Abstract
Background
Chronic obstructive pulmonary disease (COPD) has become a leading cause of morbidity and mortality in China, with tobacco smoke, air pollution, and occupational biohazards being the major risk factors.
Objectives
The REACH trial is a multicenter, prospective, randomized controlled trial undertaken in China to assess the safety and effectiveness of the Spiration® Valve System (SVS) compared to standard medical care in COPD patients with severe emphysema.
Methods
Patients with severe airflow obstruction, hyperinflation, and severe dyspnea with interlobar fissure integrity were evaluated for enrollment. A total of 107 subjects were randomized in a 2: 1 allocation ratio to either the treatment group (SVS valves and medical management) or the control group (medical management alone).
Results
The 3-month primary endpoint showed statistically significant improvement in forced expiratory volume in 1 s in the treatment group compared to the control group (0.104 ± 0.18 vs. 0.003 ± 0.15 L, p = 0.001), with the difference being durable through 6 months. Statistically significant target lobe volume reduction was achieved at 3 months (mean change 684.4 ± 686.7 mL) and through 6 months (757.0 ± 665.3 mL). Exercise function and quality of life measures improved in the treatment group, but showed a deterioration in the control group. The serious adverse event (SAE) rate was 33% in the treatment group and 24.2% in the control group. The predominance of SAEs were acute exacerbations of COPD in both groups. There was 1 death in the control group and no deaths in the treatment group.
Conclusion
The SVS represents a novel approach for the treatment of severe emphysema with a clinically acceptable risk-benefit profile.
Keywords: Chronic obstructive pulmonary disease, Emphysema, Bronchoscopic lung volume reduction, Spiration® Valve System, Endobronchial valves, Intrabronchial valves
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the world [1] and projected to be the third leading cause of death by 2020. In China, COPD has become a leading cause of morbidity and mortality, with tobacco smoke, air pollution, smoke from biofuels, and occupational biohazards being the major risk factors [2]. Moreover, COPD poses a high economic burden, with the total expenditure per patient costing 40% of an average family income in urban areas of China [3].
Current treatment guidelines for stable COPD include lifestyle modification, physical rehabilitation, and pharmacological treatment, but have ultimately failed to reverse the anatomical hyperinflation resulting in emphysema and thus to halt the progression of the disease [4]. The National Emphysema Treatment Trial (NETT) studied lung volume reduction surgery (LVRS) and established that LVRS improved survival in addition to health status, dyspnea, exercise capacity, and lung function when compared to medical treatment, with benefits restricted to the subgroup of patients with non-lower lobe predominant emphysema and low baseline exercise capacity. However, surgery was associated with significant morbidity and mortality [5], and this has not changed despite newer data on LVRS since the NETT [6].
More recently, minimally invasive bronchoscopic methods to treat emphysema have included one-way valves placed in the airways to block aeration to the hyperinflated portions of the lung in order to reduce the volume of the targeted lobe and thereby provide benefits that are comparable with LVRS [7]. The initial bronchoscopic valve studies were promising, but failed to meet their endpoints; however, subgroup analysis of the data did identify a subset of patients with complete interlobar fissure (i.e., little or no collateral ventilation) and heterogeneous distribution of emphysema in whom there was clinically significant benefit with valve therapy [8]. Subsequent studies (BeLieVeR-HIFi, STELVIO) have demonstrated effectiveness in this selected emphysema population [9, 10] and have provided compelling enough results to warrant the inclusion of bronchoscopic lung volume reduction as a treatment option in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [1].
The objective of the REACH (Research to Assess SVS [Spiration® Valve System] Safety and Effectiveness for the Treatment of Severe Emphysema in China) trial was to demonstrate the ability of the SVS (Spiration Inc./Olympus Respiratory America, Redmond, WA, USA) to be safely deployed in selected airways and to improve lung function in Chinese subjects suffering from severe emphysema.
Methods
Trial Design and Participants
REACH is a prospective, multicenter, unblinded, randomized, parallel assignment study comparing subjects treated with medical management and the SVS (treatment group) against those who received medical management alone (control group) with an allocation ratio of 2: 1, respectively. All subjects were required to have a 6-week run-in period where they achieved treatment stability without COPD exacerbation. The study was undertaken at 12 clinical sites in China.
Patients with severe dyspnea (modified Medical Research Council [mMRC] scale ≥2), severe airflow obstruction (post-bronchodilator forced expiratory volume in 1 s [FEV1] ≤45%), and hyperinflation (total lung capacity ≥100% and residual volume ≥150%) were eligible to participate in the study. All prior pulmonary function test criteria are percentages of predicted values.
High-resolution CT (HRCT) pulmonary imaging was used to ensure the following inclusion criteria: a highly diseased target lobe (≥40% emphysema involvement), high heterogeneity compared to the ipsilateral lobe (≥15% difference), and an intact interlobar fissure (≥90% complete). Visual assessment of HRCT imaging was independently evaluated by both the lead clinical site (The First Affiliated Hospital of Guangzhou) and a core laboratory (MedQIA, Los Angeles, CA, USA). Screening/baseline CT imaging from all sites was assessed by these same two reviewers to ensure consistency across the study sites. Concordant eligibility and target lobe determination was relayed to the study site. Any reviewer disagreement was arbitrated with the aid of quantitative CT (QCT) software (Apollo; VIDA, Coralville, IA, USA) using −920 Hounsfield units as a threshold. If more than one eligible lobe was identified, priority was given to fissure completeness, disease severity, and then heterogeneity to pick the target designated as primary. The site investigator could target the alternate lobe if the primary was too articulated for treatment.
A computer-generated randomization schema with a random permuted block size of 6 stratified by site was created by an independent group (the contract research organization for the study), assigned study-wide, and distributed among the 12 sites via signed and dated, opaque envelopes. To reduce selection bias, the envelopes were secured and kept by the study coordinator at each site, and the block size was not revealed to the study investigators. Randomization occurred only after a potential subject had met all the eligibility criteria for the study.
