Abstract
Purpose: To describe health care resource utilization (HCRU) and costs among patients with juvenile idiopathic arthritis (JIA) compared to patients without JIA and to describe treatment patterns among JIA patients who initiated biologic and non-biologic disease-modifying antirheumatic drugs (DMARDs).
Patients and methods: The IBM MarketScan® Commercial Database was used to identify patients aged 2–17 years with a new JIA diagnosis (index date) and 12 months continuous enrollment pre- and post-diagnosis from 2008 to 2016. JIA patients were matched to non-JIA patients on age, gender, region, and health plan type. Patients with other rheumatic or autoimmune conditions were excluded. Receipt of a biologic and/or non-biologic was evaluated on or after the new JIA diagnosis.
Results: A total of 3,815 JIA patients were matched to 11,535 non-JIA patients (mean age 10.0 [SD=4.5], 69% female). Average total costs were greater for JIA patients than non-JIA controls ($18,611 [SD=$42,104; median=$8,189] versus $2,203 [SD=$9,309; median=$649], p<0.001). Outpatient pharmacy costs were 33.6% of the total costs among JIA patients compared to 18.4% among non-JIA patients (p<0.001). The proportion of inpatient cost (11.4% versus 14.3%, p<0.001) and outpatient costs (55% versus 67.4%, p<0.001) of total costs was lower among JIA patients compared to non-JIA patients. Patients with 12 months of continuous enrollment post-treatment initiation (n=2,014) were classified as non-biologic only (n=734), biologic only (n=873), and both biologic and non-biologic (n=407) users. Among biologic and non-biologic users, 41.1% and 56.8% were persistent on their index medication for 12 months. Of patients treated with a biologic only, TNF inhibitors (TNFi) comprised 87.1% of the total treatment costs.
Conclusion: JIA is associated with increased costs and utilization in every HCRU category compared to matched non-JIA patients. While JIA-related costs varied by treatment cohort, patients on biologic DMARDs had substantially higher costs than patients on non-biologic DMARDs and fewer than one-half were persistent at 12 months after biologic initiation.
Keywords: juvenile arthritis, antirheumatic agent, medication adherence, health expenditures, administrative claims
Introduction
Juvenile idiopathic arthritis (JIA) is a diagnosis of exclusion for patients under the age of 16 with arthritis of unknown etiology lasting more than 6 weeks.1 The International League of Associations for Rheumatology has proposed seven subtypes of JIA, including systemic, oligoarticular, and polyarticular, with a combined prevalence of between 16 and 400 per 100,000.2–4 These conditions are heterogeneous in presentation and severity with complications ranging from life-threatening (macrophage activation syndrome), to disabling (visual impairment from uveitis, skeletal deformities, and chronic pain), to mild (nail pitting).1
JIA is characterized by swelling, inflammation, pain, and stiffness of the joints, and the initial goals of treatment are to reduce inflammation, prevent joint damage, and relieve pain, while longer-term objectives are complete remission and resolution of disease activity.5–7 Pharmaceutical treatments for JIA include nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids (systemic or intra-articular), and disease-modifying antirheumatic drugs (DMARDs).8 DMARDs can be biologic (ie, created by biologic processes) or non-biologic (ie, manufactured by chemical processes) and work by disrupting the development or functioning of immune cells.9
Historically, NSAIDs have been the first-line treatment with intra-articular steroid injections used to control flares of joint inflammation and prevent deformities. The non-biologic DMARD methotrexate has been the mainstay second-line agent for JIA not controlled by NSAIDs since the 1980s; however, newer targeted biologic DMARDs, such as abatacept, have generated optimism that treatment will lead to improved outcomes especially for children who cannot tolerate or are refractory to methotrexate.10–13 Even with treatment, 40–60% of the JIA patients continue to experience disease activity, joint destruction, suboptimal function, and impaired quality of life extending into adulthood.14,15
JIA is associated with high direct and indirect costs both at onset and into adulthood.16,17 Direct costs are highest among patients with more severe disease and greater disability.18,19 Although pharmacy costs account for 40–50% of the total health care costs in these studies, there is evidence that treatment with biologics can reduce the long-term complications and societal burden of JIA.20,21 In a follow-up study of adults with a childhood diagnosis of JIA, patients in remission had health care costs sevenfold less than those with active disease.15
No prior study has attempted to assess and compare the burden of JIA among insured patients and matched controls in terms of health care utilization and costs. The primary objective of this study was to compare the economic burden (including health care resource utilization [HCRU], all-cause costs, and JIA-related costs) between patients with JIA and matched non-JIA controls. An additional subanalysis was performed to describe treatment patterns, treatment prevalence, and economic outcomes among JIA patients who initiated biologic and non-biologic DMARDs.
Material and methods
Study design and data source
This retrospective, matched cohort study analyzed the demographics, treatment patterns, HCRU, and costs of JIA patients and matched controls. Treatment patterns, treatment prevalence, and economic outcome variables were also assessed based on treatment subcohorts of JIA patients who initiated biologic and non-biologic DMARDs. This analysis was performed using patient-level administrative claims data extracted from the MarketScan® Commercial Claims and Encounters Database covering April 1, 2007–December 31, 2016. The MarketScan Commercial database contains 137.6 million lives from 1995 to 2016, including 24.4 million in the 2016 database, encompassing employees, their spouses, and dependents who are covered by employer-sponsored private health insurance. Medical claims are linked to outpatient prescription drug claims and person-level enrollment information. The data that support the findings of this study are available from IBM Watson Health. Restrictions apply to the availability of these data, which were used under license for this study.
