Long noncoding RNAs (lncRNAs) are involved in various human diseases. Recently, H19 was reported to be upregulated in fibrotic rat lung and play a stimulative role in bleomycin (BLM)-induced pulmonary fibrosis in mice.
KEYWORDS: TGF-β1, lncRNA H19, miR-140, pulmonary fibrosis
ABSTRACT
Long noncoding RNAs (lncRNAs) are involved in various human diseases. Recently, H19 was reported to be upregulated in fibrotic rat lung and play a stimulative role in bleomycin (BLM)-induced pulmonary fibrosis in mice. However, its expression in human fibrotic lung tissues and mechanism of action remain unclear. Here, our observations showed that H19 expression was significantly upregulated and that of microRNA 140 (miR-140) was markedly reduced in pulmonary fibrotic tissues from idiopathic pulmonary fibrosis (IPF) patients and transforming growth factor β1 (TGF-β1)-induced HBE and A549 cells. Moreover, the expression of H19 was negatively correlated with the expression of miR-140 in IPF tissues. H19 knockdown attenuated TGF-β1-induced pulmonary fibrosis in vitro. Furthermore, animal experiments showed that H19 knockdown attenuated BLM-induced pulmonary fibrosis in mice. The study of molecular mechanisms showed that H19 functioned via reduction of miR-140 expression by binding to miR-140. The increase of miR-140 inhibited TGF-β1-induced pulmonary fibrosis, and H19 upregulation diminished the inhibitory effects of miR-140 on TGF-β1-induced pulmonary fibrosis, which was involved in the TGF-β/Smad3 pathway. Taken together, our findings showed that H19 knockdown attenuated pulmonary fibrosis via the regulatory network of lncRNA H19–miR-140–TGF-β/Smad3 signaling, and H19 and miR-140 might represent therapeutic targets and early diagnostic and prognostic biomarkers for patients with pulmonary fibrosis.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF), a common, progressive, and fatal disease with a median survival of 3 to 5 years, is characterized by phenotypic dystransition in the alveolar epithelial cells with excessive deposition of extracellular matrix collagen (1). No specific treatment for this disease exists. Undoubtedly, a better understanding of pathogenesis of pulmonary fibrosis will contribute to the development of more effective therapeutic strategies. Transforming growth factor β1 (TGF-β1), an elicitor of myofibroblast generation and deposition of extracellular matrix collagen, is recognized as a master switch of the occurrence of fibrosis. Smads (Smad2 and especially Smad3), the downstream factors in the TGF-β1 pathway, are key regulators in fibrogenesis (2, 3). Many studies have reported the roles of TGF-β/Smad3 signaling in fibrosis. For example, Qin et al. (4) reported that TGF-β/Smad3 signaling promotes renal fibrosis. Lakos et al. (5) reported that TGF-β/Smad3 signaling regulates skin fibrosis in a mouse model of scleroderma. Yu et al. (6) and Warburton et al. (7) reported that TGF-β/Smad3 signaling promotes the progression of pulmonary fibrosis. Thus, inhibition of TGF-β/Smad3 signaling may have great clinical significance for patients with pulmonary fibrosis.