SVS and Procedure
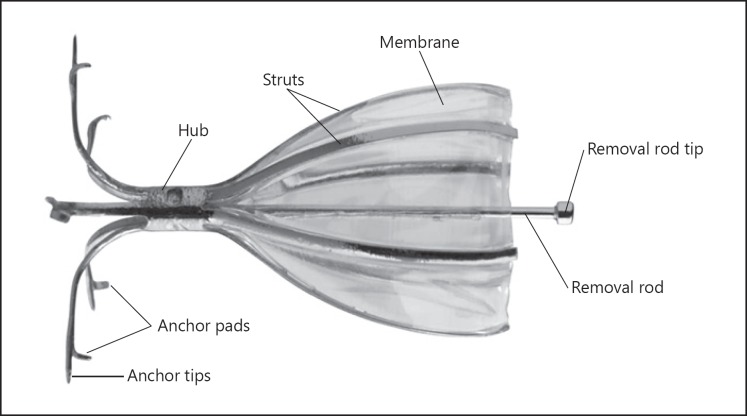
The SVS is an umbrella-shaped, one-way valve that limits airflow into targeted airways distal to the valve, but allows mucus and air movement in the proximal direction and, if needed, is removable (Fig. 1). Airways were measured using a calibrated balloon catheter to determine appropriate valve size (5, 6, or 7 mm in diameter). Valves were then placed in the airways by catheter delivery through a flexible bronchoscope. Bronchoscopy could be performed under either general anesthesia, deep sedation, and ventilatory support or moderate sedation, according to the procedures at each site. Prophylactic antibiotics were administered with an optional pulse of corticosteroids during the procedure. If any unexpected bronchoscopic findings with clinical significance were found upon initial inspection, the procedure was aborted and the subject was evaluated for rescheduling. Those subjects unsuitable for rescheduling were considered enrollment failures. The SVS was utilized to achieve total occlusion of the target lobe. There was no limit on the number of valves that could be used, and valve replacement was allowed. All procedures were proctored by trained experienced physicians from the United States.
Fig. 1.
The Spiration® Valve System.
Following SVS placement and prior to hospital discharge, subjects had chest radiographs to detect the presence of atelectasis or pneumothorax. The minimum hospital recovery duration was 3 nights, with discharge occurring at the discretion of the site investigator. Patients were scheduled for follow-up evaluations at 1, 3 (the primary endpoint for the study), 6, and 12 months. Follow-up HRCT assessments were only undertaken in the treatment group. If pulmonary function gains were less than expected, a repeat bronchoscopy could be performed at any time after the 1-month follow-up to ensure full occlusion of target lobe airways.
Follow-up of all patients was completed through the 6-month endpoint and was the primary focus of this article.
Outcomes
The primary effectiveness endpoint was the difference between treatment and control groups in the mean change in FEV1 from baseline to 3 months. The following secondary endpoints were also compared: difference between responder rates with a responder being ≥15% improvement in FEV1; target lobe volume reduction (TLVR) from baseline as measured by QCT, with minimum clinically important difference defined as a 350-mL reduction [11]; change from baseline health status as measured by Saint George's Respiratory Questionnaire (SGRQ), a disease-specific measure of health status, and the COPD Assessment Test (CAT); change from baseline dyspnea as measured by mMRC scale; change from baseline exercise capacity as measured by Six-Minute Walk Test (6MWT); and change from baseline hyperinflation as measured by residual volume. A safety assessment included incidence of device-related serious adverse events (SAEs) through 3 months. The trial did not have a Data Safety and Monitoring Board.
Statistical Methods
Using effects from earlier studies (Sciurba et al., 2010 [12] and Eberhardt et al., 2012 [13]), the FEV1 improvement between randomization groups at 3 months was expected to be 181 ± 86 mL. Assuming a minimum power of 90%, a 2: 1 treatment-to-control allocation ratio, and a dropout rate of 20%, a minimum of 38 and 19 subjects was required to detect a statistical difference (two-sided significance level of 0.05) between the treatment and control arms, respectively. A sample size of 100 subjects was chosen to provide a conservative measure of both effectiveness and safety.
Study data were processed for those subjects with both baseline and follow-up FEV1 data. For those subjects with at least one follow-up assessment, missing values were imputed using last observation carried forward. All continuous data were evaluated for normality using a Kolmogorov-Smirnov test and subsequently analyzed using the appropriate parametric (Student's t) or nonparametric (Mann-Whitney U) test. Categorical data were evaluated using Fisher's exact or Pearson's χ2 test. The significance level of all tests was 0.05.
Results
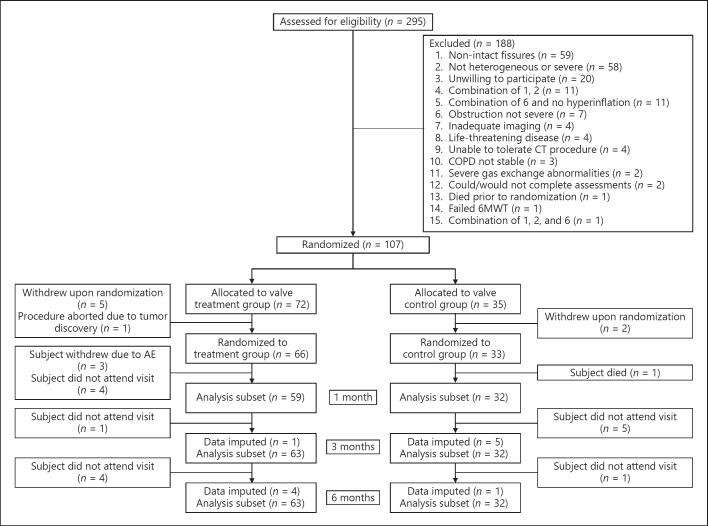
The REACH trial screened a total of 295 patients to yield 107 subjects eligible for randomization. The patient flowchart can be found in Figure 2 and shows that of the 107 randomized patients, 7 withdrew consent prior to the scheduled procedure (5 treatment patients and 2 control patients), and 1 additional patient in the treatment group was excluded from the study due to a bronchoscopic findings of lung cancer, leaving a total of 66 patients in the treatment group and 33 in the control group. There were no enrollment failures due to the inability to place SVS devices in the treatment group. Three subjects in the treatment group requested to withdraw from the study before the 1-month follow-up due to adverse events (hypoxia in 1 and acute exacerbations of COPD in the remaining 2 subjects), and at the subjects' request all valves were removed in these 3 subjects. Enrollment in the study began in November 2013 and the last follow-up was completed in March 2017.
