Abstract
Objective: Due to the prevalence of stress in modern life and its impact on spatial memory, the role of inhibitory systems in brain areas such as the nucleus accumbens (NAc) in reducing stress is important. The current study aimed to examine the response of NAc shell GABAB receptors to stress and the role of intraperitoneally (i.p.) and intra-NAc injection of the GABAB receptor agonist baclofen on spatial memory impairments in stress-exposed rats.
Methods: Eighty adult male Wistar rats were randomly divided into ten groups (n=8): two were control groups for intra-NAc and i.p baclofen; two groups were subjected to stress and injected with saline (baclofen vehicle); three groups were given baclofen (1, 5, and 10 µg/rat) intra-NAc 5 mins before stress was induced; and three groups received baclofen (1, 5, and 10 mg/kg/i.p.) 30 mins before being subjected to stress. Foot-shock stress was applied for 7 consecutive days. Behavioral assays using the Barnes maze were performed 24 hrs after the last baclofen injection.
Results: Both the intra-NAc and the i.p administration of baclofen dose-dependently reduced escape latency and total distance and increased velocity in the treatment groups in the training trials. In the probe test, the rats that had received 5 mg/kg of baclofen had the highest target frequency, but there no significant differences were observed in velocity, duration, or distance to the target between the groups.
Conclusion: According to the findings, baclofen can dose-dependently improve spatial memory, and GABAB receptor in the NAc plays an important role in spatial memory.
Keywords: baclofen, spatial memory, nucleus accumbens, stress, male rat
Introduction
Stress has a broad spectrum of harmful effects on both the health and the cognition in adult animals and humans.1,2 Stress is associated with many disorders, including post-traumatic stress disorder, depression, and schizophrenia.3
A large body of research has focused on stress and its effects on behavioral disorders. These studies have investigated the role of neurotransmitters, especially gamma-aminobutyric acid (GABA), in suppressing the effects of stress.4 The release of specific neurotransmitters and neuromodulators and the interaction between areas such as the prefrontal cortex (PFC), amygdala, hippocampus, nucleus accumbens (NAc), and hypothalamus are involved in response to a stressful situation.5
GABA is a fundamental neurotransmitter that is broadly distributed in the central nervous system.6,7 GABAB receptors are G-protein-coupled receptors that inhibit adenylate cyclase activity and mediate the slow and prolonged component of synaptic inhibition.8 Baclofen (β – chlorophenyl – GABA, Lioresal), the only clinically available GABAB receptor agonist used in the treatment of spasticity and skeletal muscle rigidity, has shown therapeutic effects in a wide range of other indications, including drug dependence, learning and memory, analgesic, anxiety disorders, and depression.9 The anxiolytic effects of baclofen have been reported in a few preclinical investigations.10 The intracerebroventricular administration of baclofen did not induce anxiolytic effects in rat,11 but baclofen was effective at reducing anxiety when it was infused into the NAc shell.12 Other studies have reported that baclofen did not affect anxiety-like behavior or learning and memory processes.13
Recent reports have documented that the NAc is a major structure that may play a critical role in the etiology and pathophysiology of depression.14,15 Stress in patients with depression and in animals leads to dysfunction of the hypothalamic-pituitary-adrenal axis,16,17 which stimulates neuron atrophy in areas of the brain such as the NAc that is involved in reward pathways.18,19
NAc is a major structure that has been recognized to play a key role in the organization and control of stress responses. Acute stress has been shown to impair spatial memory in male rats, and it has been shown that NAc neural activity plays a role in spatial memory and learning.19,20 For example, pre-training ibotenic acid lesions of the NAc impair spatial memory in rats as seen in their performance of the Morris water maze (MWM) task.21 The NAc receives dense glutamatergic projections from different brain areas, such as PFC, and from other limbic structures, such as the amygdala and hippocampus.22 Medium-sized spiny neuronsare the main cell type in the NAc and mainly express dopamine, glutamate, and GABA receptors.23
Baclofen has been used in various studies to determine the role of GABA receptors in learning and memory tasks. Deficits in passive avoidance,24 spatial memory impairments,25 and memory retention are some of the effects of baclofen on behavioral tasks.26
The impairment of learning and memory caused by baclofen is seen in the inhibition of spatial learning in mice through baclofen activating the PKA pathway. Furthermore, previous studies have indicated that baclofen plays an important role in improving cognitive deficit caused by methamphetamine (METH) in mice.27 GABAergic receptor in the NAc shell modulates METH-mediated learning deficit so that the microinjection of the GABAA receptor agonist in the NAc increases METH-induced spatial memory deficits. These findings confirm the role of NAc GABAergic receptor in spatial memory.28 Baclofen, a GABAB receptor agonist, markedly improved the spatial reference memory impairments and its efficacy may be related to the regulation of HCN channels.29 As for the role of GABAB receptors in mediating spatial memory and the involvement of NAc in this type of memory, the role of GABAergic receptor of NAc in spatial memory in stress-exposed rats is not yet clearly understood. Therefore, in the present study, the effects of local and systemic administration of baclofen on spatial memory in a stress-exposed rat model were investigated.
