Abstract
Background: Liver fibrosis occurs due to chronic liver disease due to multiple pathophysiological causes. The main causes for this condition are chronic alcohol abuse, nonalcoholic steatohepatitis, and infection due to hepatitis C virus. Currently, there is more and more information available about the molecular as well as cellular mechanisms, which play a role in the advancement of liver fibrosis. However, there is still no effective therapy against it.
Purpose: In order to find an effective treatment against liver fibrosis, our study explored whether salvianolic acid A (SA-A), a traditional Chinese medicine extracted from the plant Danshen, could effectively inhibit the liver fibrosis, which is induced by CCl4 in vivo.
Methods: The effects of SA-A were evaluated by assessing the parameters related to liver fibrosis such as body weight, histological changes, and biochemical parameters. Thereafter, the related protein or gene levels of P13K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways were determined by western blotting, real-time PCR or immunohistochemistry staining.
Results: According to the results of our study, SA-A could reduce liver fibrosis by inhibiting liver function, liver fibrosis index, collagen deposition, and improving the degree of liver fibrosis in rats. Mechanistically, the PI3K/AKT/mTOR signaling cascade was inhibited by SA-A to prevent the stimulation of hepatic stellate cell, as well as the synthesis of extracellular matrix, and regulated Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways to prevent hepatocyte apoptosis.
Conclusion: The novel findings of this study suggested that SA-A could reduce liver fibrosis and the molecular mechanisms behind it are closely associated with the regulation of PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways.
Keywords: liver fibrosis, salvianolic acid A, AKT/mTOR, Bcl-2/Bax, caspase-3/cleaved caspase-3
Introduction
Liver fibrosis is a very serious health problem all over the world with no effective treatment. Liver fibrosis is vigorous as well as a reversible process of wound healing,1,2 which is the result of tissue necrosis, where quiescent hepatic stellate cells (HSCs) proliferate and transdifferentiate into myofibroblasts which are responsible for depositing extracellular matrix (ECM) proteins (ie, collagen fibers), leading to tissue scarring.3,4 There are not any clear symptoms of liver fibrosis. However, liver fibrosis causes liver damage which then develops into the hepatitis or exacerbating cirrhosis, and the clinical manifestations of these conditions are loss in appetite, chronic indigestion, chronic gastritis, and hemorrhage. Even though a lot is known about the mechanism of liver fibrosis, such as the major reasons for the development of liver fibrosis includes chronic infection with hepatitis C virus, alcohol abuse, and also the nonalcoholic steatohepatitis,5,6 there is still no specific way to detect liver fibrosis. Thus, it is difficult to cure liver fibrosis. At present, the only treatment for the patients with liver fibrosis is liver transplantation. Although the amount of donor liver available and the exorbitant price make it difficult to achieve for every patient. Therefore, there is a need to find an effective drug against liver fibrosis.
As per our previously published studies, more and more agents are being found to have the ability to inhibit liver fibrosis.7–11 Previously we have reported that asiatic acid and tanshinol can improve carbon tetrachloride (CCl4)-induced liver fibrosis by preventing both Bcl-2/Bax as well as PI3K/AKT/mTOR signaling cascade.7,8 Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways play essential roles in the progression of liver fibrosis by preventing hepatocyte apoptosis. Therefore, regulating Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling may inhibit apoptosis of hepatocyte to reduce liver fibrosis and this may be a new anti-fibrosis strategy.12
Additionally, PI3K/AKT/mTOR signaling cascade also plays an important role in regulating the proliferation, survival, metabolism of cells and it also regulates the protein synthesis and cell cycle.13,14 In this pathway, PI3K will phosphorylate and activate the AKT protein, which is located on the plasma membrane, following which the AKT protein will activate various downstream pathways to then activate mTOR.15 In our previous research studies, we had also found out that in liver fibrosis there is an activation of the PI3K/AKT/mTOR signaling cascade by specific binding to receptors on HSCs membrane, which then induces gene translocation into the nucleus, causing the proliferation of HSCs and inducing the expression of α-smooth muscle actin (α-SMA) and other cell cytokines, as well as the production of large amount of ECM.
Salvianolic acid A (SA-A),16 a small phenolic carboxylic acid water-soluble extract from S. miltiorrhiza (Danshen), has been documented to have multiple biological activities. It can inhibit mitochondrial lipid peroxidation, sustain mitochondrial lipid peroxidation, sustain ATPase activity and eliminate super enzyme activity, and has strong antioxidant activity as well as neuroprotective effects. However, there is not any clear research on the inhibitive effect of SA-A on HSCs and liver fibrosis. Hence, the aim of this novel study was to explore the effects of SA-A in the treatment of CCl4-induced liver fibrosis in vivo and understand the molecular mechanism of SA-A in PI3K/AKT/mTOR signaling pathway.