Fig. 2.
Flowchart of the study. 6MWT, Six-Minute Walk Test; AE, adverse event; COPD, chronic obstructive pulmonary disease.
Table 1 describes the patient demographics between the randomized groups. The patient population was predominantly male (99%), reflecting the higher prevalence of COPD in men in China [2]. Smoking history was present in 97% of all patients, with no statistical difference between the randomized cohorts. Data from baseline spirometry and lung plethysmography showed no statistical differences between the groups for FEV1, total lung capacity, or residual volume. Similarly, there were no statistical differences in the baseline SGRQ, CAT, mMRC scale, and 6MWT variables between the two randomized cohorts. HRCT eligibility criteria showed no differences in emphysema involvement, heterogeneity score, or fissure integrity between the two randomized groups.
Table 1.
Patient demographics
| SVS treatment | Control | p value | |
|---|---|---|---|
| Age | 63.5±6.7 | 62.4±6.9 | 0.805 |
| Male sex | 100% | 97% | 0.344 |
| Smoking history Baseline medication | 96.7% | 97.0% | 0.729 |
| Adrenergics + corticosteroids | 85.7% | 81.2% | 0.618 |
| Anticholinergics | 79.4% | 75.8% | 0.685 |
| FEV1, L | 0.760±0.195 | 0.795±0.262 | 0.495 |
| FEV1, % predicted | 27.3±6.7 | 28.8±8.1 | 0.353 |
| Total lung capacity, % predicted | 136.0±23.6 | 137.8±23.9 | 0.775 |
| Residual volume, % predicted | 261.4±74.4 | 264.9±74.3 | 0.902 |
| Target lobe emphysema involvement, % | 62.9±12.2 | 57.7±12.1 | 0.067 |
| Heterogeneity, % | 28.4±13.9 | 24.8±10.6 | 0.280 |
| Fissure integrity, % | 97.8±4.5 | 97.0±4.9 | 0.301 |
| SGRQ score | 56.4±14.3 | 57.3±13.4 | 0.775 |
| CAT score | 20.0±6.6 | 21.6±5.6 | 0.238 |
| mMRC scale score | 2.7±0.6 | 2.6±0.6 | 0.704 |
| 6MWT, m | 338.7±94.5 | 321.5±88.0 | 0.377 |
Values are presented as mean ± SD or percentage. 6MWT, Six-Minute Walk Test; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; FEV1, forced expiratory volume in 1 s; mMRC, modified Medical Research Council; SGRQ, Saint George's Respiratory Questionnaire; SVS, Spiration® Valve System.
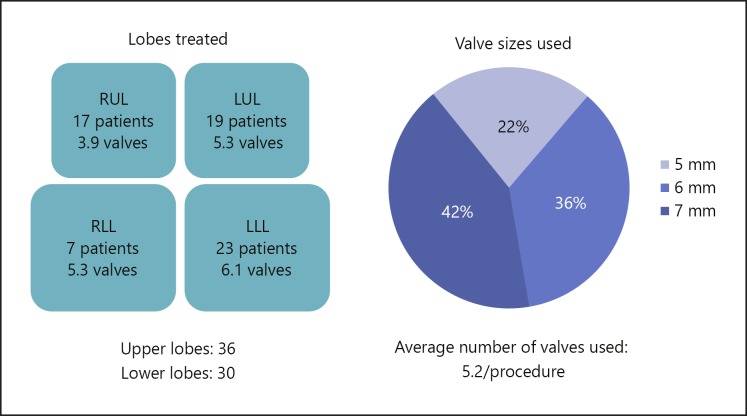
All the SVS procedures were done under general anesthesia. The distribution of target lobe treated and the average number of valves used in each lobe is shown in Figure 3. The mean bronchoscopic procedure time was 52.0 ± 18.6 min. The mean number of valves used per procedure was 5.2 ± 1.1, with the 6- and 7-mm valves being used in approximately 80% of the procedures. The mean length of hospital stay was 6.3 days.
Fig. 3.
Distribution of target lobe treated and average number of valves used in each lobe. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RUL, right upper lobe.