Materials and methods
Ethic statement and animals
All the following procedures and animal care protocols were approved by the Animal Experiment Committee at the Kerman Medical University (Ethics Code: EC/94–52/KNRC) and were performed in line with the “NIH Guide for the Care and Use of Laboratory Animals”. Eighty adult male Wistar rats (180–250 g) were obtained from the Neuroscience Research Center, Kerman, Iran, and housed in a room with a natural light cycle and constant temperature (24±2°C). Food and water were available ad libitum. All behavioral studies were performed between 08:00 and 14:00.
Experimental design
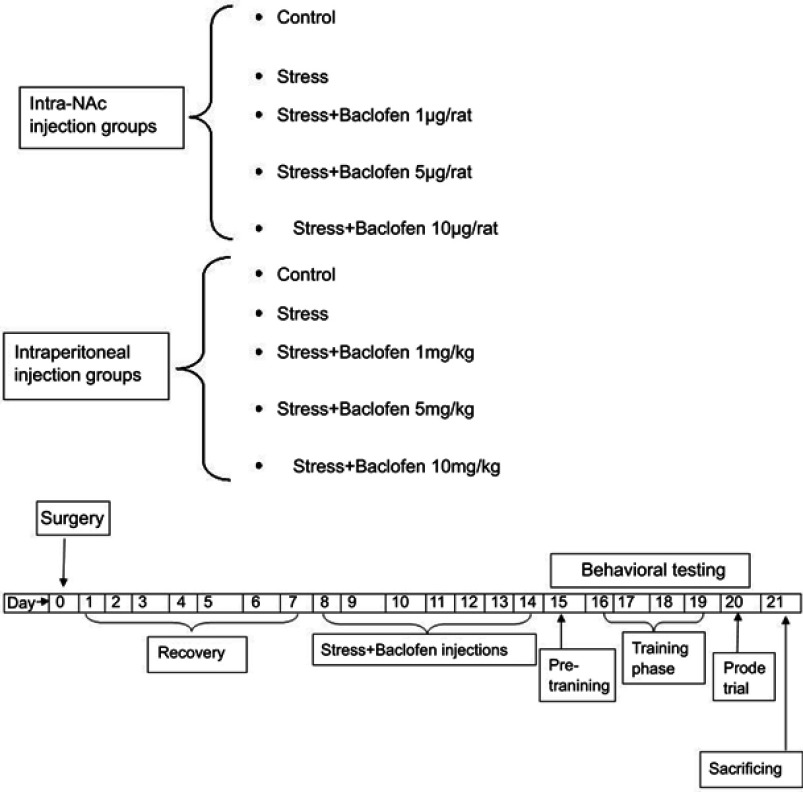
The animals were divided into ten groups (five groups i.p and five groups intra-NAc, n=8 per group): two intra-NAc and i.p. control groups. Two groups were exposed to stress and injected with saline (baclofen vehicle) intra-NAc and i.p. Three groups were given baclofen (1, 5, and 10 µg/rat) intra-NAc 5 mins before stress induction, and three groups received baclofen (1, 5, and 10 mg/kg) i.p. 30 mins before stress induction.13,37 Behavioral assays were performed 24 hrs after the last baclofen injection (8th day) (Figure 1).
Figure 1.
Experimental groups and time-line diagram showing the protocol used for all groups except for intraperitoneal injection groups that did not have surgery and cannulation.
Surgery and drug administration
After 7–10 days of acclimation to the colony room, the rats were anesthetized using i.p. injections of ketamine (75 mg/kg) and xylazine (10 mg/kg), and then a 26-gauge guide cannula (Plastics One, Roanoke, VA) was implanted bilaterally into the NAc shell (AP=1.7 mm, ML=±0.8 mm, and DV=5.6 mm). The cannula was fixed to the skull with dental acrylic, and all experiments were done one week after recovery.
Baclofen (purchased from Sigma-Aldrich, Stelnheim, Germany) was infused into the NAc through an injection needle (30-gauge, 11-mm) attached to a Hamilton syringe and polyethylene tubing.