MicroRNAs (miRNAs), small noncoding RNAs (ncRNA) of 18 to 24 nucleotides in length, can regulate a wide range of the signaling pathways related to most biological and pathological processes by binding to the 3′ untranslated region (3′-UTR) of mRNA and decreasing gene expression. Increasing evidence has suggested important roles of miRNAs in pulmonary fibrosis (8–13). Identification of pulmonary fibrosis-related miRNAs has been recognized as a promising therapeutic target and an early diagnostic and prognostic biomarker for patients with pulmonary fibrosis. For example, Duru et al. (12) reported that loss of miRNA 140 (miR-140) is a key risk factor for radiation-induced lung fibrosis through reprogramming fibroblasts and macrophages. Wang et al. (14) found that the increase of miR-140 ameliorates pulmonary fibrosis by binding to the 3′-UTR of Smad3 mRNA and suppressing the TGF-β/Smad3 pathway. Long noncoding RNAs (lncRNAs), a type of ncRNA with a length of >200 nucleotides, are important regulators of most biological and pathological processes. Many previous studies have shown that mutation and dysregulation of lncRNAs are closely associated with various human diseases, including fibrosis. For example, lncRNA APTR promotes the activation of hepatic stellate cells and the progression of liver fibrosis, lncRNA CYP4B1-PS1-001 and ENSMUST00000147869 regulate proliferation and fibrosis in diabetic nephropathy, lncRNA MIAT promotes cardiac fibrosis in postinfarct myocardium, lncRNA H19 controls the DUSP5/extracellular signal-regulated kinase 1/2 (ERK1/2) axis in cardiac fibroblast proliferation and fibrosis, and H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of EpCAM (15–20). In addition, H19 has been reported to be upregulated in fibrotic rat lung and play a stimulative role in bleomycin (BLM)-induced pulmonary fibrosis in mice (21). However, its expression in human fibrotic lung tissues and mechanism of action remain unclear. Previous studies have revealed that lncRNAs can function by interacting with miRNAs and upregulating the targets of miRNAs. We performed bioinformatics analysis of miRNA recognition sequences on H19 by starBase v2.0 and found H19 contained a binding site of miR-140. In view of these findings, it is tempting to speculate that H19 promotes pulmonary fibrosis by decreasing miR-140 expression and improving the activity of the TGF-β/Smad3 pathway.
In this study, we determined the expression levels of H19 and miR-140 in 15 IPF tissues and 15 normal lung histology samples, and we analyzed the correlation between H19 and miR-140 expression in IPF tissues. We also investigated the roles of H19 in pulmonary fibrosis using a TGF-β1-induced cell model and BLM-induced mouse model and the molecular mechanism in pulmonary fibrosis.
RESULTS
The expression and correlation of H19 and miR-140 in lung tissues of IPF patients.
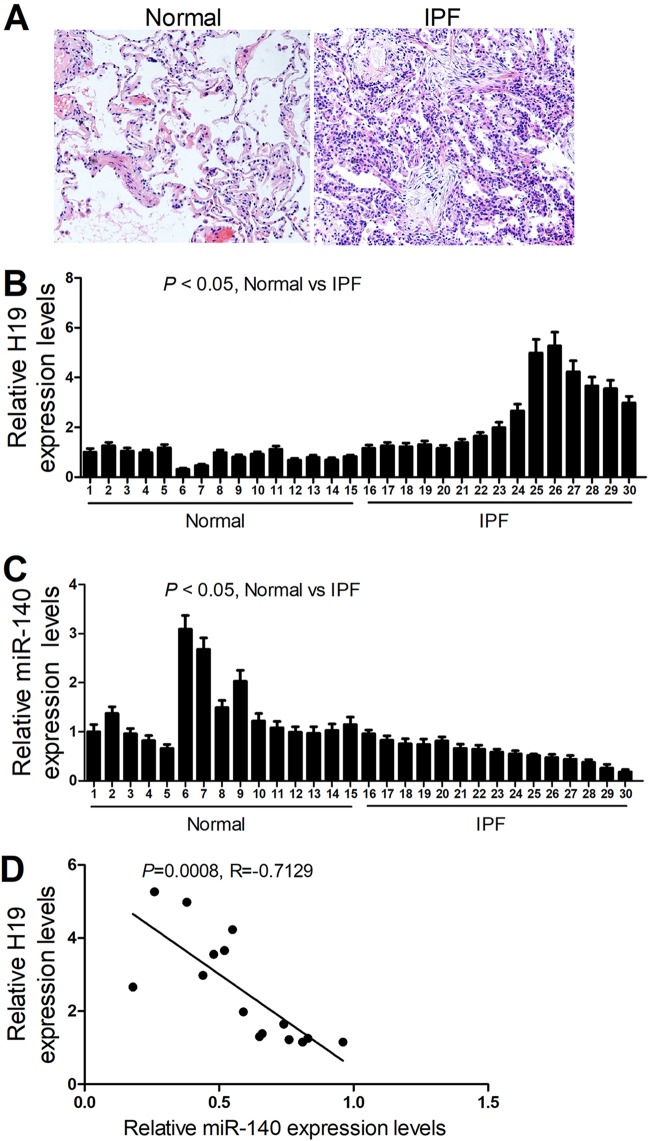
H19 and miR-140 expression levels were determined by reverse transcription-PCR (RT-PCR) in 15 normal lung tissues and 15 pulmonary fibrotic tissues from IPF patients (Fig. 1A). As shown in Fig. 1B and C, pulmonary fibrotic tissues from IPF patients had higher H19 levels and lower miR-140 levels than normal lung tissues. In addition, a negative correlation was observed between H19 and miR-140 expression in the lung tissues of IPF patients (Fig. 1D).
