPPB. The muscle strength is a determinant of physical performance in old individuals.38–40 However, to our knowledge, the relationship between PA and physical performance remains unclear. The most significant finding of our study is that gastrocnemius medialis PA was positively associated with physical performance including both gait speed and SPPB in women but not in men. The reason for this apparent sex difference is still unclear. One reason may be that women have a larger proportion of muscle mass in their lower bodies.41 In our study, sample size of male participants was smaller than that of female participants, indicating another potential reason for gender-specific differences in correlation.
This is perhaps the first study that investigated the correlation of MT and PA measured by ultrasound with muscle mass, muscle strength and physical performance. Our study demonstrated that regional MT was positively associated with RASM and handgrip strength, and gastrocnemius medialis PA was positively associated with physical performance. According to the EWGSOP, there are two criteria for diagnosing sarcopenia: low muscle mass plus either low muscle strength or low physical performance.1 Thus, our results support that for the initial diagnosis of sarcopenia, a combination of regional MT (posterior tibial MT in men, posterior fibula MT in women and anterior ulnar MT in both genders) and gastrocnemius medialis PA would be useful parameters in Chinese elderly, especially in women. Nevertheless, this study has other limitations including small sample size, single center and lack of comprehensive ultrasound information such as muscle physiological cross-sectional area, fascicle length and muscle echogenicity.
Ultrasound has been used as a safer and easily applicable method for evaluating muscle mass and quality. According to our results, we could recommend the use of ultrasound to evaluate regional MT and gastrocnemius medialis PA in the assessment of sarcopenia in Chinese elderly. However, due to the limitations such as relatively small sample size and single center, larger samples from different regions are needed to select appropriate diagnostic cutoff values for sarcopenic diagnosis. In addition, MT and PA of other regional muscles such as the thigh compartment should be evaluated in further studies.
Conclusion
In summary, sarcopenia is a public health disease that damages the quality of life and increases the mortality in the elderly. Our results suggest that a combination of posterior fibula MT, anterior ulnar MT and gastrocnemius medialis PA measured by muscle ultrasound is a promising method for the assessment of sarcopenia in Chinese elderly women. In addition, a combination of posterior tibial MT and anterior ulnar MT measured by muscle ultrasound is a promising method for the assessment of sarcopenia in Chinese elderly men. Further studies are needed to validate our conclusion.
Acknowledgments
We would like to express our sincere gratitude to all of the doctors of the Huaqiao Road Community Health Service Center, and to the participants for their cooperation. This study was funded by the grants from the National Natural Science Foundation of China (91649122) to Guoxian Ding and the National Natural Science Foundation of China (81801383) to Yunlu Sheng. These funders had no role in the design of the study, data collection, analysis, interpretation of data and in writing the manuscript.
Abbreviation list
MT, muscle thickness; PA, pennation angle; BMI, body mass index; LM, total lean mass; aLM, appendicular lean mass; RASM, relative appendicular skeletal muscle mass; SPPB, Short Physical Performance Battery.
Ethical approval
All subjects received an extensive description of the study and participated after written informed consent. The study was performed in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Author contributions
SZ, WL and SC contributed equally to this study. GD and YS were both corresponding authors of this study, designed the experiments and revised the manuscript. SZ acquired data and wrote the manuscript. WL conducted ultrasound assessment. SC conducted handgrip strength and physical function assessments. HQ and SW conducted the DXA. AZ performed statistical analysis. BL and JC checked the data of subjects and reviewed the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, et al. Mortality as an adverse outcome of sarcopenia. J Nutr Health Aging. 2013;17:259–262. doi: 10.1007/s12603-012-0434-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ticinesi A, Meschi T, Narici MV, Lauretani F, Maggio M. Muscle ultrasound and sarcopenia in older individuals: a clinical perspective. J Am Med Dir Assoc. 2017;18:290–300. doi: 10.1016/j.jamda.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 1985;89:81–88. doi: 10.1152/jappl.2000.89.1.81 [DOI] [PubMed] [Google Scholar]
- 6.Abe T, Kawakami Y, Kondo M, Fukunaga T. Comparison of ultrasound-measured age-related, site-specific muscle loss between healthy Japanese and German men. Clin Physiol Funct Imaging. 2011;31:320–325. doi: 10.1111/j.1475-097X.2011.01021.x [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Sakamaki M, Yasuda T, et al. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J Sports Sci Med. 2011;10:145–150. [PMC free article] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 10.Roubenoff R. Origins and clinical relevance of sarcopenia. Can J Appl Physiol. 2001;26:78–89. [DOI] [PubMed] [Google Scholar]