Materials and methods
Materials
SA-A was obtained from Shanghai Nature Standard R&D and Biotech Co., Ltd., Shanghai, China (molecular weight 488.70; purity 98.0%). CCl4 was obtained from Shanghai Jinghua Scientific & Technological Research Institute (Shanghai, China). Following antibodies were bought from Cell Signaling Technology (Danvers, MA, USA): monoclonal rabbit antibodies against mTOR (Cat #2983), AKT (Cat #4685), phospho-mTOR (Cat #5536), phosphor-AKT (Cat #4060), anti-caspase-3 (Cat #9665), anti-Bax (Cat #14796), anti-cleaved caspase-3 (Cat #9664), and β-actin (Cat #4970). Following antibodies were purchased from Abcam (Cambridge, MA, USA): anti-p70S6K1 (Cat #ab32359), anti-phospho-p70S6K1 (Cat #ab60948), anti-Bcl-2 (Cat #ab136285) and anti-α-SMA (Cat #ab32575) rabbit antibodies. The horseradish peroxidase (HRP)-labeled GAPDH antibody was bought from Bioworld Technology Inc. (St Louis Park, MN, USA). HRP-labeled goat anti-rabbit IgG, a secondary antibody, was bought from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Animals handling and experimental protocol
Male Sprague-Dawley rats, 8-week old (240±20 g), were obtained from the B&K Universal Group Ltd. (Shanghai, China). All animals were housed in the separate ventilated cages in SPF level on a 12 h light/dark cycle at a stable temperature of 25°C with relative humidity of 40–60%. They also had free and easy access to standard food as well as water. All animal experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals and approvals were obtained from the Animal Experimental Ethics Committee of Shanghai 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). A random division of 48 rats was done into four different groups (n=12/group): the control group; the model group; the SA-A 5 mg/kg group; and the SA-A 15 mg/kg group. Except control group, rats in other three groups received 1 mL/kg CCl4 orally (diluted in the peanut oil, 1:1) two times a week for 6 weeks to induce liver fibrosis. Moreover, the animals in the SA-A 5 mg/kg or SA-A 15 mg/kg groups were intraperitoneally administered SA-A (5 or 15 mg/kg) once daily for 6 successive weeks. At the same time animals in the control group received similar volume of peanut oil without SA-A. After 6 weeks, the living rats were weighed on empty stomach and anesthetized by using pentobarbital sodium (Shanghai Beizhuo Biochemical & Technological Co., Ltd., Shanghai, China). Blood samples were obtained from the abdominal aorta following which it was centrifuged at 1,000×g for 15 mins to isolate serum. The tissue samples of liver were taken from the same region in each group and were fixed in 10% neutral formalin. Remaining tissues from the liver were stored at −80°C for future use.
Biochemical analysis
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), important markers of liver function, were evaluated using the automatic biochemical analyzer (Beckman Coulter, Miami, FL, USA). Its principle operation was based on reflectance spectrophotometry. Hyaluronic acid (HA), laminin (LN), procollagen III N-terminal peptide (PIIINP) and collagen type IV (CIV), as four significant serum liver fibrosis indices, were evaluated by radioimmunoassay kits (Autobio Diagnostics Co., Ltd., Zhengzhou, China), according to instructions of the kits. As an important parameter reflecting liver collagen concentration, the hydroxyproline (HYP) content was detected in fresh liver samples using Hydroxyproline Testing Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in this study, and the technique was implemented based on the manufacturer’s instructions.
Histological analysis
The liver tissues were paraffin-embedded after fixation, and cut into 5-μm thick sections. Afterwards, the haematoxylin & eosin (H&E) stain was used to stain sections of liver for histological analysis according to the standard instructions. In order to randomly select microscopic areas in liver sections for examination, Ti-S inverted fluorescence microscope (Nikon, Tokyo, Japan) was used. By using following criteria, the scoring for the extent of liver fibrosis was assessed. For no presence of any obvious fibrosis (0); presence of fibrosis (1) showing the extension of collagen fibers from the central vein or portal triad to peripheral regions; mild fibrosis (2) indicating presence of limited collagen fibers that extend without any formation of compartments; moderate fibrosis (3) showing presence of collagen fibers along with the development of “pseudo leaves”; and severe fibrosis (4) showing presence of numerous collagen fibers along with stiffening of partial compartments as well as the development of “pseudo lobes”. In order to distinguish collagen fibers from tissues by histological analysis, Masson’s trichrome staining method was used. Apart from H&E staining, in order to do microscopical analysis (Olympus, Tokyo, Japan) of collagen deposition, sections of liver were stained. From each group, three slices were selected and in each slice (×100) five different areas were randomly analyzed. An expert pathologist, who was blinded to the experimental protocol, conducted these histological analysis.
Quantitative real-time PCR analysis
In order to extract total RNA from liver tissues, Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used according to the supplier's instructions. Complementary DNA (cDNA) was produced from total RNA by using the Takara PrimeScriptTMRT Master Mix (Perfect Real Time) reagent (Cat #RR047A; TaKaRa, Kyoto, Japan). Sample cDNAs were used as templates with gene-specific primers (Sangon Biotech Co. Ltd., Shanghai, China) (Table S1) and RT-PCR was performed using SYBR® Premix Ex TaqTMII (Tli RNaseH Plus) (Cat #RR820A; TaKaRa) and LightCycler 480 instrument (Roche, Basel, Switzerland). By using the relative quantitative formula 2−ΔΔCT, the detected levels of main mRNAs were calculated after normalization with GAPDH. With the help of Sangon Biotech Co. Ltd., the sequences of PCR primers were designed as well as synthesized. The list of primers is provided in Table S1.
Table S1.