FIG 1.
H19 is significantly upregulated in pulmonary fibrotic tissues from IPF patients and is inversely correlated with the expression level of miR-140. (A) Representative images of normal and fibrotic tissues in lung from H&E staining assays. (B and C) qRT-PCR assays showed that pulmonary fibrotic tissues from IPF patients have higher H19 levels and lower miR-140 levels than normal lung tissues. (D) A negative correlation was observed between H19 and miR-140 expression in the lung tissues of IPF patients.
H19 and miR-140 expression in TGF-β1-treated HBE and A549 cells.
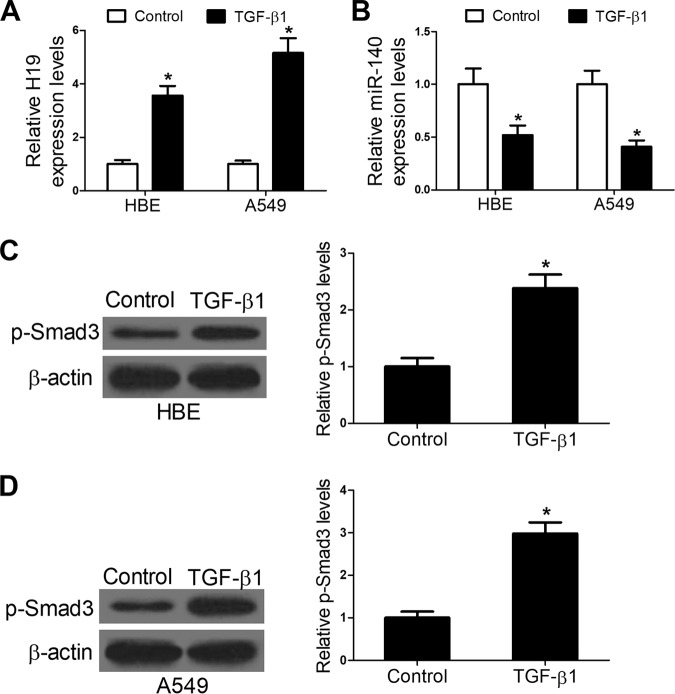
TGF-β1 is one of the major mediators of pulmonary fibrosis and can induce fibrosis in vitro. After exposure to TGF-β1 (10 ng/ml) for 48 h, HBE and A549 cells were collected, and H19 as well as miR-140 expression in these cells was determined. The results showed that TGF-β1 treatment enhanced H19 expression (Fig. 2A) and reduced miR-140 expression in HBE and A549 cells (Fig. 2B). In addition, Western blot assays were conducted to detect the effects of TGF-β1 treatment on expression of the TGF-β1 signaling activator phosphorylation of Smad3 (p-Smad3). The results showed that TGF-β1 treatment increased the expression levels of p-Smad3 (Fig. 2C and D). Collectively, these results indicate that H19 and miR-140 expression is associated with pulmonary fibrosis in vitro.
FIG 2.
TGF-β1 treatment enhances H19 and p-Smad3 expression and reduces miR-140 expression in HBE and A549 cells. (A and B) qRT-PCR assays showed that TGF-β1 treatment enhanced H19 expression and reduced miR-140 expression in HBE and A549 cells. (C and D) Western blot assays showed that TGF-β1 treatment enhanced p-Smad expression in HBE and A549 cells. *, P < 0.05.