Patient selection and cohort assignment
Patients aged 2–17 years were eligible for inclusion in the JIA cohort if, between April 1, 2008, and December 31, 2016, they had either ≥2 distinct non-diagnostic medical claims (occurring at least 7 but not more than 183 days apart) with a diagnosis code for JIA or ≥1 non-diagnostic medical claim with a diagnosis code for JIA followed by a claim for a biologic DMARD (abatacept, adalimumab, etanercept, infliximab, anakinra, tocilizumab, or intravenous immunoglobulin [IVIG]) within 183 days. The remaining patients aged 2–17 years between April 1, 2008, and December 1, 2016, were eligible for the control cohort if they did not have any medical claims with a diagnosis code for JIA or pharmacy claims for DMARDs. The index date of patients in the JIA cohort was the date of the first observed medical claim with a diagnosis code for JIA. The index date of patients in the control cohort was randomly assigned to match the index date distribution of the qualified JIA cohort.
In addition, all eligible patients were aged 2–17 as of the index date and had continuous enrollment in medical and pharmacy benefits for 12 months before (baseline period) and 12 months after (follow-up period) the index date. Patients with evidence of other rheumatic disease (systemic lupus erythematosus and other diffuse connective tissue diseases, vasculitis, and sarcoidosis) or non-JIA autoimmune conditions (chronic lymphocytic leukemia, Crohn’s disease, non-Hodgkin’s lymphoma, plaque psoriasis, polyarteritis nodosa, ulcerative colitis, or Wegener’s granulomatosis) during in the baseline period were excluded from the study. Patients with evidence of two or more biologics on the index date were also excluded. After attrition, control patients were randomly selected without replacement and matched 3:1 to JIA patients based on age, gender, region, and insurance type.
For the sub-analysis, patients in the JIA cohort were assigned to treatment subcohorts based on DMARD utilization and available enrollment data. Each JIA patient record was examined for evidence of ≥1 pharmacy or medical claim for a biologic DMARD (including abatacept, adalimumab, etanercept, infliximab, anakinra, tocilizumab, and intravenous immunoglobulin [IVIG]) or non-biologic DMARD (methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, azathioprine, penicillamine, and thalidomide). The date of the first pharmacy or medical claim for a DMARD was defined as the treatment start date. Patients were considered eligible non-biologic or biologic DMARD users if they had continuous enrollment with medical and pharmacy benefits from the index date through 12 months after the treatment start date. Patients with evidence of DMARD utilization and available enrollment data were assigned to one of three mutually exclusive treatment subcohorts (biologic only users, biologic and non-biologic users, non-biologic only users). The remaining JIA patients were assigned to the other JIA cohort if they did not have any medical claims with a diagnosis code for JIA or pharmacy claims for DMARDs.
Outcomes
Demographic characteristics were measured on the index date and included age, age group, sex, geographic region, and health plan type. Clinical characteristics were measured during the 12-month baseline period and included Deyo-Charlson Comorbidity Index (DCCI), comorbid conditions (attention-deficit hyperactivity disorder [ADHD], anxiety, asthma, depression, diabetes), and concomitant medications (ADHD medications, anti-depressants, anti-diabetic agents, anxiolytics, corticosteroids, NSAIDs, opioids).
For all JIA patients and matched controls, total all-cause and JIA-related costs and HCRU, including inpatient admissions, emergency room (ER) visits, and outpatient services such as physician office visits, laboratory tests, radiology exams, and prescription fills, were evaluated over the 12-month follow-up period. Costs were inflated to 2017 dollars using the Medical Care Component of the Consumer Price Index.22 For patients with biologic use, the outpatient DMARD treatment costs were measured, covering both the cost of the pharmaceutical agent and the cost of administration, in the 12 months following the treatment start date. JIA-related claims were identified by any of the following: (1) ≥1 non-diagnostic medical claim with JIA in any position on an outpatient claim; (2) ≥1 inpatient claim with JIA as the primary diagnosis; (3) ≥1 pharmacy claim for a biologic or non-biologic DMARD.
Treatment patterns for all patients in the three treatment subcohorts were assessed for the 12-month period following the treatment start date. Measured treatment characteristics included the proportion of patients prescribed each DMARD subtype (biologic or non-biologic) agent, the proportion of patients prescribed each drug as their first DMARD agent, and the time from JIA diagnosis to treatment start date. Also measured during the treatment follow-up period were adherence (a medication possession ratio ≥0.80), discontinuation (a gap of more than 60 days or more in the index therapy following the run-out of the previous days’ supply or duration of clinical benefit), and switching within class (discontinuation of the index DMARD and subsequent initiation of a different DMARD of the same class).
Statistical analysis
The mean and standard deviation (SD) were reported for all continuous variables while frequencies and percentages were reported for categorical variables. All data analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC). Multivariate analysis was used to examine primary HCRU and cost outcomes while controlling for baseline demographic and clinical characteristics. The outcomes analyzed in separate generalized linear models included total health care costs, inpatient admissions, cost of inpatient admission, ER visits, cost of ER visits, office visits, cost of office visits, cost of other outpatient services, number of outpatient prescription fills, and cost of outpatient prescriptions. Cost models used a log link and gamma error distribution. Logistic regression was used to model odds of an event occurring, and Poisson regression was used for count models. The main explanatory item of interest was patient cohort (JIA cohort [all, biologic only, biologic and non-biologic, non-biologic only, other] vs Non-JIA control). Each model adjusted for age, sex, geographic region, health plan, urbanicity, index year, DCCI, comorbid conditions, and concomitant medications.