Primer sequences for real-time PCR assay
| Gene | Forward primer (5ʹ–3ʹ) | Reverse primer (5ʹ–3ʹ) |
|---|---|---|
| ɑ-SMA | CCAGGGAGTGATGGTTGGA | CCGTTAGCAAGGTCGGATG |
| Caspase-3 | ATGGAGAACAACAAAACCTCAGT | TTGCTCCCATGTATGGTCTTTAC |
| PDGF-βR | TTCCAGGAGTGATACCAGCTT | AGGGGGCGTGATGACTAGG |
| CTGF | CAACTATGATGCGAGCCAAC | TCCAGCCTGCAGAAGGTATT |
| Desmin | CCGATCCAGACCTTCTCTGC | TCTCCATCCCGGGTCTCAAT |
| Vimentin | TGAGATCGCCACCTACAGGA | GAGTGGGTGTCAACCAGAGG |
| TGF-β1 | CTTGCCCTCTACAACCAACA | ACTTGCGACCCACGTAGTAGA |
| GAPDH | TGGATTTGGACGCATTGGTC | TTTGCACTGGTACGTGTTGAT |
Immunohistological staining
In order to determine the expressions of proteins, immunohistological analysis was performed in sample tissues of liver. The liver slices were first deparaffinized and then to inhibit the activity of endogenous peroxidase they were treated with 3% H2O2. Citrate buffer was used for antigen retrieval process. After cooling, in order to occlude any non-specific protein binding, the liver sections were then treated with 5% BSA. The sections of liver were incubated overnight (4°C) with various primary antibodies such as Bcl-2 (1:200), α-SMA, Bax (1:200), p-AKT, cleaved caspase-3, p-p70S6K1, and p-mTOR. Negative controls were set up by incubating the sections with only PBS. Next day, the liver sections were washed by PBS following which they were further incubated with a biotinylated secondary antibody (1:1). Following this step, the sections were further incubated with an avidin-biotin-peroxidase complex, and then stained with 3,3'-diaminobenzidine. Images of sections were taken by microscope (Nikon).
Western blotting analysis
As a first step, total proteins were extracted from tissue samples of liver and quantification was done with the help of a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China) as per the manufacturer’s protocol. SDS-PAGE was used to separate the total proteins and then by using a wet transfer system proteins were transferred on to PVDF membranes. The PVDF membranes containing proteins were blocked by using 5% non-fat milk that was made in TBS buffer and then incubated with primary antibodies overnight (4°C). Various primary antibodies were used such as cleaved caspase-3, caspase-3, Bax, Bcl-2, AKT, p-AKT, mTOR, p-mTOR, p70S6K1, p-p70S6K1, and α-SMA. After overnight incubation with primary antibodies, next day the PVDF membranes were incubated at room temperature with respective secondary antibodies. By using an enhanced chemiluminescence system (Fusion FX7 Spectra, Vilber Lourmat, Eberhardzell, Germany), the protein bands were detected. The analysis was conducted by using Quantity One (Bio-Rad Laboratories Inc., Hercules, CA, USA) as per the standard method.
Statistical analysis
The data in this study are presented as the mean ± S.D. One-way ANOVA statistical test was used for the analysis of comparisons. For all analysis, GraphPad Prism (Version 5; GraphPad Software, Inc., La Jolla, CA, USA) statistical software was used. Statistical significance was achieved with a P<0.05.
Results
SA-A reduced the clinical symptoms in CCL4-induced liver fibrosis rats
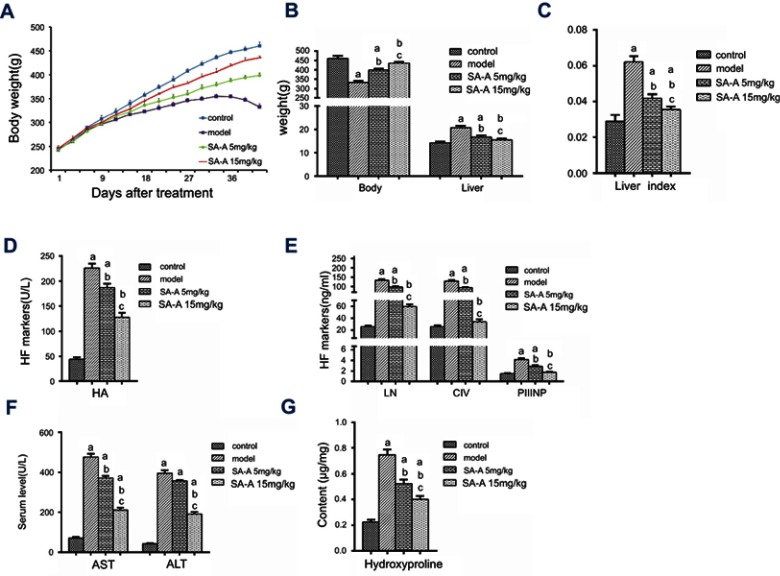
CCl4 was used to induce liver fibrosis in rats to assess the SA-A’s protecting effect and inhibition of chronic liver fibrosis. This rat model was used for histopathological as well as biochemical analysis. During our experiment, no death was recorded in the control group, 2 deaths were recorded in the model group, and 1 death in each SA-A group. Control group grew well, gaining weight faster than the remaining three other groups (P<0.05), while the model group began losing hair and appetite, and gained weight slower after 2 weeks. As for the SA-A groups, the low dose (5 mg/kg) group began losing hair, appetite and weight around 3–6 weeks. However, the rats still looked healthier than the model group but not as good as the control group. The high dose (15 mg/kg) group showed up significantly less symptoms than the model group, but weight gain was still slower than the control group, faster than the model and low dose SA-A groups (P<0.05, Figure 1A).
Figure 1.