H19 knockdown represses pulmonary fibrosis in vitro.
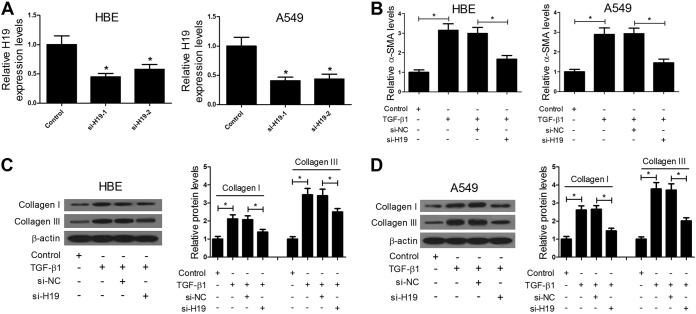
To further investigate the roles of H19 in pulmonary fibrosis, H19 expression was decreased by short interfering RNA (siRNA) transfection in HBE and A549 cells. As shown in Fig. 3A, compared with siRNA H19-2 (si-H19-2) transfection, si-H19-1 transfection decreased H19 expression to a greater extent, which was the reason why si-H19-1 transfection was performed for follow-up experiments. Myofibroblast generation and deposition of extracellular matrix collagen are major characteristics of pulmonary fibrosis. Thus, we further investigated the effects of H19 reduction on pulmonary fibrosis via detection of myofibroblast marker alpha smooth-muscle actin (α-SMA) levels by enzyme-linked immunosorbent assay (ELISA) and collagen I and collagen III expression by Western blotting. The results showed that H19 knockdown blocked TGF-β1-induced α-SMA and collagen I and III expression in HBE and A549 cells (Fig. 3B to D). Taken together, these results indicate that H19 knockdown represses pulmonary fibrosis in vitro.
FIG 3.
H19 knockdown represses pulmonary fibrosis in vitro. (A) qRT-PCR assays showed that transfection of si-H19-1 or si-H19-2 significantly decreased H19 expression in HBE and A549 cells, especially in the si-H19-1 group. (B to D) ELISA and Western blot assays showed that H19 knockdown blocked TGF-β1-induced α-SMA and collagen I and III expression in HBE and A549 cells. *, P < 0.05.
H19 functions by targeting miR-140.
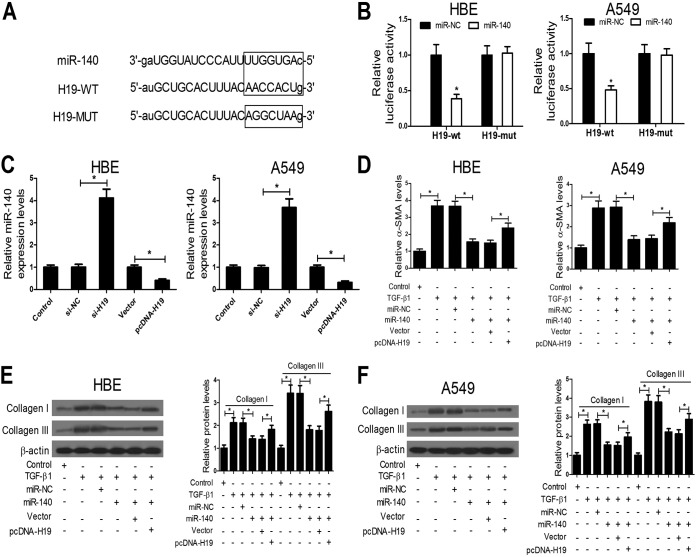
The online software starBase v2.0 was employed to predict miRNA recognition elements on H19, and the results showed that miR-140 could bind to complementary sequences in H19 (Fig. 4A). The H19 sequence with wild-type (WT) or mutant (MUT) binding sites of miR-140 was inserted into luciferase reporter plasmids, and then the plasmids (H19-WT or H19-MUT) were cotransfected with miR-140 mimic or miRNA negative control (miR-NC) into HBE and A549 cells. Compared with miR-NC, miR-140 mimic transfection significantly reduced the luciferase activity of the wild-type reporter but did not affect the luciferase activity of the mutant reporter (Fig. 4B). In addition, the miR-140 expression level was increased by H19 inhibition, while it was decreased by H19 overexpression (Fig. 4C). These data suggest that H19 can directly target miR-140 and downregulate its expression. We further investigated whether H19 functions by targeting miR-140. miR-140 mimic and pcDNA-H19 were transfected into HBE and A549 cells individually or in combination, and then these cells were exposed to TGF-β1 (10 ng/ml). After 48 h, α-SMA, collagen I, and collagen III expression was determined. The results showed that the increase of miR-140 blocked TGF-β1-induced α-SMA and collagen I and III expression, while H19 upregulation diminished the effects of miR-140 on TGF-β1-induced α-SMA and collagen I and III expression (Fig. 4D to F). Collectively, these results indicate that H19 functions by targeting miR-140 in vitro.