Histology
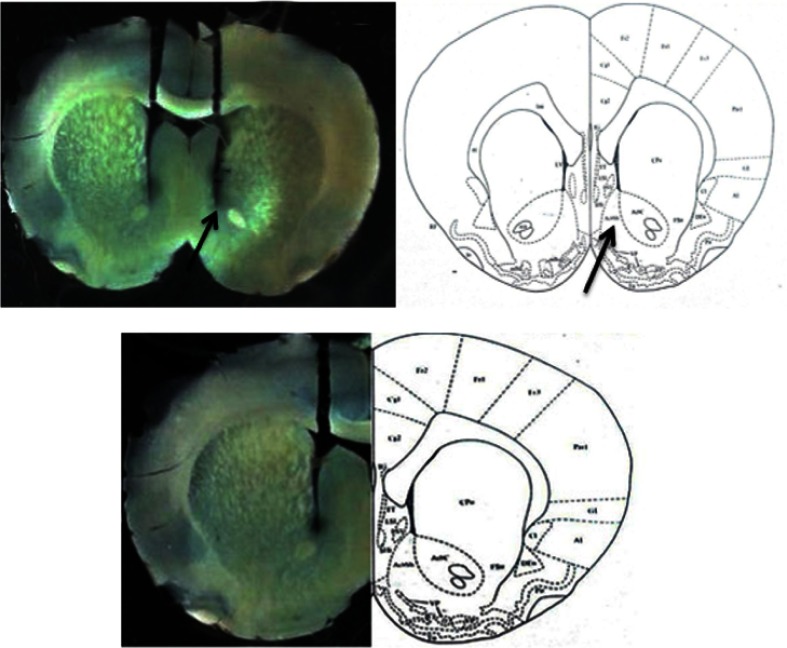
After completion of the behavioral tests, rats were euthanized with an overdose of ketamine and their brains were removed. The location of the cannula was determined using a light microscope and atlas plates (Figure 2).29
Figure 2.
Location of microinjection cannula tips in the brain for all animals used for intra-nucleus accumbens shell injections in this study.
Foot-shock stress
The apparatus used for stress induction consisted of plexiglas supplied by the Borje-Sanat Corporation, Tehran, Iran30 and nine equal compartments (16×16×54 cm). The apparatus floor was equipped with stainless steel rods (4 mm in diameter) placed 1.3 cm apart. The rods were attached to a generator that was controlled by a computer. Electric foot-shock stress was applied for 7 consecutive days between 08:00 and 13:00. Animals were placed in a stress box 20 mins before exposure to stress. During the session in the foot-shock box, rats received five foot shocks (voltage of 60 V and frequency of 10 Hz for 10 s). The animals in the control group were placed in the compartments without being subjected to foot shock. The time for stress induction for each animal was randomly selected for minimum stress adaptation.
Behavioral testing
Apparatus
Spatial learning in the rats was assessed using a Barnes maze,31 made from white round Plexiglas with a diameter of 92 cm. Twenty holes (8 cm in diameter) were installed on the perimeter at a distance of 2 cm from the edge. Under the escape hole, a dark Plexiglas chamber that could be changed was set between the holes. This circular platform was then mounted on a stand 100 cm above the ground and balanced. A 120 W light was placed on top of the maze. The rats were trained to find the escape hole according to visual cues (eg, squares, circles, or triangles) attached to the walls around the room. During testing, the experimenter stood in a fixed position in one corner of the room 1.5 m away from the maze. A video camera (Logitech High Definition Webcam) was suspended 144.8 cm above the center of the maze, and a laptop computer that operated the video camera was located next to the investigator. The testing room was located inside a larger behavioral suite that included a separate, central room where the rats were kept in home cages before and between trials.
Procedure
At day 0 (habituation), each rat was placed in the maze for 4 mins with the lamp turned off. On the training day, each trial began by the rat being put in a black starting cylinder (∅: 8 cm, height: 12.5 cm) which was placed in the center of the platform and removed after 10 s, allowing rats to freely explore the apparatus. The animals were given 90 s to find the target hole. If rats could not successfully find the target hole, they were gently guided into it. After finding the main hole, the rat remained in the same state for 60 s. This protocol was repeated for 4 consecutive days (four training sessions per day). After testing each rat, the maze around the target hole was cleaned using ethanol. In the training phase, the escape latency, velocity, and total distance to reach the target hole were measured for each trial of each individual rat.
Twenty-four hours after the last training day (the probe day), each rat was placed inside the center of the maze and given 2 mins to explore it. On the probe day, animals cannot escape through the target hole. After 2 mins, the buzzer was turned off and the rat was transferred to its cage. During the probe phase, the distance traveled by the animal to reach the target, target frequency, velocity in target, and time to reach the target hole were recorded.
Statistical analysis
All of the statistical analyses were performed using IBM SPSS Statistics 20 software (Chicago, IL, United States). All data were expressed as mean±SEM The Barnes maze training in the acquisition phase was analyzed using a two-way ANOVA along with repeated measures to determine the differences of the learning rates of the groups. The obtained data from probe trials were analyzed using one-way ANOVA. In all cases, Tukey post-hoc test was used for multiple comparisons. The accepted level of significance for all tests was P<0.05.