Results
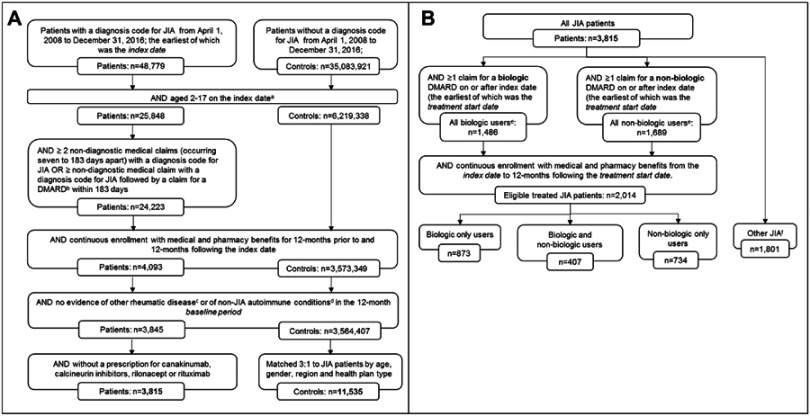
A total of 3,815 JIA patients were identified and matched to 11,535 non-JIA control patients (Figure 1A). Among the JIA patients, 2,014 patients were eligible for inclusion in 1 of the 3 DMARD treatment subcohorts: 873 (43.4%) biologic only users, 407 (20.2%) biologic and non-biologic users, and 734 (36.4%) non-biologic only users (Figure 1B).
Figure 1.
Patient attrition flow charts for (A) JIA cases and matched controls and (B) identification of JIA DMARD treatment subcohorts.
Notes: aControl patients were randomly assigned an index date maintaining the index date distribution of the qualified JIA cohort. bCannot have >1 biologic DMARD on the same day that could be considered as an index DMARD claim. cSystemic lupus erythematosus and other connective tissue disease, vasculitis, and sarcoidosis. dChronic lymphocytic leukemia, Crohn’s disease, polyarteritis nodosa, Non-Hodgkin’s lymphoma, plaque psoriasis, ulcerative colitis, or Wegener’s granulomatosis. ePatients may qualify as both biologic and non-biologic users. fComprised of all JIA patients not included in the 3 treatment subcohorts.
Abbreviations: DMARD, disease-modifying antirheumatic drugs; JIA, juvenile idiopathic arthritis.
JIA patients and their matched controls had an average age of 10.0±4.5 years, and 69% were female. The frequency of all examined comorbid conditions and usage of concomitant medication was higher in the JIA cohort compared the control cohort in the baseline period (Table 1). The overall frequency of all baseline comorbidities was below 10% with asthma being the most common (8.9% JIA vs 6.3% controls) followed by anxiety (3.6% JIA vs 1.6% controls) and ADHD (3.5% JIA vs 2.6% controls). JIA patients were nearly 12 times more likely to use NSAIDs (75.1% JIA vs 6.3% controls) and over 2 times more likely to use corticosteroids (43.9% JIA vs 16.0% controls) or opioids (23.6% JIA vs 11.7% controls) in the baseline period compared to control patients (Table 1).
Table 1.
Baseline demographic and clinical characteristics
| All JIA patients | Treatment subcohorts | Matched controls | ||||
|---|---|---|---|---|---|---|
| Biologic only | Biologic & non-biologic | Non-biologic only | Other JIA casesa | |||
| n=3,815 | n=873 | n=407 | n=734 | n=1,801 | n=11,535 | |
| Age, mean (SD) | 10 (4.5) | 11 (4.5) | 12 (3.8) | 11 (4.2) | 9 (4.6) | 10 (4.5) |
| Age group, years, n (%) | ||||||
| 2–5 | 740 (19.4%) | 127 (14.6%) | 28 (6.9%) | 100 (13.6%) | 485 (26.9%) | 2,235 (19.4%) |
| 6–10 | 981 (25.7%) | 194 (22.2%) | 103 (25.3%) | 178 (24.3%) | 506 (28.1%) | 2,970 (25.8%) |
| 11–15 | 1,543 (40.5%) | 371 (42.5%) | 203 (49.9%) | 340 (46.3%) | 629 (34.9%) | 4,653 (40.3%) |
| 16–17 | 551 (14.4%) | 181 (20.7%) | 73 (17.9%) | 116 (15.8%) | 181 (10.1%) | 1,677 (14.5%) |
| Female, n (%) | 2,631 (69.0%) | 616 (70.6%) | 278 (68.3%) | 539 (73.4%) | 1,198 (66.5%) | 7,956 (69.0%) |
| Health plan type, n (%) | ||||||
| Comprehensive/Indemnity | 55 (1.4%) | 16 (1.8%) | 2 (0.5%) | 13 (1.8%) | 24 (1.3%) | 165 (1.4%) |
| EPO/PPO | 2,261 (59.3%) | 513 (58.8%) | 227 (55.8%) | 422 (57.5%) | 1,099 (61.0%) | 6,837 (59.3%) |
| POS/POS w/capitation | 344 (9.0%) | 78 (8.9%) | 38 (9.3%) | 72 (9.8%) | 156 (8.7%) | 1,041 (9.0%) |
| HMO | 661 (17.3%) | 151 (17.3%) | 81 (19.9%) | 146 (19.9%) | 283 (15.7%) | 1,992 (17.3%) |
| CDHP/HDHP | 381 (10.0%) | 92 (10.5%) | 49 (12.0%) | 62 (8.5%) | 178 (9.9%) | 1,158 (10.0%) |
| Other/Unknown | 113 (3.0%) | 23 (2.6%) | 10 (2.5%) | 19 (2.6%) | 61 (3.4%) | 342 (3.0%) |
| Region, n (%) | ||||||
| Northeast | 714 (18.7%) | 140 (16.0%) | 76 (18.7%) | 118 (16.1%) | 380 (21.1%) | 2,151 (18.7%) |
| North Central | 1,021 (26.8%) | 199 (22.8%) | 118 (29.0%) | 251 (34.2%) | 453 (25.2%) | 3,087 (26.8%) |
| South | 1,239 (32.5%) | 341 (39.1%) | 113 (27.8%) | 215 (29.3%) | 570 (31.7%) | 3,741 (32.4%) |
| West | 775 (20.3%) | 178 (20.4%) | 93 (22.9%) | 136 (18.5%) | 368 (20.4%) | 2,358 (20.4%) |