FIG 4.
H19 functions by targeting miR-140. (A) Bioinformatics analyses showed the predicted H19 binding sites in miR-140. (B) Luciferase reporter assays revealed that cotransfection with miR-140 significantly reduced luciferase activity of the wild-type reporter, but it had no effect on the luciferase activity of the mutant reporter in HBE and A549 cells. (C) qRT-PCR assays showed that the miR-140 expression level was increased by H19 inhibition while it was decreased by H19 overexpression. (D to F) ELISA and Western blot assays showed that the increase of miR-140 blocked TGF-β1-induced α-SMA and collagen I and III expression, while H19 upregulation diminished the effects of miR-140 on TGF-β1-induced α-SMA and collagen I and III expression. *, P < 0.05.
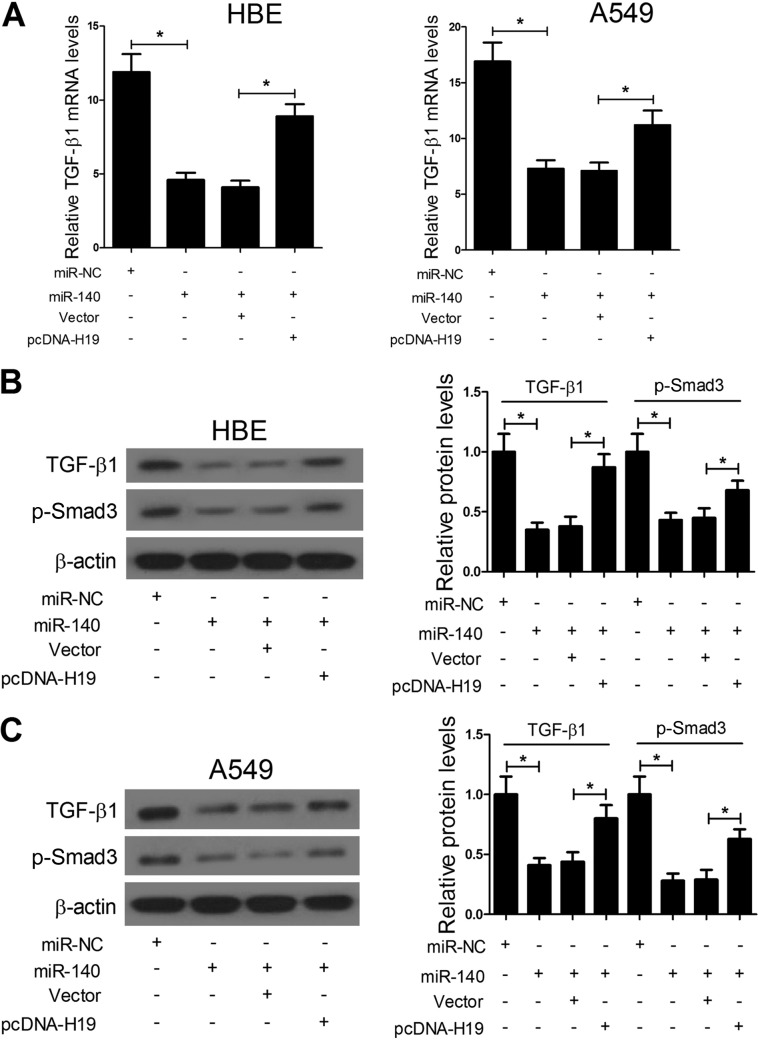
H19 targets miR-140 and regulates the TGF-β/Smad3 pathway.