Results
Effects of stress and baclofen administration on spatial learning in the training trial
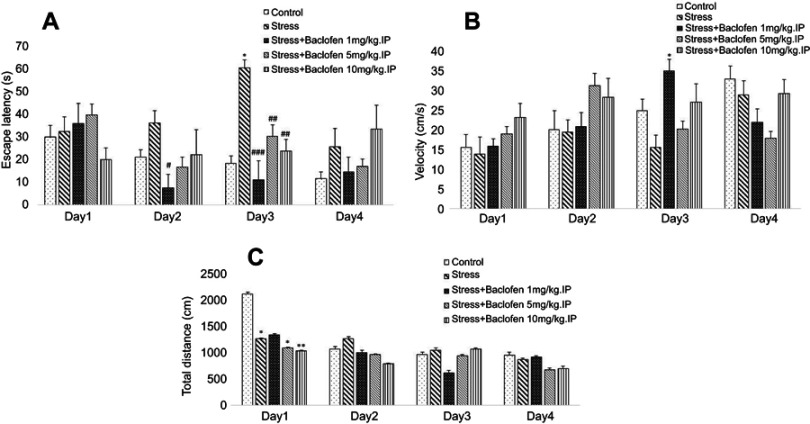
The effect of i.p injection (1, 5, and 10 mg/kg) and intra-NAc administration (1, 5, and 10 µg/rat) of baclofen before inducing stress on spatial memory was determined using the Barnes maze. The data on the i.p. administration groups are shown in Figure 3A–C. Two-way ANOVA indicated that the escape latency (Figure 3A) was significantly higher in stress-exposed rats than in rats of the control group during training trials (p<0.05), showing spatial learning impairment in the stress-exposed rats. The administration of baclofen in the three groups (baclofen 1, 5, 10 mg/kg) improved stress-induced impairment of spatial learning (p<0.05). The velocity rates for the i.p. administration groups are shown in Figure 3B. No significant difference was observed among any of the groups except the stress+baclofen1 mg/kg.IP group, which showed a significantly higher velocity than the stress-exposed rats group during the 3rd-day training trials (Figure 3B); P<0.05).
Figure 3.
The effects of foot-shock stress and baclofen (1, 5, and 10 mg/kg, i.p) on escape latency (A), velocity (B), and total distance (C) in Barnes maze task. Two-way ANOVA and repeated measure used for the analysis of these data. Data are shown as mean±SEM (n=8). (*) p<0.05, (**) p<0.01, vs control group. (#) p<0.05, (##) p<0.01, (###) p<0.001, vs stress group.
The results for total distance for the i.p. administration groups are shown in Figure 3C. The total distance was lower in the stress-exposed rats than in the rats of the control group on day one of training (P<0.05). The administration of baclofen in the treatment groups (baclofen 5, and 10 mg/kg) lowered total distance compared to the control group (P<0.05).
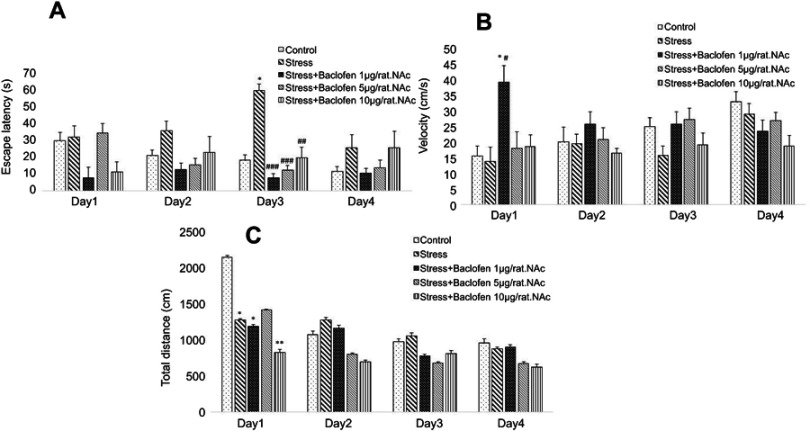
Data for the intra-NAc baclofen administration groups are shown in Figure 4A–C. Two-way ANOVA indicated that escape latency (Figure 4A) was significantly higher in the stress-exposed rats than in the rats of the control group during training trials (P<0.05), showing spatial learning impairment in the stress-exposed rats. The administration of baclofen in the three groups (1, 5, and 10 µg/rat.NAc) improved the stress-induced impairment of spatial learning (p<0.01).
Figure 4.
The effects of foot-shock stress and baclofen (1, 5 and 10 µg/rat, intra-NAc) on escape latency (A), velocity (B), and total distance (C) in Barnes maze task. Two-way ANOVA and repeated measure used for the analysis of these data. Data are shown as mean±SEM (n=8). (*) p<0.05, (**) p<0.01, vs control group. (#) p<0.05, (##) p<0.01, (###) p<0.001, vs stress group.
The velocity rates for the intra-NAc baclofen administration groups are shown in Figure 4B. There was a significantly higher velocity rate in the baclofen1.intra-NAc group than in the stress-exposed rats and the control groups during the first-day training trials (Figure 4B); p<0.05.
The total distances for the intra-NAc administration groups are shown in Figure 4C. Total distance was lower in the stress-exposed rats than in the rats of the control group during training (p<0.05). The administration of baclofen in the treatment groups (stress+baclofen 1, 10 µg/rat) reduced the total distance compared to the control group (p<0.05).