(A) Body weight in the four groups after treatment. Effects of SA-A on (B) body weight, liver weight, (C) liver index, on serum level of (D) HA, (E) LN, CIV, PIIINP, (F) AST, ALT, and on the tissue content of (G) hydroxyproline. aP<0.05 as compared with control group, bP <0.05 as compared with model group, cP <0.05 as compared with SA-A 5 mg/kg group.
Abbreviations: ALT, alanineaminotransferase; AST, aspartateaminotransferase; CIV, Type IV collagen; HA, hyaluronicacid; LN, laminin; PIIINP, procollagen III N-terminal peptide; SA-A, salvianolic acid A.
After anatomical dissection, we found out that the liver tissues from the control group were soft, red, and smooth while the liver tissues from the model group had rough surfaces and hard texture. Compared to the model group, the high dose SA-A group had significantly better liver tissues. As mentioned in Figure 1B and C, livers in the model group gained more weight (P<0.05) and had increased liver index (P<0.05). However, with concomitant intraperitoneal injection of SA-A, nearly all these signs were reduced, especially in the high dose SA-A group.
SA-A alleviated liver injury as well as fibrosis after the use of CCL4 in rats
In medicine, the most popular index reflecting liver damage is the quantity of aminotransferase in blood.17 Hence during the in vivo experiment, blood was collected from every rat from the abdominal aorta to test for the liver enzymes that include ALT, AST and serum markers of liver fibrosis such as HA, CIV, LN and PIIINP. As mentioned in Figure 1D–F, the ALT, AST, HA, CIV, LN and PIIINP markers in the model group were all statistically significantly greater compared to the control group (P<0.05); while the SA-A group, specially the high dose group showed significantly lower levels of these indicators when compared with the model group.
According to these compared test results, we can conclude that SA-A can effectively reduce the advancement of the liver fibrosis in CCl4-induced rats, and there was dose dependency between SA-A and the treatment effect. This also proved that SA-A may be a useful drug to prevent liver damage.
Effect of SA-A on serum level of hydroxyproline
HYP is a unique metabolite of collagen in vivo. Liver fibrosis is caused by activation of quiescent HSCs and their trans-differentiation into myofibroblasts which then produce vast quantity of collagen fibers that increase HYP concentration.18 Hence detection of the concentration of the HYP in liver tissues can help diagnose liver fibrosis. As mentioned in Figure 1G, compared to the control group, the concentration of HYP in the model group was higher. However, in the SA-A group, especially the high dose group, the concentration of HYP was lower than the model group despite being a little higher than the control group. These results prove that SA-A can decrease liver fibrosis that was induced by CCl4 in rats.
Histological analysis after the treatment with SA-A in CCL4-induced liver fibrosis rats model
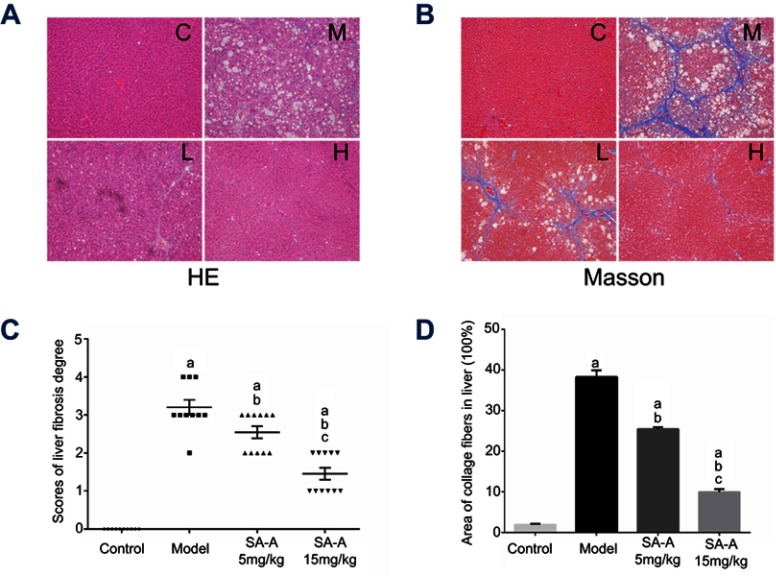
In order to determine the degree of liver damage and fibrosis in the rats due to the use of CCl4, H&E staining and Masson staining were used to detect the pathological state of the livers tissue. H&E staining and Masson staining are common staining methods in clinical pathology. Hematoxylin is a dye that binds to chromatin in the basophilic nucleus and appears blue, and Eosin binds to the protein in the cytoplasm and appears pink. Through this method, the degree of the liver damage and inflammation can be observed through the change of the structure of hepatic lobules. Moreover, the degree of the liver fibrosis can also be estimated by these methods. In Masson staining, the collagen fibers are colored blue while the muscle fibers are colored red, which can be used to visualize the grade of liver fibrosis accurately.
Combined results of H&E and Masson staining are represented in Figure 2. The degree of denaturation and necrosis of hepatocytes was increased obviously in the model group. Moreover, both infiltration of inflammatory cells and the distribution of collagen fibers were expanded extensively, resulting in the formation of typical pseudo-lobules in the model group. Compared to the situation in the model group, treatment with SA-A could ameliorate the degree of injuries and fibrogenesis obviously induced by CCl4. The high dose of SA-A significantly decreased inflammatory cell infiltration and collagen deposition (P<0.05). Our findings further illustrate that SA-A could efficiently control the liver fibrosis.
Figure 2.