Effectiveness
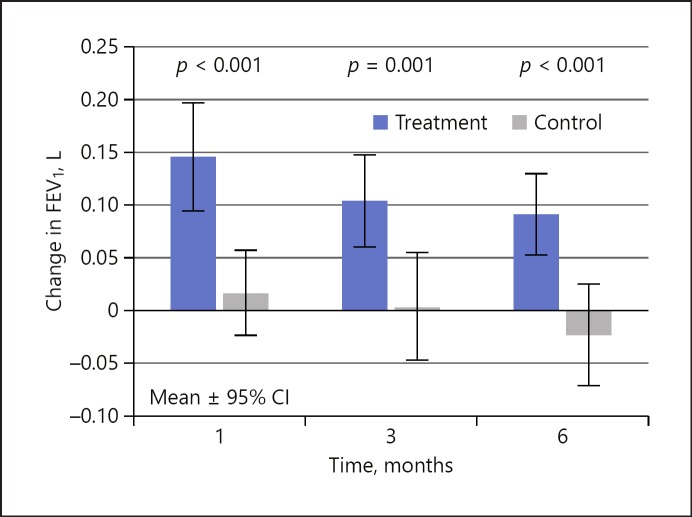
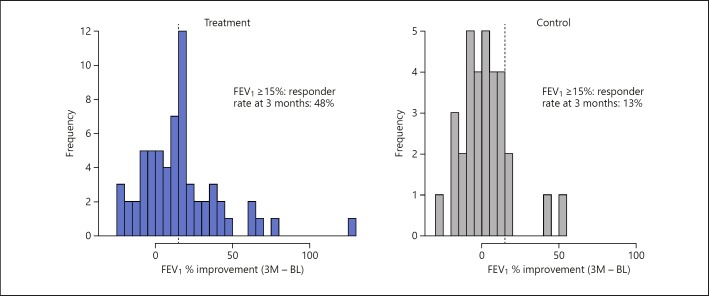
Table 2 shows the results of the primary and secondary effectiveness outcomes at baseline and follow-up, including the change from baseline. The treatment and control arms had mean FEV1 improvements from baseline to 3 months of 0.104 ± 0.178 and 0.003 ± 0.147 L, respectively, satisfying the primary effectiveness endpoint (p = 0.001), as shown in Figure 4. The relative percent improvement from follow-up to baseline between the two groups was 16.5, 13.5, and 15.2% at 1, 3, and 6 months, respectively. Using a threshold of ≥15% improvement in FEV1 from baseline, the treatment group responder rate was 49, 48, and 41% compared to 22, 13, and 21% in the control group. Figure 5 plots the percentage improvement in FEV1 at 3 months in a graphical form and clearly shows the difference in the ≥15% responder rate of 48% in the treatment group compared to 13% in the control group.
Table 2.
Primary and secondary effectiveness results
| SVS treatment |
Control |
p value1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean | SD | –95% CI | +95% CI | n | mean | SD | –95%CI | +95% CI | ||
| FEV1, L | |||||||||||
| Baseline | 63 | 0.760 | 0.196 | 0.712 | 0.808 | 33 | 0.796 | 0.262 | 0.706 | 0.885 | |
| 1 month | 59 | 0.914 | 0.283 | 0.842 | 0.986 | 32 | 0.804 | 0.256 | 0.716 | 0.893 | |
| 3 months | 63 | 0.864 | 0.246 | 0.803 | 0.925 | 32 | 0.791 | 0.267 | 0.698 | 0.883 | |
| 6 months | 63 | 0.851 | 0.245 | 0.790 | 0.911 | 33 | 0.772 | 0.262 | 0.682 | 0.861 | |
| Delta | |||||||||||
| B – 1 month | 59 | 0.146 | 0.201 | 0.095 | 0.197 | 32 | 0.017 | 0.117 | –0.024 | 0.057 | <0.001 |
| B – 3 months | 63 | 0.104 | 0.178 | 0.060 | 0.148 | 32 | 0.003 | 0.147 | –0.047 | 0.054 | 0.001 |
| B – 6 months | 63 | 0.091 | 0.156 | 0.052 | 0.129 | 33 | –0.024 | 0.142 | –0.072 | 0.024 | <0.001 |
| | |||||||||||
| SGRQ, points | |||||||||||
| Baseline | 63 | 56.42 | 14.33 | 52.88 | 59.96 | 32 | 57.28 | 13.39 | 52.64 | 61.92 | |
| 1 month | 60 | 45.36 | 15.77 | 41.37 | 49.35 | 32 | 56.40 | 16.70 | 50.61 | 62.19 | |
| 3 months | 63 | 48.50 | 16.92 | 44.32 | 52.68 | 32 | 56.51 | 20.07 | 49.56 | 63.46 | |
| 6 months | 63 | 48.04 | 18.60 | 43.45 | 52.63 | 33 | 58.86 | 18.20 | 52.65 | 65.07 | |
| Delta | |||||||||||
| B – 1 month | 60 | –11.18 | 18.12 | –15.76 | –6.60 | 31 | –0.31 | 13.28 | –4.98 | 4.37 | 0.005 |
| B – 3 months | 63 | –7.92 | 17.18 | –12.17 | –3.68 | 31 | –0.73 | 16.90 | –6.68 | 5.22 | 0.058 |
| B – 6 months | 63 | –8.39 | 17.43 | –12.69 | –4.08 | 32 | 2.11 | 17.24 | –3.87 | 8.08 | 0.007 |
| | |||||||||||
| CAT score | |||||||||||
| Baseline | 63 | 20.06 | 6.65 | 18.42 | 21.70 | 33 | 21.58 | 5.55 | 19.69 | 23.47 | |
| 1 month | 60 | 17.48 | 7.36 | 15.62 | 19.34 | 32 | 22.12 | 5.85 | 20.09 | 24.15 | |
| 3 months | 63 | 18.22 | 6.38 | 16.64 | 19.80 | 32 | 22.31 | 6.22 | 20.15 | 24.47 | |
| 6 months | 63 | 17.89 | 7.84 | 15.95 | 19.83 | 33 | 23.52 | 5.58 | 21.62 | 25.42 | |
| Delta | |||||||||||
| B – 1 month | 60 | –2.82 | 8.61 | –5.00 | –0.64 | 32 | 0.50 | 4.54 | –1.07 | 2.07 | 0.075 |
| B – 3 months | 63 | –1.83 | 8.16 | –3.85 | 0.18 | 32 | 0.69 | 6.34 | –1.51 | 2.88 | 0.130 |
| B – 6 months | 63 | –2.17 | 8.57 | –4.28 | –0.05 | 33 | 1.94 | 6.32 | –0.22 | 4.10 | 0.017 |
| | |||||||||||
| mMRC scale score | |||||||||||
| Baseline | 63 | 2.68 | 0.59 | 2.54 | 2.83 | 33 | 2.64 | 0.60 | 2.43 | 2.84 | |
| 1 month | 60 | 2.07 | 0.96 | 1.82 | 2.31 | 32 | 2.34 | 0.79 | 2.07 | 2.62 | |