In view of the previous report, which showed that miR-140 ameliorates pulmonary fibrosis by targeting Smad3 and suppressing the TGF-β/Smad3 pathway, we further investigated whether H19 targeted miR-140 and regulated the TGF-β/Smad3 pathway in HBE and A549 cells. We detected the expression of TGF-β1 and p-Smad3, two important factors in the TGF-β/Smad3 pathway, in response to the alteration of H19 and miR-140 expression. The results showed that miR-140 significantly repressed the mRNA and protein expression of TGF-β1, while H19 upregulation relieved the inhibitory effect of miR-140 on TGF-β1 expression (Fig. 5A to C). Similarly, miR-140 significantly repressed the protein expression of p-Smad3, while H19 upregulation relieved the inhibitory effect of miR-140 on p-Smad3 expression (Fig. 5B and C). Taken together, these data indicate that H19 targets miR-140 and regulates the TGF-β/Smad3 pathway in HBE and A549 cells.
FIG 5.
H19 targets miR-140 and regulates the TGF-β/Smad3 pathway. (A to C) qRT-PCR and Western blot assays showed that miR-140 significantly represses the mRNA and protein expression of TGF-β1 and p-Smad3, while H19 upregulation relieves the inhibitory effect of miR-140 on TGF-β1 and p-Smad3 expression. *, P < 0.05.
H19 knockdown inhibits pulmonary fibrosis via the TGF-β/Smad3 pathway in vivo.
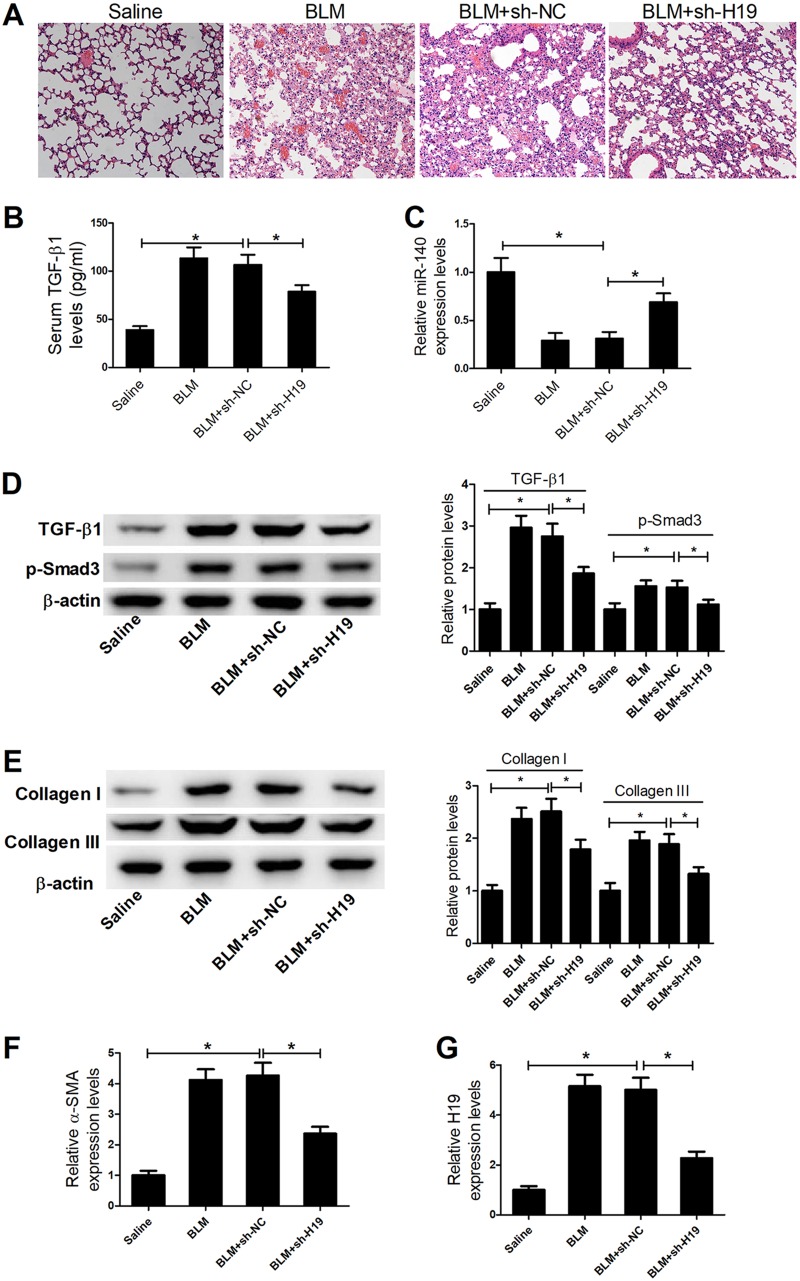
To further characterize the function of H19 in pulmonary fibrosis, BLM treatment was used to establish pulmonary fibrosis mouse models. The established pulmonary fibrosis mouse models were verified by hematoxylin and eosin (H&E) staining assays. H&E staining assays also showed marked attenuation of BLM-induced pulmonary fibrosis in the mice treated with short hairpin RNA H19 (sh-H19) (Fig. 6A). The serum TGF-β1 level was significantly increased in a BLM-induced mouse model of pulmonary fibrosis compared with that of the saline control, while sh-H19 treatment decreased TGF-β1 levels in a BLM-induced mouse model of pulmonary fibrosis (Fig. 6B). In addition, the expression of miR-140, TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA in the lung tissues of four groups was also detected. The