SA-A protects against CCl4-induced liver fibrosis in rats. (A) H&E-staining of liver sections, magnification: ×100. (B) Masson-staining of liver sections, magnification: ×100. (C) The scores of the degree of liver fibrosis. (D) The area of collagen fibers in liver (%). Data are expressed as the mean ± S.D. aP<0.05 as compared with control (C) group, bP <0.05 as compared with model (M) group, cP <0.05 as compared with SA-A 5 mg/kg (L) group.
Abbreviations: H&E, Haematoxylin & eosin; SA-A, salvianolic acid A.
SA-A affected expression of ɑ-SMA in CCL4-induced liver fibrosis
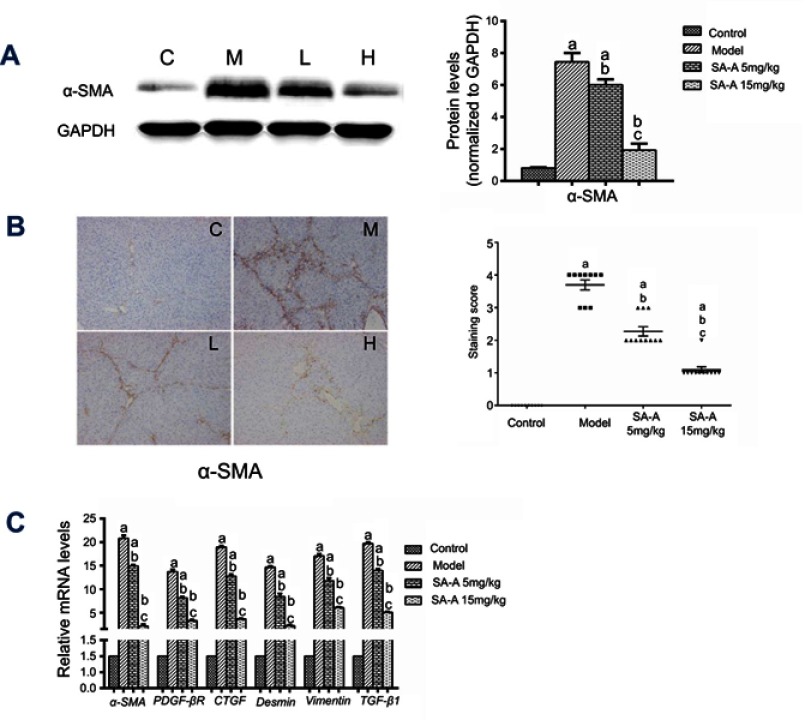
Alpha-SMA is an important marker of stimulated HSCs.19,20 In the liver, no other cells express intense cytoplasmic α-SMA immunoreactivity except the vascular smooth muscle cells and activated HSCs. Therefore, α-SMA expression can be used to identify activated HSCs that show myofibroblastic phenotype and the detection of the levels of α-SMA expression in liver tissues can be used to determine the extent of liver damage.
In this study, western blot analysis (Figure 3A) was used together with immunohistochemistry (Figure 3B) and qPCR (Figure 3C) to detect the α-SMA protein expression as well as its mRNA level in liver tissue samples. As mentioned in Figure 3A and C, the model group showed significant over-expression of α-SMA protein and mRNA levels compared to the control group (P<0.05), but when SA-A was used at a high dose, α-SMA protein expression as well as mRNA levels have clearly decreased (P<0.05). Figure 3B also confirmed these results. Meanwhile, we have also detected the gene expressions of inflammatory factors (TGF-β1) and other HSCs’ markers (Figure 3C) such as PDGF-βR, CTGF, Desmin and Vimentin which also showed that high dose of SA-A could effectively alleviate liver fibrosis in the liver fibrosis that is induced by CCl4 in rats. Even though the low dose SA-A was also effective but to a lesser extent.
Figure 3.
SA-A inhibits HSCs activation to prevent liver fibrosis. (A) Effects of SA-A on the expression of a-SMA in the liver tissues were measured by western blot analysis. (B) Immunohistochemistry staining of a-SMA in the liver tissues, magnification: ×100. (C) Relative mRNA levels of α-SMA, PDGF-βR, CTGF, Desmin, Vimentin, and TGF-β1. Data are expressed as the mean ± S.D.. aP<0.05 as compared with control (C) group, bP<0.05 as compared with model (M) group, cP<0.05 as compared with SA-A 5 mg/kg (L) group.
Abbreviations: HSCs, hepatic stellate cells; SA-A, salvianolic acid A.