| 3 months | 63 | 1.95 | 0.92 | 1.72 | 2.18 | 32 | 2.25 | 0.92 | 1.93 | 2.57 | |
| 6 months | 63 | 1.95 | 1.11 | 1.68 | 2.23 | 33 | 2.27 | 1.13 | 1.89 | 2.66 | |
| Delta | |||||||||||
| B – 1 month | 60 | –0.60 | 0.80 | –0.80 | –0.40 | 32 | –0.31 | 0.64 | –0.54 | –0.09 | 0.077 |
| B – 3 months | 63 | –0.73 | 0.87 | –0.94 | –0.52 | 32 | –0.41 | 0.84 | –0.70 | –0.12 | 0.076 |
| B – 6 months | 63 | –0.73 | 0.92 | –0.96 | –0.50 | 33 | –0.36 | 1.03 | –0.71 | –0.01 | 0.091 |
| | |||||||||||
| 6MWT, m | |||||||||||
| Baseline | 63 | 338.7 | 94.5 | 315.4 | 362.0 | 33 | 321.5 | 88.0 | 291.5 | 351.5 | |
| 1 month | 60 | 362.4 | 100.1 | 337.1 | 387.7 | 32 | 329.7 | 78.9 | 302.4 | 357.0 | |
| 3 months | 63 | 365.9 | 88.3 | 344.1 | 387.7 | 32 | 326.5 | 90.9 | 295.0 | 358.0 | |
| 6 months | 63 | 359.5 | 102.7 | 334.1 | 384.9 | 33 | 305.9 | 110.5 | 268.2 | 343.6 | |
| Delta | |||||||||||
| B – 1 month | 60 | 20.56 | 75.90 | 1.35 | 39.77 | 32 | 10.73 | 40.20 | –3.20 | 24.66 | 0.419 |
| B – 3 months | 63 | 27.17 | 71.97 | 9.40 | 44.94 | 32 | 7.50 | 50.42 | –9.97 | 24.97 | 0.126 |
| B – 6 months | 63 | 20.82 | 86.65 | –0.58 | 42.22 | 33 | –15.58 | 71.91 | –40.12 | 8.96 | 0.042 |
| | |||||||||||
| Residual volume, L | |||||||||||
| Baseline | 63 | 6.04 | 1.62 | 5.64 | 6.44 | 33 | 5.97 | 1.59 | 5.43 | 6.51 | |
| 1 month | 58 | 5.48 | 1.53 | 5.09 | 5.87 | 32 | 5.52 | 1.33 | 5.06 | 5.98 | |
| 3 months | 62 | 5.53 | 1.19 | 5.23 | 5.82 | 32 | 5.74 | 1.45 | 5.23 | 6.24 | |
| 6 months | 63 | 5.62 | 1.74 | 5.19 | 6.05 | 33 | 5.92 | 1.28 | 5.48 | 6.36 | |
| Delta | |||||||||||
| B – 1 month | 58 | –0.59 | 1.71 | –1.03 | –0.15 | 32 | –0.48 | 1.18 | –0.89 | –0.07 | 0.469 |
| B – 3 months | 62 | –0.52 | 1.43 | –0.87 | –0.16 | 32 | –0.26 | 1.42 | –0.75 | 0.23 | 0.629 |
| B – 6 months | 63 | –0.42 | 1.84 | –0.87 | 0.03 | 33 | –0.05 | 1.33 | –0.50 | 0.41 | 0.114 |
6MWT, Six-Minute Walk Test; B, baseline; CAT, Chronic Obstructive Pulmonary Disease Assessment Test; mMRC, modified Medical Research Council; SGRQ, Saint George's Respiratory Questionnaire; SVS, Spiration® Valve System.
Comparison between groups.
Fig. 4.
Change in FEV1 for the treatment and control arms. FEV1, forced expiratory volume in 1 s.
Fig. 5.
Percentage improvement in FEV1 at 3 months. 3M, 3 months; BL, baseline; FEV1, forced expiratory volume in 1 s.
The target lobe volume results of the treatment group are shown in Table 3 and indicate statistically significant mean reductions of 684 and 757 mL at 3 and 6 months, respectively. Using a TLVR threshold of 350 mL, 52.5 and 66.1% of treatment patients were responders at the 3- and 6-month timepoints. The rate of complete target lobe atelectasis was 26.2% (16 of 61 patients) at 3 months and 28.8% (17 of 59 patients) at 6 months. Residual volume as measured by plethysmography showed a reduction of 0.42 L from baseline to 6 months in the treatment group, while the control group showed only a change of 0.08 L over the same time interval; this difference between the groups was not statistically significant.
Table 3.
Target lobe volume results of the treatment group
| Target lobe volume, mL | n | Mean | SD | p value |
|---|---|---|---|---|
| Baseline | 66 | 1,795.16 | 502.66 | |
| 3 months | 61 | 1,063.94 | 794.06 | |
| 6 months | 59 | 993.57 | 777.04 | |
| Delta | ||||
| Baseline – 3 months | 61 | 684.38 | 686.65 | <0.001 |
| Baseline – 6 months | 59 | 757.00 | 665.27 | <0.001 |
CT was only done at 3- and 6-month follow-ups.