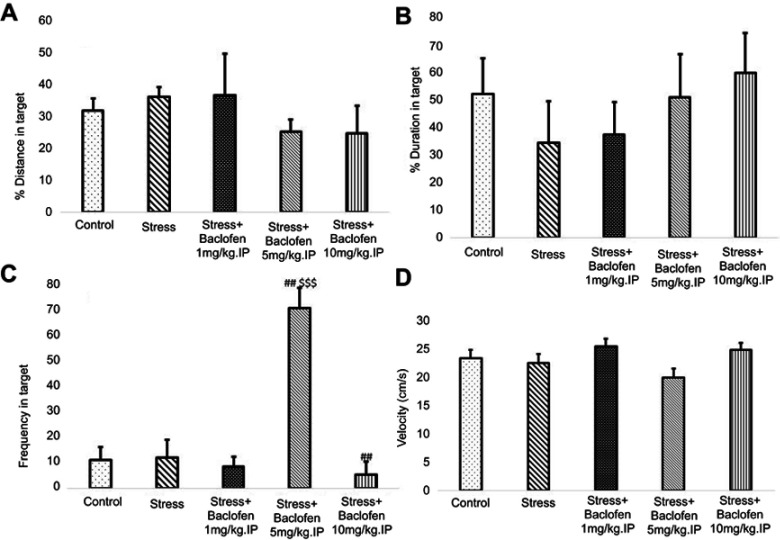
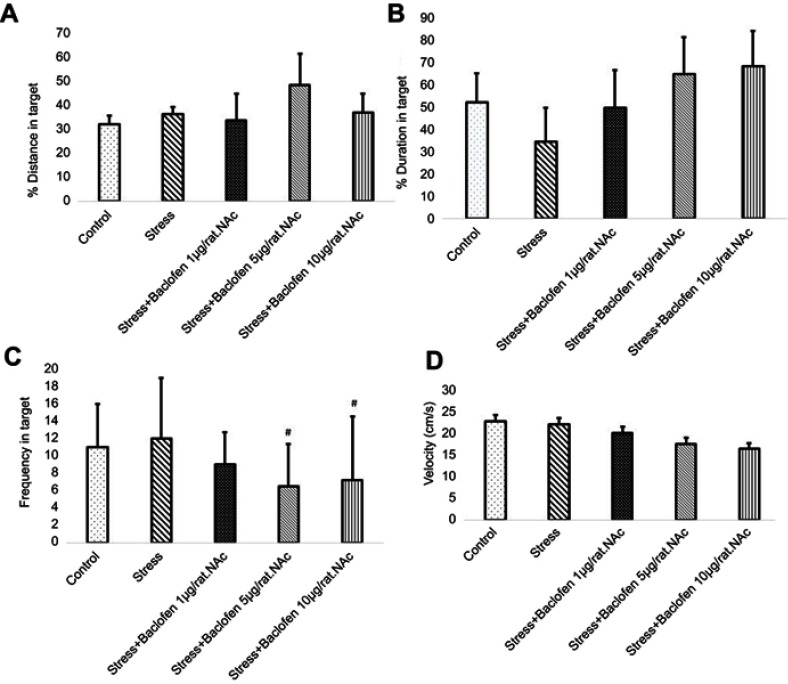
Effects of stress and baclofen administration on spatial learning in the probe trial are shown in Figure 5A–D. The probe test was conducted 24 hrs after the last training trial, and the mean percentages of distance, time in the target quadrant, frequency, and velocity in the target quadrant were analyzed to assess spatial memory retention. The Tukey’s test following one-way ANOVA indicated that time in the target quadrant in the i.p. baclofen administration group (stress+baclofen 5 mg/kg. IP) was significantly longer compared to the stress-exposed rats (p<0.01) (Figure 5C). A comparison of the treatments groups indicated that time in the target quadrant was significantly longer in the (stress+baclofen 5 mg/kg. IP) group than in the other treatment groups (stress+baclofen 1 and 10 mg/kg. IP) (p<0.001) (Figure 5C); however, in the (stress+baclofen 10 mg/kg. IP) group, the time in the target quadrant was significantly less compared to the stress-exposed rats (p<0.01) (Figure 5C). Effects of stress and baclofen administration on spatial learning in the probe trial in NAc groups are shown in Figure 6A–D. Time in the target quadrant in the intra-NAc baclofen administration groups (5 and 10 ug/rat. NAc) was significantly less compared to the stress-exposed rats (p<0.05) (Figure 6C).
Figure 5.
The effects of foot-shock stress and baclofen (1, 5, and 10 mg/kg, i.p) on traveled distance (A), duration (B), frequency (C), and velocity (D) in the target quadrant in Barnes maze task. One-way ANOVA and post hoc Tukey’s multiple comparison tests used for the analysis of these data. Data are shown as mean±SEM (n=8). (##) p<0.01, vs stress group. ($$$) p<0.001 vs stress+baclofen 10 mg/kg.IP and stress+baclofen 1 mg/kg.IP groups.
Figure 6.
The effects of foot-shock stress and baclofen (1, 5, and 10 ug/rat, intra-NAc) on traveled distance (A), duration (B), frequency (C), and velocity (D) in the target quadrant in Barnes maze task. One-way ANOVA and post hoc Tukey’s multiple comparison tests used for the analysis of these data. Data are shown as mean±SEM (n=8). (#) p<0.05, vs stress group.