- 11.Beaudart C, McCloskey E, Bruyere O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. doi: 10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerreiro AC, Tonelli AC, Orzechowski R, et al. Bedside ultrasound of quadriceps to predict rehospitalization and functional decline in hospitalized elders. Front Med (Lausanne). 2017;4:122. doi: 10.3389/fmed.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe T, Ogawa M, Loenneke JP, Thiebaud RS, Loftin M, Mitsukawa N. Relationship between site-specific. loss of thigh muscle and gait performance in women: the HIREGASAKI study. Arch Gerontol Geriatr. 2012;55:e21–e25. doi: 10.1016/j.archger.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 14.Abe T, Thiebaud RS, Loenneke JP, Ogawa M, Mitsukawa N. Association between forearm muscle thickness and age-related loss of skeletal muscle mass, handgrip and knee extension strength and walking performance in old men and women: a pilot study. Ultrasound Med Biol. 2014;40:2069–2075. doi: 10.1016/j.ultrasmedbio.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 15.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between. Ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr). 2013;35:2377–2388. doi: 10.1007/s11357-013-9517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuyumcu ME, Halil M, Kara O, et al. Ultrasonographic evaluation of the calf muscle mass and architecture in elderly patients with and without sarcopenia. Arch Gerontol Geriatr. 2016;65:218–224. doi: 10.1016/j.archger.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: macarthur studies of successful aging. Aging (Milano). 1994;6:410–419. [DOI] [PubMed] [Google Scholar]
- 18.Takai Y, Ohta M, Akagi R, et al. Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J Nutr Health Aging. 2014;18:579–585. doi: 10.1007/s12603-013-0419-7 [DOI] [PubMed] [Google Scholar]
- 19.Abe T, Kawakami Y, Bemben MG, Fukunaga T. Comparison of age-related, site-specific muscle loss between young and old active and inactive Japanese women. J Geriatr Phys Ther. 2011;34:168–173. doi: 10.1519/JPT.0b013e31821c9294 [DOI] [PubMed] [Google Scholar]
- 20.Paris MT, Lafleur B, Dubin JA, Mourtzakis M. Development of a bedside viable ultrasound protocol to quantify appendicular lean tissue mass. J Cachexia Sarcopenia Muscle. 2017;8:713–726. doi: 10.1002/jcsm.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 22.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are. greater in hypertrophied than in normal muscles. J Appl Physiol (1985). 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740 [DOI] [PubMed] [Google Scholar]
- 25.Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol. 2007;100:613–619. doi: 10.1007/s00421-007-0481-0 [DOI] [PubMed] [Google Scholar]
- 26.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol (1985). 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003 [DOI] [PubMed] [Google Scholar]
- 27.Binzoni T, Bianchi S, Hanquinet S, et al. Human gastrocnemius medialis pennation angle as a function of age: from newborn to the elderly. J Physiol Anthropol Appl Human Sci. 2001;20:293–298. [DOI] [PubMed] [Google Scholar]
- 28.Abe T, Patterson KM, Stover CD, et al. Site-specific thigh muscle loss as an independent phenomenon for age-related muscle loss in middle-aged and older men and women. Age (Dordr). 2014;36:9634. doi: 10.1007/s11357-014-9634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger J, Bunout D, Barrera G, et al. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch Gerontol Geriatr. 2015;61:33–38. doi: 10.1016/j.archger.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Thomaes T, Thomis M, Onkelinx S, Coudyzer W, Cornelissen V, Vanhees L. Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD-patients. BMC Med Imaging. 2012;12:7. doi: 10.1186/1471-2342-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986 [DOI] [PubMed] [Google Scholar]
- 32.Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24–31. doi: 10.1007/s00421-005-0061-0 [DOI] [PubMed] [Google Scholar]
- 33.Tandon P, Low G, Mourtzakis M, et al. A model to identify Sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480. doi: 10.1016/j.cgh.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 34.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of. human skeletal muscle size. Eur J Appl Physiol. 2004;91:116–118. doi: 10.1007/s00421-003-0961-9 [DOI] [PubMed] [Google Scholar]
- 35.Abe T, Counts BR, Barnett BE, et al. Associations between handgrip. Strength and ultrasound-measured muscle thickness of the hand and forearm in young men and women. Ultrasound Med Biol. 2015;41:2125–2130. doi: 10.1016/j.ultrasmedbio.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271–276. doi: 10.1097/MCO.0b013e328337819e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KE, Jang SN, Lim S, et al. Relationship between muscle mass and physical performance: is it the same in older adults with weak muscle strength? Age Ageing. 2012;41:799–803. doi: 10.1093/ageing/afs115 [DOI] [PubMed] [Google Scholar]
- 38.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x [DOI] [PubMed] [Google Scholar]
- 39.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. [DOI] [PubMed] [Google Scholar]
- 40.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 41.Gallagher D, Visser M, de Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229 [DOI] [PubMed] [Google Scholar]