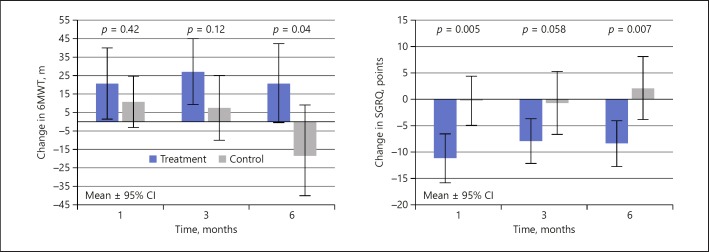
The changes in 6MWT and SGRQ are shown in Figure 6 and Table 2. Exercise function showed mean improvements in the treatment group at all timepoints; however, statistical significance between groups only occurred at the 6-month follow-up when the control group showed a marked deterioration. The relative percent improvement from follow-up to baseline between the two groups was 3.0, 8.4, and 15.5% over the 1-, 3-, and 6-month follow-ups, respectively, and was statistically significantly different at 6 months. The SGRQ showed a similar trend over time with relative differences between the groups of 10.9, 7.2, and 10.5 points at 1, 3, and 6 months, respectively, being statistically significant at the 1-month (p = 0.005) and 6-month (p = 0.007) timepoints.
Fig. 6.
Changes in 6MWT and SGRQ. 6MWT, Six-Minute Walk Test; SGRQ, Saint George's Respiratory Questionnaire.
Though both CAT and mMRC scale showed trends toward improvement in the treatment group compared to the control group (Table 2), statistical significance was only shown for the CAT at the 6-month (p = 0.017) timeframe.
Bronchoscopy for revision/replacement of valves occurred in 12 treatment patients (18.2%); 2 of these patients required an additional bronchoscopy procedure for a total of 14 additional procedures. Repeat bronchoscopy occurred a mean of 81.4 days (range 27–152 days) after the initial procedure. A total of 29 additional valves were placed, with 4 valves removed (3 in 1 patient). Bronchoscopy can reveal migration or dysfunction of valves and granulation in the airway, which can reduce the effectiveness of valves. Thus, repeat bronchoscopy is recommended in clinical practice in case of loss of effectiveness after the treatment, and this occurred in 8 patients, resulting in an FEV1 improvement of 8.2 ± 3.76 (SE) mL (χ2[1] = 4.29, p = 0.038). Although significant, these gains are an order of magnitude less than the treatment group as a whole. The repeat bronchoscopies occurred within 1 month of the original valve placement. The average TLVR at the 3- and 6-month timeframes was on average 200 mL less than that obtained by the entire treatment population. Thus, even after valve adjustment, these patients had an attenuated response mechanistically (as evidenced by the smaller TLVR response), but also as noted in their FEV1 response.
Safety
A total of 40 SAEs were noted in the entire study population. There were no unanticipated adverse device effects during the clinical study. Table 4 documents all SAEs in both study groups through the 6-month follow-up period. The treatment group is further categorized by device and procedure relatedness.
Table 4.
SAEs through 6 months
| Treatment (n = 66) | Control (n = 33) | |
|---|---|---|
| Total SAEs | 30/22 (33.3%) | 10/8 (24.2%) |
| Device-related | 8/6 (9.1%) | |
| Acute exacerbations of COPD | 7/5 (7. 6%) | |
| Pneumothorax | 1/1 (1. 5%) | |
| Procedure-related | 3/3 (4. 5%) | |
| Anesthesia | 2/2 (3.0%) | |
| Acute heart failure | 1/1 (1. 5%) | |
| Device- and procedure-related | 5/5 (7.6%) | |
| Pneumothorax | 4/4 (6.1%) | |
| Anesthesia | 1/1 (1. 5%) | |
| Unrelated | 14/11 (16.7%) | 10/8 (24.2%) |
| Acute exacerbations of COPD | 9/8 (12.1%) | 4/4 (12.1%) |
| Pneumonia | 1/1 (1. 5%) | – |
| Other | 4/2 (3.0%) | 5/3 (9.1%) |
| Death | – | 1/1 (3.0%)1 |
Values are presented as number of events/number of patients (%). COPD, chronic obstructive pulmonary disease; SAEs, serious adverse events.
Acute exacerbations of COPD resulting in death.
In the treatment population (n = 66), there were a total of 30 SAEs documented in 22 patients, for an overall SAE rate of 33%, the majority of these events being acute exacerbations of COPD. Pneumothorax was the second most frequent event in the treatment group, occurring in 5 patients (7.6%). In 3 cases the event occurred within the first 30 days, with the remaining two events occurring at 60 and 150 days after valve implantation. Four of these events were designated as device- and procedure-related, and the remaining event (occurring at day 150 after the procedure) was designated as only device-related. Early-onset pneumothorax in the treated population was probably the result of valve placement leading to changes in the conformation of the lung because of acute reduction in lung volume [14]. Late-onset pneumothorax was most likely due to ongoing complications associated with COPD. The length of hospital stay for these patients was prolonged to a median of 16 days, but no valves had to be removed either because of or to treat the pneumothorax events.
There were 15 events in 12 patients (18.2%) that were unrelated to either device or procedure, with acute exacerbations of COPD again comprising the majority of these unrelated events. Of note, no deaths occurred in the treatment group. All device and procedure relationship assignments were based on site investigator assessment.
There was no migration (defined as major movement of the device from the initial implant location) or expectoration of valves noted in the study by review of CT and/or X-ray data.
In the control group (n = 33), there were 10 SAEs in 8 patients for an overall SAE incidence rate of 24.2%. Five of these events were either acute exacerbations of COPD or COPD events, with the remaining SAEs being unrelated to the underlying disease state. There was 1 death (an acute exacerbation of COPD event which led to respiratory failure and multiorgan failure) reported in the control group for a mortality rate of 3%.
Discussion
Previous multicenter valve trials such as the IBV Valve Trial [15] and VENT [12] included patients with collateral ventilation in whom the derived benefit, in particular far less lobar atelectasis, was a critical determinant in the reduced lung function response [12] and survival [16]. By prospectively classifying in favor of patients with heterogeneous disease and radiologically intact fissures, the REACH study has shown that implantation of SVS valves yields improvements in lung function, exercise capacity, and quality of life when compared to a randomized control cohort. These improvements in lung function are similar in magnitude to those obtained with LVRS, but with markedly less morbidity [5, 17, 18].