| Unknown | 66 (1.7%) | 15 (1.7%) | 7 (1.7%) | 14 (1.9%) | 30 (1.7%) | 198 (1.7%) |
| DCCI, mean (SD) | 0.23 (0.49) | 0.30 (0.59) | 0.27 (0.52) | 0.24 (0.50) | 0.18 (0.42) | 0.08 (0.31) |
| Comorbid conditions, n (%) | ||||||
| ADHD | 133 (3.5%) | 32 (3.7%) | 17 (4.2%) | 21 (2.9%) | 63 (3.5%) | 299 (2.6%) |
| Anxiety | 138 (3.6%) | 37 (4.2%) | 19 (4.7%) | 22 (3.0%) | 60 (3.3%) | 180 (1.6%) |
| Asthma | 341 (8.9%) | 83 (9.5%) | 42 (10.3%) | 61 (8.3%) | 155 (8.6%) | 722 (6.3%) |
| Depression | 85 (2.2%) | 33 (3.8%) | 14 (3.4%) | 14 (1.9%) | 24 (1.3%) | 181 (1.6%) |
| Diabetes | 35 (0.9%) | 11 (1.3%) | 4 (1.0%) | 6 (0.8%) | 14 (0.8%) | 38 (0.3%) |
| Concomitant medications, n (%) | ||||||
| ADHD Medications | 234 (6.1%) | 57 (6.5%) | 27 (6.6%) | 47 (6.4%) | 103 (5.7%) | 602 (5.2%) |
| Anti-depressants | 307 (8.1%) | 97 (11.1%) | 52 (12.8%) | 55 (7.5%) | 103 (5.7%) | 460 (4.0%) |
| Anti-diabetic agents | 48 (1.3%) | 14 (1.6%) | 5 (1.2%) | 11 (1.5%) | 18 (1.0%) | 61 (0.5%) |
| Anxiolytics | 296 (7.8%) | 93 (10.7%) | 43 (10.6%) | 57 (7.8%) | 103 (5.7%) | 372 (3.2%) |
| Corticosteroids | 1,675 (43.9%) | 477 (54.6%) | 246 (60.4%) | 358 (48.8%) | 594 (33%) | 1,843 (16.0%) |
| NSAIDs | 2,864 (75.1%) | 608 (69.6%) | 347 (85.3%) | 609 (83.0%) | 1,300 (72.2%) | 721 (6.3%) |
| Opioids | 901 (23.6%) | 251 (28.8%) | 118 (29.0%) | 180 (24.5%) | 352 (19.5%) | 1,345 (11.7%) |
Note: aOther JIA subcohort contains all JIA patients not included in the treatment subcohorts.
Abbreviations: ADHD, attention-deficit hyperactivity disorder; CDHP, consumer-driven health plan; DCCI, Deyo-Charlson comorbidity index; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; JIA, juvenile idiopathic arthritis; NSAIDs, nonsteroidal anti-inflammatory drugs; POS, point of service; PPO, preferred provider organization; SD, standard deviation.
Patients treated with DMARDs were more likely than the average JIA patient to be 11 or older (63.8% treated vs 54.9% all JIA). Among DMARD treated patients, the frequency of asthma, anxiety, and ADHD in the baseline period were highest in the subcohort who received both biologic and non-biologic DMARDs: 10.3%, 4.7%, and 4.2%, respectively. This subcohort also had the highest baseline utilization of NSAIDs (85.3%), corticosteroids (60.4%), and opioids (29.0%) (Table 1). For patients who received both a biologic and non-biologic, the time from index date until treatment initiation was 11 months shorter for non-biologic use compared to biologics.
Patients were more likely to be adherent to biologics (46.6% biologics vs 33.2% non-biologics) yet more likely to be persistent with non-biologics (41.1% biologics vs 56.8% non-biologics) (Table 2). Within class, switching was common, with 11.7% of the non-biologic users switching to an alternate non-biologic within the first year and 14.9% of the biologic users switching to an alternate biologic. Among patients treated with DMARDs, the tumor necrosis factor inhibitors (TNFi) etanercept (58.4% biologic only and 62.7% biologic and non-biologic) and adalimumab (21.0% biologic only and 25.7% biologic and non-biologic) were the most common first biologic agents used (Table 3). Methotrexate was the most common first non-biologic agent used (68.8% non-biologic only and 82.6% biologic and non-biologic).
Table 2.
DMARD treatment patterns
| All biologic users | All non-biologic usersa | |
|---|---|---|
| n=1,280 | n=1,124 | |
| Adherence, n (%) | 597 (46.6%) | 373 (33.2%) |
| Persistence, n (%) | 526 (41.1%) | 638 (56.8%) |
| Switchingb within class, n (%) | 191 (14.9%) | 132 (11.7%) |
| Months to DMARD initiation,c mean (SD) | 15.4 (18.3) | 4.5 (10.2) |
Notes: aSeventeen patients who received non-biologics had multiple drugs on their non-biologic treatment start date and were excluded from the treatment pattern analysis. bConverting route of administration did not constitute a switch in therapy. cNumber of months between index date and treatment start date for patients who received both biologic and non-biologic DMARDs.