Discussion
In this study, whether dose-dependent administration of baclofen would alleviate spatial memory in rats following exposure to stress was investigated. The findings showed that intra-NAc administration of 1 µg/rat baclofen and i.p administration of 1 mg/kg baclofen before the training trials improved spatial memory and cognitive flexibility by decreasing escape latency on the 3rd day and the 2nd day of training in the Barnes maze task, respectively. The dosage had no significant effects on spatial memory on the other 3 days of training. In both the intra-NAc and the i.p. administration of baclofen groups, total distance decreased on the 1st day of training in the Barnes maze task. The current results are consistent with the previous report that bilateral occlusion of the common carotid arteries (two-vessel occlusion, 2VO) to induce chronic cerebral hypoperfusion could cause PFC-dependent spatial working memory impairment and increase escape latency in MWM, and this impairment could be restored by treatment with baclofen. The biologically plausible mechanisms of baclofen efficacy may be through the upregulation of the decreased HCN2 channel expression in the PFC.32
Velocity to find the target hole was increased on the 1st day of training by baclofen 1 µg/rat in the intra-NAc group, but it increased on the 3rd day of training by baclofen 1 mg/kg in the i.p. group. The training procedure employed in the present study indicated that administration of the highest and middle doses of baclofen (5, 10 mg/kg and µg/rat) exerted non-specific effects on velocity during the 4 training days. Chronic baclofen administration had no effect on the subsequent expression of spatial memory in the Barnes maze and no effect on the latency to enter the escape box.33 However, the current study showed that acute baclofen administration reduced escape latency in this test, although the other parameters showed no significant differences.
The present study showed that rats in the i.p. administration of baclofen (5 mg/kg) group spent the longest time in the target quadrant during the probe trial. Although no significant differences in duration, distance, or velocity in the target quadrant between the baclofen-treated groups were observed, rats treated with baclofen 10 mg/kg i.p. and the intra-NAc administration of baclofen (5, 10 µg/rat) showed a statistically significant decrease in the time in the target quadrant. It may be this decrease is correlated with impairment of spatial memory that is probably caused by the inhibitory role of GABAergic receptor of the NAc in spatial memory.34 Baclofen 10 mg/kg disrupted memory maintenance in rats performing the passive avoidance task.35 Castellano et al have provided evidence that baclofen at 10 and 30 mg/kg in different memory tests and dose-dependently disrupted learning and memory in rodents when administered systemically.36 In previous studies, baclofen infused subcutaneously (1 mg/kg and 5 mg/kg) did not influence spatial learning in any of the tested doses during the training trial of the MWM test; however, the highest dose of baclofen (10 mg/kg) increased escape latency and distance traveled in the training and reduced the time spent in the correct MWM quadrant in the probe compared with the controls and the animals receiving lower doses of baclofen (1 mg/kg and 5 mg/kg).37 The difference between the current results and the results presented in the mentioned articles can be explained by differences in the methods that were used. In the present study, the role of stress, treatment dosage, behavioral test, and the duration of the experiment was important. According to our opinion, the dose of baclofen is very important but we suggested that the activation of GABAergic receptor in the NAc is related to the effects of stress on spatial memory. Generally stress plays a key role in the interacting of baclofen effects on spatial memory.
Pilipenko et al demonstrated that rats given baclofen at a dose of 0.05 mg/kg showed significantly shorter escape latency compared with the streptozotocin group on training day 1, whereas lower doses of baclofen (0.025 mg/kg) protected the rats against STZ-induced spatial learning impairments on training days 2–4. This data indicates the memory-enhancing effects of very low doses of GABA-B receptor agonists.38 Recently, a very low dose (0.01 mg/kg) of baclofen was shown to promote cell survival in a mouse model of Huntington’s disease which exhibited an improvement in behavior and enhanced activity of the ubiquitin-proteasome system.39
The current study showed spatial memory impairment in the stress group through increased escape latency and decreased velocity in the Barnes maze task. This finding is consistent with previous findings referring to the vulnerability of GABAergic receptor to many factors such as stress.40,41 Here, it is suggested that the activation of GABAergic receptor in the NAc is related to the effects of reduced stress on spatial memory.
It is well known that GABAergic receptors as inhibitory receptors are abundant in brain structures such as the ventral tegmental area, NAc, amygdala, and medial PFC, and impairment of these neurons is related to major depression.42,43 It seems that the activation of GABAergic receptors in the NAc and different doses of baclofen reduced the destructive effects of stress.