The FEV1 responder rate in our study (47.6% at the 3-month primary endpoint) was comparable with that in the BeLieVeR-HIFi [9] (47% when collateral ventilation-positive patients were excluded) and STELVIO (59%) [10] single-center studies and the multicenter TRANSFORM [19] (55% using a ≥12% improvement threshold) and LIBERATE [20] (47.7% at 12 months) studies. Moreover, QCT was sufficient to screen responders, without the need for adjunctive collateral ventilation assessments, as required in other studies [9, 10].
Of those patients in the treatment arm, a post hoc analysis showed that target location significantly affected FEV1 outcomes (χ2[3] = 19.1, p < 0.001). A larger study will be needed to ascertain whether treatment of a particular lobe represents a negative predictor of outcome. It is not clear either whether there is a phenotypic difference among the Chinese COPD population, which may have influenced the ratio of lower to upper lobe treatment.
The pneumothorax rate in this study (7.6% in the treatment group) was comparable to frequencies reported in the BeLieVeR-HIFi trial [9], which also used fissure integrity as a surrogate assessment for collateral ventilation, but lower than seen in the recent TRANSFORM and LIBERATE multicenter trials [19, 20], which showed a 21.5 and 34.4% rate of serious pneumothorax events, respectively. The markedly lower pneumothorax rate in our study may be attributable to a very conservative postoperative care regimen, with patients staying in hospital for a median of 6 days post intervention and exposed to limited activities of daily living, which has been previously shown to reduce pneumothorax incidence [21]. Gompelmann et al. [22] have also noted that with greater emphysematous destruction of the untreated ipsilateral lobe there is an increased incidence of pneumothorax. In this study, the average emphysema severity in the ipsilateral lobe was 34.6%, with an emphysema heterogeneity between lobes of 28.4% (Table 2). In contrast, the ipsilateral lobe emphysema severity in the TRANSFORM [19] and LIBERATE [20] studies was 45.4 and 47.5%, respectively, and this may account for the comparatively lower pneumothorax rate seen in the REACH study. Lastly, it should be noted that the lower TLVR (compared to other valve trials), possibly associated with the relative lack of experience in the use of endobronchial valves, could also have attributed to the lower pneumothorax rate. All cases of pneumothorax in our study were successfully managed according to published guidelines [23], with all patients requiring a chest drain. Notably, the occurrence of pneumothorax does not appear to negatively impact clinical outcomes in studies using valve therapy [14] and was not associated with any cases of mortality in our study.
The present study, with a representative sampling of emphysema sufferers in China, is generalizable to that population, with some limitations. The trial was unblinded without a sham group, creating possible physician and/or subject bias, although the mechanistic changes in TLVR in the treatment group would be difficult to attribute to subject or measurement bias. As this randomized controlled trial was started in 2013, TLVR with a minimum clinically important difference defined as a 350-mL reduction was chosen based on the results of VENT [12] and European VENT [11]. However, more recent experience suggests that a higher TLVR is necessary to achieve a clinically relevant benefit [24, 25]. Only 1 female was included, but this seems to reflect the much higher ratio of males with emphysema in China [2]. The current study was undertaken at multiple sites in China with physicians who had little or no previous experience with endobronchial valve placement. Despite this, there were very few procedural adverse events and no mortality in the treatment population, indicating the relative simplicity and generalizability of the valve procedure for bronchoscopic lung volume reduction. Lastly, the trial was undertaken before the availability of the larger 9-mm SVS valve, which would have significantly reduced the number of valves used per procedure and thus the overall length of the procedure.
The REACH trial achieved its primary endpoint with FEV1 improvement at 3 months and showed durabil ity of effects through 6 months. In addition, the trial results demonstrated TLVR, indicating that the valve had achieved its goal of reducing hyperinflation in the targeted lobe, an effect that was durable through the 6-month timeframe post valve deployment. The study results also showed improvements in exercise function (6MWT) and quality of life (SGRQ and CAT) parameters. Retrospective studies have shown a survival advantage associated with TLVR and atelectasis [26, 27], and while the significant improvements seen in our study may be a good predictor for long-term survival, this will need to be confirmed in future studies with longer-term follow-up than in the current study [28].
The SVS represents a novel approach for the treatment of severe heterogeneous emphysema with a clinically acceptable risk-benefit profile. The recent results from Valipour et al. [29], who also used valves for bronchoscopic lung volume reduction, suggest that patients with homogenous emphysema may also benefit from this interventional treatment approach.
Statement of Ethics
The study was undertaken following ethics committee ap proval. All subjects provided written informed consent prior to participation. The study is registered at ClinicalTrials.gov, NCT01989182.
Disclosure Statement
Nanshan Zhong, MD is the overall study principal investigator and has no conflict of interest to disclose. Felix J.F. Herth, MD has acted in a consultant capacity for Spiration Inc./Olympus Respiratory America. All other coauthors have no conflicts of interest to disclose.
Funding Sources
Spiration Inc./Olympus Respiratory America funded the REACH trial. Spiration was involved in the design of the study. N. Zhong, S. Li, and G. Wang had full access to all the data. N. Zhong had final responsibility to submit the paper for publication.