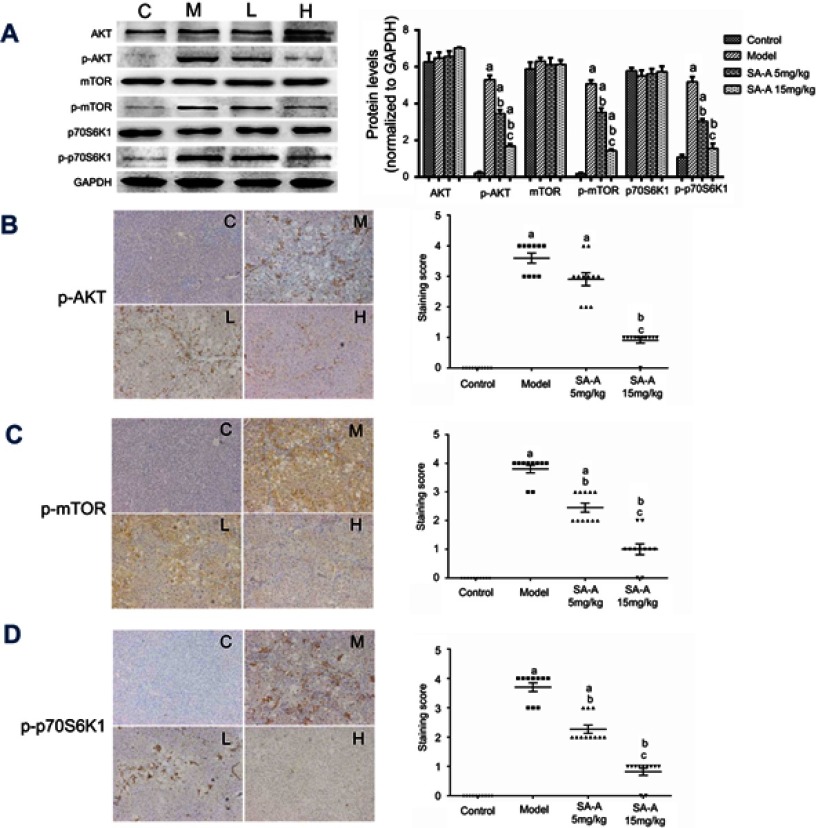
SA-A effectively inhibited the activation of PI3K/AKT/mTOR cell signaling mechanism in liver tissues of CCL4-induced liver fibrosis rats
From our previous study, we have found that PI3K/AKT/mTOR signaling cascade has a crucial role in activating HSCs which is a necessary step in liver fibrosis, so in this study western blot analysis (Figure 4A) and immunohistochemistry (Figure 4B–D) techniques were used to determine the expression of the activated AKT (p-AKT) as well as mTOR (p-mTOR) proteins along with the p-p70S6K1,21 which is a hallmark of stimulation by mTOR, in liver tissues. From the immunohistochemistry results, we found that the model group showed higher expression of p-AKT, p-mTOR and p-p70S6K1, and a lower expression in low dose SA-A group, especially in high dose SA-A group. The western blots showed that when comparing the SA-A groups to the CCl4-induced liver fibrosis rats group a statistically significant decrease was observed in the p-AKT, p-mTOR and p-p70S6K1 expressions in vivo specially in the high dose group which meant that SA-A can attenuate the stimulation of the PI3K/AKT/mTOR signaling mechanism, which then reduces the clinical symptoms of liver fibrosis.
Figure 4.
SA-A inhibits PI3K/AKT/mTOR signaling pathway in CCl4-induced liver fibrosis. (A) Effects of SA-A on the expression of p-AKT, AKT, p-mTOR, mTOR, p-p70S6K1, and p70S6K1 in the liver tissues were measured by western blot analysis. Immunohistochemistry of (B) p-AKT, (C) p-mTOR, and (D) p-p70S6K1 in the liver tissues, magnification: ×100. Data are expressed as the mean ± S.D. aP<0.05 as compared with control (C) group, bP<0.05 as compared with model (M) group, cP<0.05 as compared with SA-A 5mg/kg (L) group.
Abbreviations: CCl4, carbon tetrachlorid; SA-A, salvianolic acid A.
All these results confirmed that SA-A could effectively inhibit the progression of CCl4-induced fibrosis of liver by preventing the stimulation of the PI3K/AKT/mTOR signaling pathways.
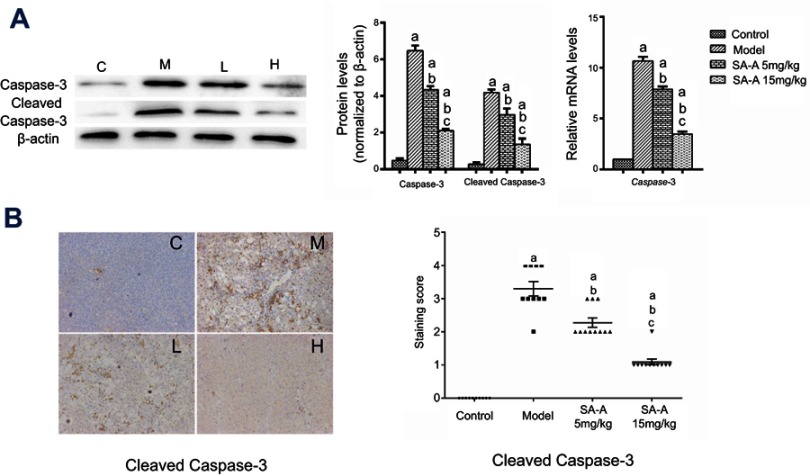
SA-A decreased CCL4-induced hepatocyte apoptosis
In order to detect the hepatocyte apoptosis in the CCl4-induced liver fibrosis, western blot analysis, real-time PCR and immunochemistry were used to analyze the cleaved-caspase 3 and caspase 3 expressions. Figure 5A and B showed that in CCl4-induced liver fibrosis model there was a higher caspase 3 and cleaved-caspase 3 expression, while in the high dose SA-A group there was nearly the same level as in the control group. The low dose SA-A group had lower expression levels than the CCl4 group, indicating that SA-A could protect the hepatocyte from apoptosis while the liver fibrosis accelerated cell apoptosis. Furthermore, in order to further understand the pathways of the anti-apoptotic effect of SA-A in liver fibrosis, the western blot analysis and immunohistochemistry techniques were used to determine the expression of Bcl-2 protein, an anti-apoptosis protein, as well as Bax protein which is a pro-apoptosis protein in vivo. As represented in Figure 6, Bcl-2 had lower protein expression while Bax had higher protein expression in the CCl4 group, and when SA-A was used, Bcl-2 expression was augmented and Bax expression was reduced, recovering the balance of Bcl-2/Bax ratio. All these results confirmed that SA-A decreased the hepatocyte apoptosis induced by CCl4-induced liver fibrosis.