Abbreviations: DMARD, disease modifying anti-rheumatic drug; SD, standard deviation.
Table 3.
First DMARD used by each treatment subcohort
| Biologic only users | Biologic & non-biologic users | Non-biologic only users | All treated patients | |
|---|---|---|---|---|
| n=873 | n=407 | n=734 | n=2,014 | |
| Biologics, n (%) | ||||
| Abatacept | 15 (1.7%) | 6 (1.5%) | - | 21 (1.0%) |
| Adalimumab | 188 (21.0%) | 106 (25.7%) | - | 294 (14.4%) |
| Anakinra | 43 (4.8%) | 9 (2.2%) | - | 52 (2.5%) |
| Etanercept | 523 (58.4%) | 259 (62.7%) | - | 782 (38.3%) |
| Infliximab | 71 (7.9%) | 20 (4.8%) | - | 91 (4.5%) |
| Intravenous immunoglobulin | 17 (1.9%) | 1 (0.2%) | - | 18 (0.9%) |
| Tocilizumab | 16 (1.8%) | 6 (1.5%) | - | 22 (1.1%) |
| Non-biologics, n (%) | ||||
| Hydroxychloroquine | - | 31 (7.5%) | 149 (20.2%) | 180 (8.8%) |
| Methotrexate | - | 341 (82.6%) | 506 (68.8%) | 847 (41.4%) |
| Sulfasalazine | - | 42 (10.2%) | 75 (10.2%) | 117 (5.7%) |
| Other (azathioprine, leflunomide) | - | 5 (1.2%) | 15 (2.0%) | 20 (1.0%) |
Abbreviation: DMARD, disease-modifying anti-rheumatic drug.
Compared to matched controls, JIA patients were more likely to have at least one inpatient admission (4.6% vs 1.5%) and were more likely to have at least one emergency room visit (26.3% vs 16.2%). JIA patients also had on average (mean [SD]) more outpatient physician office visits (10.8 [6.6] vs 3.1 [3.2]) and more outpatient prescriptions (19.0 [17.8] vs 3.7 [6.2]) than matched controls. Among JIA patients, the mean (SD) number of JIA-related outpatient office visits was 5.0 (4.4) in biologic-only users, 6.0 (3.8) in biologic and non-biologic users, and 4.9 (3.2) in non-biologic only users. Additionally, the mean (SD) number of JIA-related outpatient prescriptions was 8.4 (5.9) in biologic-only users, 10.1 (6.9) in biologic and non-biologic users, and 5.9 (5.1) in non-biologic-only users (Table 4).
Table 4.
All-cause and JIA-related health care resource utilization
| All JIA patients |
Treatment subcohorts | Controls | ||||
|---|---|---|---|---|---|---|
| Biologic only users | Biologic & non-biologic users | Non-biologic only users | Other JIA cases | |||
| n=3,815 | n=873 | n=407 | n=734 | n=1,801 | n=11,535 | |
| All-cause health care utilization | ||||||
| Patients with inpatient admission, n (%) | 177 (4.6%) | 72 (8.3%) | 15 (3.7%) | 25 (3.4%) | 65 (3.6%) | 167 (1.5%) |
| Patients with emergency room visit, n (%) | 1,003 (26.3%) | 265 (30.4%) | 104 (25.6%) |
174 (23.7%) | 460 (25.5%) | 1,871 (16.2%) |
| Average office visits per patient, mean (SD) | 10.8 (6.6) | 12.1 (7.4) | 12.8 (7.2) | 10.7 (6.0) | 9.7 (6.0) | 3.1 (3.2) |
| Average outpatient prescriptions per patient, mean (SD) | 19.0 (17.8) | 26.5 (21.1) | 30.8 (19.8) | 22.9 (15.3) | 11.2 (11.9) | 3.7 (6.2) |
| JIA-related health care utilization | ||||||
| Patients with inpatient admission, n (%) | 25 (0.7%) | 15 (1.7%) | 2 (0.5%) | 5 (0.7%) | 3 (0.2%) | n/a |
| Patients with emergency room visit, n (%) | 213 (5.6%) | 61 (7.0%) | 24 (5.9%) | 48 (6.5%) | 80 (4.4%) | n/a |
| Average office visits per patient, mean (SD) | 4.6 (3.5) | 5.0 (4.4) | 6.0 (3.8) | 4.9 (3.2) | 4.0 (2.9) | n/a |
| Average outpatient prescriptions per patient, mean (SD) | 4.3 (5.9) | 8.4 (5.9) | 10.1 (6.9) | 5.9 (5.1) | 0.3 (1.7) | n/a |
Abbreviations: JIA, juvenile idiopathic arthritis; n/a, not applicable; SD, standard deviation.