Author Contributions
N. Zhong was involved in study design and manuscript writing as the overall study principal investigator. As the corresponding author, he was provided full access to the data and had final responsibility for the decision to submit this original research for publication. F.J.F. Herth was involved in the study design as well as review and editing of the manuscript. All other coauthors were involved in data collection as principal investigators at their respective sites and as reviewers of the manuscript. Spiration Inc./Olympus Respiratory America funded the REACH trial and helped in trial design and review of the manuscript.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2018 Report www.goldcopd.org/gold-reports/ [Google Scholar]
- 2.Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest. 2011 Apr;139((4)):920–9. doi: 10.1378/chest.10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He QY, Zhou X, Xie CM, Liang ZA, Chen P, Wu CG. [Impact of chronic obstructive pulmonary disease on quality of life and economic burden in Chinese urban areas] Zhonghua Jie He He Hu Xi Za Zhi. 2009 Apr;32((4)):253–7. [PubMed] [Google Scholar]
- 4.Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–94. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003 May;348((21)):2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 6.Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2014 Dec;148((6)):2651–8.e1. doi: 10.1016/j.jtcvs.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herth FJ, Slebos DJ, Criner GJ, Shah PL. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2017. Respiration. 2017;94((4)):380–8. doi: 10.1159/000479379. [DOI] [PubMed] [Google Scholar]
- 8.Eberhardt R, Gompelmann D, Herth FJ, Schuhmann M. Endoscopic bronchial valve treatment: patient selection and special considerations. Int J Chron Obstruct Pulmon Dis. 2015 Oct;10:2147–57. doi: 10.2147/COPD.S63473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015 Sep;386((9998)):1066–73. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 10.Klooster K, Hartman JE, Ten Hacken NH, Slebos DJ. One-Year Follow-Up after Endobronchial Valve Treatment in Patients with Emphysema without Collateral Ventilation Treated in the STELVIO Trial. Respiration. 2017;93((2)):112–21. doi: 10.1159/000453529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. International VENT Study Group Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012 Jun;39((6)):1334–42. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 12.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. VENT Study Research Group A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010 Sep;363((13)):1233–44. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt R, Gompelmann D, Schuhmann M, Reinhardt H, Ernst A, Heussel CP, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest. 2012 Oct;142((4)):900–8. doi: 10.1378/chest.11-2886. [DOI] [PubMed] [Google Scholar]
- 14.Gompelmann D, Herth FJ, Slebos DJ, Valipour A, Ernst A, Criner GJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration. 2014;87((6)):485–91. doi: 10.1159/000360641. [DOI] [PubMed] [Google Scholar]
- 15.Wood DE, Nader DA, Springmeyer SC, Elstad MR, Coxson HO, Chan A, et al. IBV Valve Trial Research Team The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol. 2014 Oct;21((4)):288–97. doi: 10.1097/LBR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 16.Hopkinson NS, Toma TP, Hansell DM, Goldstraw P, Moxham J, Geddes DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med. 2005 Mar;171((5)):453–60. doi: 10.1164/rccm.200407-961OC. [DOI] [PubMed] [Google Scholar]
- 17.Clark SJ, Zoumot Z, Bamsey O, Polkey MI, Dusmet M, Lim E, et al. Surgical approaches for lung volume reduction in emphysema. Clin Med (Lond) 2014 Apr;14((2)):122–7. doi: 10.7861/clinmedicine.14-2-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) Part II: lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med. 2011 Oct;184((8)):881–93. doi: 10.1164/rccm.201103-0455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter RCT of Zephyr® Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM) Am J Respir Crit Care Med. 2017 Sep;••• doi: 10.1164/rccm.201707-1327OC. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. LIBERATE Study Group A Multicenter RCT of Zephyr® Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE) Am J Respir Crit Care Med. 2018 May;•••:rccm.201803-0590OC. doi: 10.1164/rccm.201803-0590OC. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Herzog D, Poellinger A, Doellinger F, Schuermann D, Temmesfeld-Wollbrueck B, Froeling V, et al. Modifying post-operative medical care after EBV implant may reduce pneumothorax incidence. PLoS One. 2015 May;10((5)):e0128097. doi: 10.1371/journl.pone.0128097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gompelmann D, Lim HJ, Eberhardt R, Gerovasili V, Herth FJ, Heussel CP, et al. Predictors of pneumothorax following endoscopic valve therapy in patients with severe emphysema. Int J Chron Obstruct Pulmon Dis. 2016 Aug;11:1767–73. doi: 10.2147/COPD.S106439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valipour A, Slebos DJ, de Oliveira HG, Eberhardt R, Freitag L, Criner GJ, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema—potential mechanisms, treatment algorithm, and case examples. Respiration. 2014;87((6)):513–21. doi: 10.1159/000360642. [DOI] [PubMed] [Google Scholar]
- 24.Welling JB, Hartman JE, van Rikxoort EM, Ten Hacken NH, Kerstjens HA, Klooster K, et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology. 2018 Mar;23((3)):306–10. doi: 10.1111/resp.13178. [DOI] [PubMed] [Google Scholar]
- 25.Gompelmann D, Kontogianni K, Schuhmann M, Eberhardt R, Heussel CP, Herth FJ. The minimal important difference for target lobe volume reduction after endoscopic valve therapy. Int J Chron Obstruct Pulmon Dis. 2018 Feb;13:465–72. doi: 10.2147/COPD.S152029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkinson NS, Kemp SV, Toma TP, Hansell DM, Geddes DM, Shah PL, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J. 2011 Jun;37((6)):1346–51. doi: 10.1183/09031936.00100110. [DOI] [PubMed] [Google Scholar]
- 27.Garner J, Kemp SV, Toma TP, Hansell DM, Polkey MI, Shah PL, et al. Survival after Endobronchial Valve Placement for Emphysema: A 10-Year Follow-up Study. Am J Respir Crit Care Med. 2016 Aug;194((4)):519–21. doi: 10.1164/rccm.201604-0852LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skowasch D, Fertl A, Schwick B, Schäfer H, Hellmann A, Herth FJ, LIVE Study Investigators A Long-Term Follow-Up Investigation of Endobronchial Valves in Emphysema (the LIVE Study): Study Protocol and Six-Month Interim Analysis Results of a Prospective Five-Year Observational Study. Respiration. 2016;92((2)):118–26. doi: 10.1159/000448119. [DOI] [PubMed] [Google Scholar]
- 29.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. IMPACT Study Team Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med. 2016 Nov;194((9)):1073–82. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]