Figure 5.
SA-A decreases CCl4-induced hepatocyte apoptosis. (A) Effects of SA-A on the expression of caspase-3 and cleaved caspase-3 in the liver tissues were measured by western blot analysis and real-time PCR; (B) Immunohistochemistry staining of caspase-3 and cleaved caspase-3 in the liver tissues, magnification: ×100. Data are expressed as the mean ± S.D. aP<0.05 as compared with control (C) group, bP<0.05 as compared with model (M) group, cP<0.05 as compared with SA-A 5 mg/kg (L) group.
Abbreviations: CCl4, carbon tetrachlorid; SA-A, salvianolic acid A.
Figure 6.
SA-A decreases CCl4-induced hepatocyte apoptosis. (A) Effects of SA-A on the expression of Bcl-2 and Bax in the liver tissues were measured by western blot analysis. Immunohistochemistry staining of (B) Bcl-2 and (C) Bax in the liver tissues, magnification: ×100. Data are expressed as the mean ± S.D. aP<0.05 as compared with control (C) group, bP<0.05 as compared with model (M) group, cP<0.05 as compared with SA-A 5mg/kg (L) group.
Abbreviations: CCl4, carbon tetrachlorid; SA-A, salvianolic acid A.
Discussion
Chronic liver diseases cause persistent or recurrent inflammation which leads to advanced fibrosis and lastly to cirrhosis.22,23 Patients suffering from cirrhosis have a high possibility of development of various complications such as liver failure, esophageal varices, encephalopathy, ascites, and hepatocellular carcinoma.24 All over the world, it is difficult to detect and effectively treat liver fibrosis.25 Recent research studies have been focusing on the assessment of various noninvasive procedures to evaluate the liver fibrosis. However, it is also essential to find a novel drug as a therapy for liver fibrosis. In the present study, we have identified a possible functioning drug extracted from traditional Chinese medicinal herb: SA-A. In this study, we collected the blood from every rat from their abdominal aorta to detect the liver enzymes such as ALT, AST; as well as serum markers of liver fibrosis such as HA, CIV, LN and PIIINP. In addition, H&E and Masson staining were implemented to detect the effect of SA-A on liver fibrosis. According to the results of our study, SA-A could reduce liver fibrosis by inhibiting liver function, liver fibrosis index, collagen deposition and improving the extent of liver fibrosis in the animals.
Stimulation of quiescent HSCs into contractile, fibrogenic, and proliferative cells after liver injury is still the main mechanism behind liver fibrosis, which makes HSC a major target as an anti-fibrotic treatment.26 On one hand, the activated HSCs are known to secrete TGF-β1 that is the most effective fibrogenetic factor, which induces accumulation of ECM and production of collagen.27,28 However, on the other hand, stimulated HSCs also hinder the stimulation of matrix metalloproteinases by up-regulating the tissue inhibitors metalloproteinases, and thus lead to a decrease of matrix degradation.29 Activated HSCs then transform into the myofibroblasts, which cause an increased expression of collagen fibers with an overexpression of HYP, which then overloads the liver’s capacity to function properly and finally developing into liver fibrosis. Thus, HYP’s concentration in liver tissue can be used as a marker to judge the extent of liver damage. Our novel findings also confirmed that SA-A could decrease the concentration of the HYP in CCl4-induced liver tissue in a dose-dependent manner, indicating that SA-A can reduce liver fibrosis.
Also, introduction of SA-A leads to substantial downregulation of α-SMA, a biomarker of the HSCs that is probably the outcome of decreased activation of HSCs. By detecting the α-SMA protein expression through the western blot technique and immunohistochemistry, we determined that SA-A reduced the α-SMA protein expression in vivo further confirming that SA-A could inhibit CCl4-induced liver fibrosis by preventing the activation of the HSCs.
Recently, multiple studies had reported that along with the HSC activation and proliferation and ECM synthesis, the PI3K/AKT/mTOR signaling cascade is important for the development of liver fibrosis.30 The whole process is activated by AKT signaling mechanism via PI3K that eventually causes activation of mTOR to increase the transcription of activation as well as proliferation-related genes.31 Combined with previous studies, we also found that SA-A inhibited HSCs stimulation as well as synthesis of ECM, by blocking the PI3K/AKT/mTOR signaling cascade. Moreover, in order to understand the mode of action of how SA-A activates HSC, we also determined if the PI3K/AKT/mTOR signaling cascade was hampered due to the treatment with SA-A. By immunohistochemically as well as using western blot analysis, we tried to explore the underlying molecular mechanisms. We found that SA-A effectively decreased the stimulation of the PI3K/AKT/mTOR signaling cascade in CCl4-induced liver fibrosis. These results revealed that SA-A can work as a prospective therapeutic drug to treat liver fibrosis by regulation of PI3K/AKT/mTOR signaling cascade.
Apart from all results above, we also tried to understand whether SA-A could affect apoptosis in CCl4-induced liver fibrosis model. Expressions of Bcl-2/Bax and caspase-3/cleaved caspase-3 proteins were tested in vivo using western blot analysis and immunohistochemistry. The results indicated that SA-A obviously augmented the expression of Bcl-2, and decreased the expression of Bax and caspase-3/cleaved caspase-3 when compared with CCl4-induced liver fibrosis, meaning that SA-A could effectively inhibit apoptosis induced by the fibrosis process.