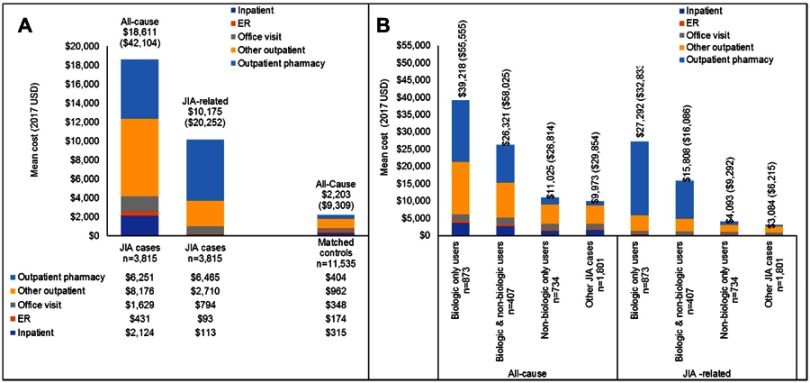
Total unadjusted all-cause health care costs (mean [SD]) were over eight times higher for JIA patients ($18,611 [$42,104], median=$8,189) compared with matched controls ($2,203 [$9,309], median=$649) (Figure 2A). JIA-related costs made up over half of the total all-cause costs for JIA patients ($10,175 [$20,252], median=$2,656). In the 12 months following the index date, the biologic only subcohort had the highest all-cause and JIA-related costs of all the treatment subcohorts (Figure 2B). For this subcohort, outpatient pharmacy costs comprised 45% of the mean all-cause costs and 79% of the mean JIA-related costs. In the 12 months following treatment initiation, the mean annual unadjusted all-cause health care costs for the biologic only cohort ranged from $17,083 to $48,048 depending on index biologic agent (Table 5). Of the 19.4 million dollars spent on the treatment of these 873 biologic-only patients, 87.1% was spent on TNFis.
Figure 2.
Annual unadjusted all-cause and JIA-related health care costs for (A) JIA patients and matched controls and (B) treatment subcohorts.
Note: Numbers in brackets above the bars represent the standard deviation values.
Abbreviations: ER, emergency room; JIA, juvenile idiopathic arthritis.
Table 5.
Treatment costs for the 12 months post-treatment initiation with a biologic DMARD
| Category | First agent used | n | Mean | Median | Cumulative costs | % of cumulative costs | Total % by category |
|---|---|---|---|---|---|---|---|
| TNFi | Adalimumab | 188 | $26,938 | $15,495 | $5,064,410 | 26.1% | 87.1% |
| Etanercept | 523 | $17,083 | $15,545 | $8,934,571 | 46.0% | ||
| Infliximab | 71 | $41,136 | $26,236 | $2,920,667 | 15.0% | ||
| Non-TNFi | Abatacept | 15 | $21,825 | $17,129 | $327,371 | 1.7% | 8.7% |
| Anakinra | 43 | $20,523 | $13,349 | $882,504 | 4.5% | ||
| Tocilizumab | 16 | $30,175 | $20,269 | $482,795 | 2.5% | ||
| Other | Intravenous immunoglobulin | 17 | $48,048 | $36,297 | $816,818 | 4.2% | 4.2% |
| Totals | Biologic only users | 873 | $19,429,134 |
Abbreviations: DMARD, disease modifying anti-rheumatic drug; TNFi, tumor necrosis factor inhibitor.
After adjusting for covariates, JIA patients were 1.69 times more likely to have an inpatient admission (p=0.0012), had 2.62 times more outpatient physician office visits (p<0.0001), and had 3.24 times more outpatient prescription fills (p<0.0001) than patients without JIA (Table 6). JIA patients total all-cause costs were 6.58 times higher, inpatient costs were 5.39 times higher, emergency room costs were 1.27 times higher, office visit costs were 3.44 times higher, other outpatient services costs were 6.54 times higher, and outpatient prescription costs were 16.19 times higher than matched control patients (all p<0.0001).
Table 6.
Estimates of annual health care resource utilization and costs for JIA patients vs matched controls
| JIA cohort vs matched controls | Point estimator | Lower 95% CL | Upper 95% CL | P-value |
|---|---|---|---|---|
| Total all-cause costs | CR=6.58 | 6.17 | 7.02 | <0.0001 |
| Inpatient costs | CR=5.39 | 4.64 | 6.27 | <0.0001 |
| Probability of inpatient admission | OR =1.69 | 1.23 | 2.33 | 0.0012 |
| Emergency room costs | CR=1.27 | 1.13 | 1.43 | <0.0001 |
| Probability of emergency room visit | OR =1.07 | 0.93 | 1.22 | 0.3376 |
| Office visit costs | CR=3.44 | 3.24 | 3.64 | <0.0001 |
| Number of office visits | VR =2.62 | 2.53 | 2.71 | <0.0001 |
| Other outpatient services costsa | CR=6.54 | 6.06 | 7.06 | <0.0001 |
| Outpatient prescription costs | CR=16.19 | 14.82 | 17.70 | <0.0001 |
| Outpatient prescription fills | FR =3.24 | 3.06 | 3.43 | <0.0001 |
Notes: Adjusted for age, sex, geographic region, health plan, urbanicity, index year, DCCI, comorbid conditions, and concomitant medications; In all cases, ratios were calculated as [JIA]/[controls]; aOther outpatient services consist of laboratory, radiology, and hospital outpatient facility costs.
Abbreviations: CL, confidence level; CR, cost ratio; OR, odds ratio; FR, prescription fill ratio; VR, visits ratio.
Discussion
This analysis used a large, geographically diverse commercial insurance database that includes data from over 200 self-insured employers or health plans making it more generalizable than an analysis from only a single health plan or employer. Despite the fact that JIA is a rare condition, the size of the underlying database allowed for subcohort analysis as well as robust estimates of the study outcomes due to larger sample sizes than reported in prior studies. To our knowledge, no prior studies have compared treatment patterns, HCRU and costs among insured JIA patients matched to non-JIA patients. Thus, this study fills a gap in the literature assessing the burden of JIA. In the 12 months following diagnosis, patients with JIA incurred substantially higher costs compared to matched non-JIA patients. This was evident in every HCRU category, and the high annual expenditures were driven by outpatient services, such as diagnostic services and outpatient pharmacy costs. Initial JIA treatment is predominately methotrexate, etanercept, and adalimumab, with the majority of biologic costs coming from TNFis. Among patients using only biologics, patients on TNFis accounted for 87% of the annual biologic spending. In addition, receipt of other supportive medications (corticosteroids and NSAIDs) and medication for pain management (opioids) was common among JIA patients.