Consistent with previous reports, it was found that, depending on the dose, baclofen can impair or improve spatial memory in rats by decreasing distance traveled and escape latency in the Barnes maze task.44 As discussed in some of the literature, the different effects of baclofen on learning and memory tasks may be related to the side effects of this drug or the complexity of inhibitory currents in metabotropic receptors on learning and memory tasks.45 Taken together, it is determined that GABAB receptors play an important role in the formation of spatial memory. Furthermore, baclofen dose-dependently decreased the impairing effect of stress on spatial memory, its efficacy may be related to the regulation of HCN channels. According to our findings, baclofen can dose-dependently improve spatial memory deficit in stress-exposed rats. Both intra-NAc and i.p. administrations of baclofen mediate the memory improvement by decreasing escape latency and total distance and increasing velocity in the training trials to find the escape box in the Barnes maze task and increased the frequency in the probe trial.
In summary, the present study shows the GABAB receptor activation ameliorates spatial memory impairments in stress-exposed rats. Baclofen can dose-dependently improve spatial memory, and GABAB receptor in the NAc plays an important role in spatial memory. Findings of the present study could have important implications for the development of novel strategies for the treatment of stress-related disorders. Increased understanding of the mechanisms involved in spatial memory may be useful in the treatment of psychiatric disorders.
Acknowledgments
This work was supported by the Kerman Neuroscience Research Center, Kerman, Iran; under grant [9452]; and Baqiyatallah Neuroscience Research Center, Tehran, Iran; under grant [349]. This article is extracted from the elite research project belong to Mr Majid Askaripour in the field of Physiology.
Disclosure
No potential conflicts of interest in this work were reported by the authors.
References
- 1.Braastad BO. Effects of prenatal stress on behaviour of offspring of laboratory and farmed mammals. Appl Anim Behav Sci. 1998;61(2):159–180. doi: 10.1016/S0168-1591(98)00188-9 [DOI] [Google Scholar]
- 2.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev. 2002;26(4):457–470. [DOI] [PubMed] [Google Scholar]
- 3.Peterson RA. Handbook of stress, medicine and health. J Health Psychol. 2000;5(2):255–256. doi: 10.1177/135910530000500215 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs EH, Wardeh G, Smit AB, Schoffelmeer AN. Morphine causes a delayed increase in glutamate receptor functioning in the nucleus accumbens core. Eur J Pharmacol. 2005;511(1):27–30. doi: 10.1016/j.ejphar.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459–466. doi: 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston GA. Neuropharmacology of amino acid inhibitory transmitters. Annu Rev Pharmacol Toxicol. 1978;18:269–289. doi: 10.1146/annurev.pa.18.040178.001413 [DOI] [PubMed] [Google Scholar]
- 7.Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. [DOI] [PubMed] [Google Scholar]
- 8.Bowery N, Enna SJ, Olsen RW. Six decades of GABA. Biochem Pharmacol. 2004;68(8):1477–1478. doi: 10.1016/j.bcp.2004.07.033 [DOI] [PubMed] [Google Scholar]
- 9.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84(3):835–867. doi: 10.1152/physrev.00036.2003 [DOI] [PubMed] [Google Scholar]
- 10.Amikishieva AV, Semendyaeva SN. Effects of baclofen on anxiety, sexual motivation, and olfactory perception in male mice in different psychoemotional states. Neurosci Behav Physiol. 2007;37(9):929–937. doi: 10.1007/s11055-007-0101-9 [DOI] [PubMed] [Google Scholar]
- 11.Zarrindast M, Rostami P, Sadeghi-Hariri M. GABA(A) but not GABA(B) receptor stimulation induces antianxiety profile in rats. Pharmacol Biochem Behav. 2001;69(1–2):9–15. [DOI] [PubMed] [Google Scholar]
- 12.Lopes AP, Ganzer L, Borges AC, et al. Effects of GABA ligands injected into the nucleus accumbens shell on fear/anxiety-like and feeding behaviours in food-deprived rats. Pharmacol Biochem Behav. 2012;101(1):41–48. doi: 10.1016/j.pbb.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Risbrough VB, Cates-Gatto C, et al. Comparison of the effects of the GABAB receptor positive modulator BHF177 and the GABAB receptor agonist baclofen on anxiety-like behavior, learning, and memory in mice. Neuropharmacology. 2013;70:156–167. doi: 10.1016/j.neuropharm.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. [DOI] [PubMed] [Google Scholar]
- 15.Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23(10):475–482. [DOI] [PubMed] [Google Scholar]
- 16.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–1180. doi: 10.1017/S1461145708009309 [DOI] [PubMed] [Google Scholar]
- 17.Strekalova T, Couch Y, Kholod N, et al. Update in the methodology of the chronic stress paradigm: internal control matters. Behav Brain Funct. 2011;7:9. doi: 10.1186/1744-9081-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banasr M, Dwyer JM, Duman RS. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol. 2011;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918 [DOI] [PubMed] [Google Scholar]