To conclude, this study has reported that CCl4 induces liver fibrosis via the stimulation of PI3K/AKT/mTOR signaling cascade, CCl4 also increases protein expression of α-SMA and HYP, the biomarkers of the liver fibrosis, and can also increase the expression of the Bax as well as Caspase-3/cleaved-caspase-3 protein, reduce Bcl-2 protein expression which shows that CCl4 can induce liver cell apoptosis. However, when SA-A is used, especially at a high dose, SA-A can prevent these situations, the stimulation of PI3K/AKT/mTOR is inhibited, the expression of α-SMA, HYP and the protein Bax, caspase-3/cleaved caspase-3 all reduced while the expression of Bcl-2 increased. The findings from this novel study suggest that SA-A is a potential treatment option for liver fibrosis, and could be developed into an effective drug to treat this kind of disease.
Acknowledgments
This project was financially supported by the National Natural Science Foundation of China (81803815) and the Fund of Shanghai Jiao Tong University School of Medicine (TM201714).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
References
- 1.Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S60–S63. doi: 10.1016/j.clinre.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atta HM. Reversibility and heritability of liver fibrosis: implications for research and therapy. World J Gastroentero. 2015;21(17):5138–5148. doi: 10.3748/wjg.v21.i17.5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao YL, Ma X, Wang JB, et al. Curcumin protects against CCl4-induced liver fibrosis in rats by inhibiting HIF-1alpha through an ERK-dependent pathway. Molecules. 2014;19(11):18767–18780. doi: 10.3390/molecules191118767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuppan D. Liver fibrosis: common mechanisms and antifibrotic therapies. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S51–S59. doi: 10.1016/j.clinre.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 6.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22(7):512–518. doi: 10.1002/jhbp.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei LW, Chen QS, Guo AJ, Fan J, Wang R, Zhang H. Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol. 2018;60:1–8. doi: 10.1016/j.intimp.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Peng RQ, Wang SZ, Wang R, Wang YY, Wu Y, Yuan YF. Antifibrotic effects of tanshinol in experimental hepatic fibrosis by targeting PI3K/AKT/mTOR/p70S6K1 signaling pathways. Discov Med. 2017;23(125):81–94. [PubMed] [Google Scholar]
- 9.Wang YY, Wang R, Wang YJ, Peng RQ, Wu Y, Yuan YF. Ginkgo biloba extract mitigates liver fibrosis and apoptosis by regulating p38 MAPK, NF-κB/IκBɑ, and Bcl-2/Bax signaling. Drug Des Devel Ther. 2015;9:6303–6317. doi: 10.2147/DDDT.S93732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R, Zhang H, Wang YY, Song FX, Yuan YF. Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-κB/IκBɑ, p38 MAPK, and Bcl-2/Bax signaling. Int Immunopharmacol. 2017;47:126–133. doi: 10.1016/j.intimp.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Wang J, Song FX, Li SN, Yuan YF. Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBɑ signaling pathway. Drug Des Devel Ther. 2018;12:1281–1292. doi: 10.2147/DDDT.S159546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30(4):402–410. doi: 10.1055/s-0030-1267540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polo ML, Riggio M, May M, et al. Activation of PI3K/Akt/mTOR signaling in the tumor stroma drives endocrine therapy-dependent breast tumor regression. Oncotarget. 2015;6(26):22081–22097. doi: 10.18632/oncotarget.4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5(5):1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 15.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11(7):1946–1954. doi: 10.1039/c5mb00101c [DOI] [PubMed] [Google Scholar]
- 16.Oh KS, Oh BK, Mun J, Seo HW, Lee BH. Salvianolic acid A suppress lipopolysaccharide-induced NF-kappaB signaling pathway by targeting IKKbeta. Int Immunopharmacol. 2011;11(11):1901–1906. doi: 10.1016/j.intimp.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 17.Hassan S, Syed S, Kehar SI. Review of diagnostic techniques of hepatic fibrosis. J Pak Med Assoc. 2014;64(8):941–945. [PubMed] [Google Scholar]
- 18.Lee HS, Shun CT, Chiou LL, Chen CH, Huang GT, Sheu JC. Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: emphasis on sampling variability. J Gastroenterol Hepatol. 2005;20(7):1109–1114. doi: 10.1111/j.1440-1746.2005.03901.x [DOI] [PubMed] [Google Scholar]
- 19.Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143(4):1073–1083. doi: 10.1053/j.gastro.2012.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. doi: 10.1016/j.bpg.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–1656. doi: 10.2353/ajpath.2010.090885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787–799. doi: 10.1016/j.mcna.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol. 2016;13(7):402–411. doi: 10.1038/nrgastro.2016.86 [DOI] [PubMed] [Google Scholar]
- 24.Herrmann E, de Lédinghen V, Cassinotto C, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67(1):260–272. doi: 10.1002/hep.29179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–13056. doi: 10.1073/pnas.0804280105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31(5):1094–1106. doi: 10.1053/he.2000.6126 [DOI] [PubMed] [Google Scholar]
- 28.Galli A, Crabb DW, Ceni E, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122(7):1924–1940. doi:10.1053/gast.2002.33666 [DOI] [PubMed] [Google Scholar]
- 29.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urtasun R, Lopategi A, George J, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55(2):594–608. doi: 10.1002/hep.24701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664 [DOI] [PubMed] [Google Scholar]