Only 47% of the biologic users and 33% of the non-biologic users were adherent to their medication regime in the first year following initiation. The limited existing literature relies on self-reported compliance and reports adherence rates of 76−92% depending on country of residence.23,24 Self-reported adherence is often higher than claims-based measurements because of biases in both methods.25 The low persistence observed in this study suggests that patients do not stay on one therapy very long with the majority having a significant or complete gap in therapy within the first year of initiation. Because of this, therapies with different mechanism of action MOAs should be considered for patients, particularly those who either do not respond to or cannot tolerate a TNFi. Further research is warranted to determine factors associated with improved adherence or persistence among JIA patients taking into account MOA.
Though rare, JIA is a high-cost disease state. In this study, JIA patients had significantly higher all-cause, inpatient, emergency room, office visit, and outpatient prescription costs compared to matched non-JIA patients. The average unadjusted cost for all JIA patients was $18,611 with greater HCRU in every category. This is consistent with recent findings on the cost of JIA in the European Union which reported annual direct health care costs ranging from €11,068 to €22,138 ($13,044 to $26,090) depending on country of treatment.26 After adjusting for covariates, JIA patients had 6.58 times higher total health care costs than healthy controls and spent 16.19 times more on outpatient prescriptions.
In the analysis, the overwhelming majority of treatment costs were from TNFi use. High disease and treatment costs paired with low treatment adherence and persistence at one year suggest a need for employing a broader range of treatment options. During the 9 years covered by this study and beyond, the biologic treatment options for JIA have expanded, and several biosimilars to existing drugs have been released.27 Early, aggressive treatment, including first-line use of biologics, is now the recommended therapeutic approach with the objective of achieving full disease remission.28 Payers should consider incorporating JIA treatment outcomes into formulary decisions particularly due to the poor treatment patterns and cost outcomes associated with the current predominant therapies. While biologic JIA treatment is driven by etanercept and adalimumab, patients may benefit from utilizing other biologics, including abatacept.
Limitations
There are several limitations associated with this study. First, as administrative claims data are not collected for research purposes, the data are more prone to miscoding or undercoding compared to data collected for clinical trials. Second, this study used data from commercially insured patients, and the findings may not be generalizable to the uninsured or those with other insurance. Third, this analysis was limited to patients with 24 months of continuous enrollment, and the results may not be applicable to patients with less stable health insurance coverage. Fourth, even after adjusting for available baseline characteristics the analysis may be subject to residual confounding variables such as disease severity and duration. To mitigate the impact of these confounding variables, we used DCCI, comorbid conditions, and concomitant medication usage as proxy measures of overall health and disease severity when constructing the multivariate models. Fifth, claims data only identifies that a medication was dispensed and not that the medication was administered or taken as prescribed. Additionally, medication dispensed without the filing of an insurance claim would not have been recorded in the dataset; however, non-filing is unlikely due to the high cost of JIA medications and standard billing procedures.
Conclusions
JIA patients had significantly higher all-cause, inpatient, emergency room, office visit, and outpatient prescription costs compared to matched non-JIA patients. Costs were driven by significantly higher utilization of inpatient admissions, physician office visits, and outpatient pharmacy prescription claims in JIA patients compared to matched non-JIA patients. Patients on biologic DMARDs had substantially higher costs than patients on non-biologic DMARDs, with the majority of biologic treatment costs coming from TNFi use. Biologic and non-biologic patients have low adherence and persistence on therapy. Therefore, high disease and treatment costs combined with low treatment adherence and persistence suggest a need for use of broader JIA treatment options.
Acknowledgments
Editorial support was provided by Jessamine P. Winer-Jones, Ph.D. of Truven Health Analytics, an IBM Watson Health business. These services were paid for by Bristol-Myers Squibb. This study was funded by Bristol-Myers Squibb.
Abbreviation list
ADHD, attention-deficit hyperactivity disorder; CDHP, consumer-driven health plan; CR, cost ratio; DCCI, Deyo-Charlson comorbidity index; DMARD, disease-modifying antirheumatic drugs; EPO, exclusive provider organization; FR, prescription fill ratio; HDHP, high-deductible health plan; HMO, health maintenance organization; JIA, juvenile idiopathic arthritis; MOA, mechanism of action; NSAIDs, nonsteroidal anti-inflammatory drugs; OR, odds ratio; POS, point of service; PPO, preferred provider organization; SD, standard deviation; TNFi, Tumor necrosis factor inhibitors; VR, visit ratio.
Ethics approval and informed consent
All study data were accessed with protocols compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 regulations (HIPAA). As all databases used in the study are fully de-identified and compliant with the HIPPA, this study was exempted from Institutional Review Board approval.
Disclosure
Alexander Marshall, Kiran Gupta, and Michael Pazirandeh are employed by Bristol-Myers Squibb. Machaon Bonafede and Donna McMorrow are employed by Truven Health Analytics, an IBM Watson Health business and received funding from Bristol-Myers Squibb to conduct this study. The authors report no other conflicts of interest in this work. This research was presented in part at ISPOR 2018 in Baltimore, MD, USA and at the Annual European Congress of Rheumatology in Amsterdam, Netherlands.