- 20.Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15(4):271–280. doi: 10.1101/lm.721108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annett LE, McGregor A, Robbins TW. The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behav Brain Res. 1989;31(3):231–242. [DOI] [PubMed] [Google Scholar]
- 22.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29(3):503–537. [DOI] [PubMed] [Google Scholar]
- 23.Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol. 2006;4(4):277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson LH, Bettler B, Kaupmann K, Cryan JF. Behavioral evaluation of mice deficient in GABA(B(1)) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology. 2007;190(4):541–553. doi: 10.1007/s00213-006-0631-9 [DOI] [PubMed] [Google Scholar]
- 25.McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53(2):303–308. [DOI] [PubMed] [Google Scholar]
- 26.Pitsikas N, Rigamonti AE, Cella SG, Muller EE. The GABAB receptor and recognition memory: possible modulation of its behavioral effects by the nitrergic system. Neuroscience. 2003;118(4):1121–1127. [DOI] [PubMed] [Google Scholar]
- 27.Voigt RM, Herrold AA, Riddle JL, Napier TC. Administration of GABA(B) receptor positive allosteric modulators inhibit the expression of previously established methamphetamine-induced conditioned place preference. Behav Brain Res. 2011;216(1):419–423. doi: 10.1016/j.bbr.2010.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heysieattalab S, Naghdi N, Zarrindast MR, Haghparast A, Mehr SE, Khoshbouei H. The effects of GABAA and NMDA receptors in the shell-accumbens on spatial memory of METH-treated rats. Pharmacol Biochem Behav. 2016;142:23–35. doi: 10.1016/j.pbb.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. Gulf professional publishing; Houston, TX, USA: 2004. [Google Scholar]
- 30.Hooshmandi Z, Rohani AH, Eidi A, Fatahi Z, Golmanesh L, Sahraei H. Reduction of metabolic and behavioral signs of acute stress in male Wistar rats by saffron water extract and its constituent safranal. Pharm Biol. 2011;49(9):947–954. doi: 10.3109/13880209.2011.558103 [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld CS, Ferguson SA. Barnes maze testing strategies with small and large rodent models. J Vis Exp. 2014;26(84):e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo P, Chen C, Lu Y, et al. Baclofen ameliorates spatial working memory impairments induced by chronic cerebral hypoperfusion via up-regulation of HCN2 expression in the PFC in rats. Behav Brain Res. 2016;308:6–13. doi: 10.1016/j.bbr.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 33.Gill KM, Grace AA. Differential effects of acute and repeated stress on hippocampus and amygdala inputs to the nucleus accumbens shell. Int J Neuropsychopharmacol. 2013;16(9):2013–2025. doi: 10.1017/S1461145713000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebrahimi-Ghiri M, Rostampour M, Jamshidi-Mehr M, Nasehi M, Zarrindast MR. Role of CA1 GABAA and GABAB receptors on learning deficit induced by D-AP5 in passive avoidance step-through task. Brain Res. 2018;1678:164–173. doi: 10.1016/j.brainres.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Swartzwelder HS, Tilson HA, McLamb RL, Wilson WA. Baclofen disrupts passive avoidance retention in rats. Psychopharmacology. 1987;92(3):398–401. [DOI] [PubMed] [Google Scholar]
- 36.Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52(2):170–179. [DOI] [PubMed] [Google Scholar]
- 37.Holajova M, Franek M. Effect of short- and long-term administration of baclofen on spatial learning and memory in rats. Physiol Res. 2018;67(1):133–141. doi: 10.33549/physiolres.933554 [DOI] [PubMed] [Google Scholar]
- 38.Pilipenko V, Narbute K, Beitnere U, et al. Very low doses of muscimol and baclofen ameliorate cognitive deficits and regulate protein expression in the brain of a rat model of streptozocin-induced Alzheimer’s disease. Eur J Pharmacol. 2018;818:381–399. doi: 10.1016/j.ejphar.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 39.Kim W, Seo H. Baclofen, a GABAB receptor agonist, enhances ubiquitin-proteasome system functioning and neuronal survival in Huntington’s disease model mice. Biochem Biophys Res Commun. 2014;443(2):706–711. doi: 10.1016/j.bbrc.2013.12.034 [DOI] [PubMed] [Google Scholar]
- 40.Akaike N. Time-dependent rundown of GABA response in mammalian cns neuron during experimental anoxia. Obes Res. 1995;3 Suppl 5:769s–777s. [DOI] [PubMed] [Google Scholar]
- 41.Wang J-H, Lu W, Wen B. Neuron-specific mechanisms for epilepsy self-termination. Mol Cell Epilepsy. 2015;3, e716. doi:10.14800/mce.716 [Google Scholar]
- 42.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13(4):411–420. doi: 10.1017/S1461145709990587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu A, Cui S, Wang JH. Incoordination among subcellular compartments is associated with depression-like behavior induced by chronic mild stress. Int J Neuropsychopharmacol. 2016;19(5). doi: 10.1093/ijnp/pyv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng PY, Xiao Z, Yang C, et al. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63(2):230–243. doi: 10.1016/j.neuron.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaney CF, Bolton MM, Murtishaw AS, Sabbagh JJ, Magcalas CM, Kinney JW. Baclofen administration alters fear extinction and GABAergic protein levels. Neurobiol Learn Mem. 2012;98(3):261–271. doi: 10.1016/j.nlm.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]