References
- 1.Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–778. doi: 10.1016/S0140-6736(07)60363-8 [DOI] [PubMed] [Google Scholar]
- 2.Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 3.Oberle EJ, Harris JG, Verbsky JW. Polyarticular juvenile idiopathic arthritis – epidemiology and management approaches. Clin Epidemiol. 2014;6:379–393. doi: 10.2147/CLEP.S53168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manners PJ, Diepeveen DA. Prevalence of juvenile chronic arthritis in a population of 12-year-old children in urban Australia. Pediatrics. 1996;98:84–90. [PubMed] [Google Scholar]
- 5.Beukelman T, Patkar Nivedita M, Saag KG, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011;2011(63):465–482. doi: 10.1002/acr.20460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringold S, Weiss PF, Beukelman T, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65:2499–2512. doi: 10.1002/art.38092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dueckers G, Guellac N, Arbogast M, et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin Immunol. 2012;142:176–193. doi: 10.1016/j.clim.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 8.McMahan R, Balfe LM, Greene L. Summary of AHRQ’s comparative effectiveness review of disease-modifying antirheumatic drugs for children with juvenile idiopathic arthritis. J Manag Care Pharm. 2012;18:1–16. doi: 10.18553/jmcp.2012.18.S1-B.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemper A, Coeytaux R, Sanders GD, et al. AHRQ Comparative Effectiveness Reviews. Disease-Modifying Antirheumatic Drugs (DMARDS) in Children with Juvenile Idiopathic Arthritis (JIA). Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 10.Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138–2149. doi: 10.1016/S0140-6736(11)60244-4 [DOI] [PubMed] [Google Scholar]
- 11.Lovell DJ, Ruperto N, Mouy R, et al. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol. 2015;67:2759–2770. doi: 10.1002/art.39234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Truckenbrodt H, Häfner R. Methotrexate therapy in juvenile rheumatoid arthritis: a retrospective study. Arthritis Rheum. 1986;29:801–807. [DOI] [PubMed] [Google Scholar]
- 13.Ramanan AV, Whitworth P, Baildam EM. Use of methotrexate in juvenile idiopathic arthritis. Arch Dis Child. 2003;88:197–200. doi: 10.1136/adc.88.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravelli A. Toward an understanding of the long-term outcome of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:271–275. [PubMed] [Google Scholar]
- 15.Minden K, Niewerth M, Listing J, Biedermann T, Schöntube M, Zink A. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63:836–842. doi: 10.1136/ard.2003.008516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorthy LN, Peterson MGE, Hassett AL, Lehman TJA. Burden of childhood-onset arthritis. Pediatr Rheumatol Online J. 2010;8:20. doi: 10.1186/1546-0096-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allaire SH, DeNardo BS, Szer IS, Meenan RF, Schaller JG. The economic impacts of juvenile rheumatoid arthritis. J Rheumatol. 1992;19:952–955. [PubMed] [Google Scholar]
- 18.Minden K, Niewerth M, Listing J, et al. The economic burden of juvenile idiopathic arthritis-results from the german paediatric rheumatologic database. Clin Exp Rheumatol. 2009;27:863–869. [PubMed] [Google Scholar]
- 19.Bernatsky S, Duffy C, Malleson P, Feldman DE, St Pierre Y, Clarke AE. Economic impact of juvenile idiopathic arthritis. Arthritis Rheum. 2007;57:44–48. doi: 10.1002/art.22463 [DOI] [PubMed] [Google Scholar]
- 20.Minden K, Niewerth M, Zink A, et al. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology (Oxford). 2012;51:1407–1415. doi: 10.1093/rheumatology/kes019 [DOI] [PubMed] [Google Scholar]
- 21.Gidman W, Meacock R, Symmons D. The humanistic and economic burden of juvenile idiopathic arthritis in the era of biologic medication. Curr Rheumatol Rep. 2015;17:31. doi: 10.1007/s11926-015-0508-1 [DOI] [PubMed] [Google Scholar]
- 22.United States Department of Labor, Bureau of Labor Statistics. Consumer Price index detailed report: annual average of 2017 tables. Available from: https://www.bls.gov/cpi/tables/home.htm. Accessed February22, 2018.
- 23.Kabir MH, Laila K, Haque M, Rahman SA. Drug compliance in children with juvenile idiopathic arthritis and reasons for poor compliance. Am JClin Exp Med. 2017;5:15–18. doi: 10.11648/j.ajcem.20170501.14 [DOI] [Google Scholar]
- 24.Pelajo CF, Sgarlat CM, Lopez-Benitez JM, et al. Adherence to methotrexate in juvenile idiopathic arthritis. Rheumatol Int. 2012;32:497–500. doi: 10.1007/s00296-010-1774-x [DOI] [PubMed] [Google Scholar]
- 25.Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum. 2009;38:396–402. doi: 10.1016/j.semarthrit.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhlmann A, Schmidt T, Treskova M, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. 2016;17(Suppl 1):79–87. doi: 10.1007/s10198-016-0786-1 [DOI] [PubMed] [Google Scholar]
- 27.Poddighe D, Romano M, Gattinara M, Gerloni V. Biologics for the treatment of juvenile idiopathic arthritis. Curr Med Chem. 2018;25(42):5860–5893. doi: 10.2174/0929867325666180522085716 [DOI] [PubMed] [Google Scholar]
- 28.Ravelli A, Consolaro A, Horneff G, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77(6):819–828. doi: 10.1136/annrheumdis-2018-213030 [DOI] [PubMed] [Google Scholar]