results showed that miR-140 expression was decreased; TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA expression was increased in a BLM-induced pulmonary fibrosis mouse model compared with the control, while sh-H19 treatment enhanced miR-140 expression and reduced TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA expression in the BLM-induced mouse model of pulmonary fibrosis (Fig. 6C to F). To ensure the efficiency of sh-H19 in the BLM-induced mouse model of pulmonary fibrosis, quantitative RT-PCR (qRT-PCR) was used to detect the expression of H19 in different groups after transfection with sh-H19. The results showed that sh-H19 could downregulate the expression of H19 in the BLM-induced mouse model (Fig. 6G). Overall, these results indicate that H19 knockdown represses pulmonary fibrosis by regulating miR-140 expression and inhibiting the TGF-β/Smad3 pathway in vivo and in vitro.
FIG 6.
H19 knockdown inhibits pulmonary fibrosis in vivo. (A) H&E staining assays showed marked attenuation of BLM-induced pulmonary fibrosis in the mice treated with sh-H19. (B) ELISAs showed that the serum TGF-β1 level was significantly increased in BLM-induced mouse model of pulmonary fibrosis compared to that of the saline control, while sh-H19 treatment decreased TGF-β1 levels in the BLM-induced mouse model of pulmonary fibrosis. (C to F) qRT-PCR and Western blot assays showed that miR-140 expression was decreased and TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA expression levels were increased in BLM-induced pulmonary fibrosis mouse models compared with those of the control, while sh-H19 treatment enhanced miR-140 expression and reduced TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA expression in BLM-induced mouse models of pulmonary fibrosis. (G) qRT-PCR assays showed that H19 expression was decreased in BLM-induced pulmonary fibrosis mouse models after transfection with sh-H19. *, P < 0.05.
DISCUSSION
Pulmonary fibrosis represents the most aggressive form of interstitial lung disease, with a poor prognosis and high mortality rate. Increasing evidence has suggested that miRNAs, small ncRNAs, are involved in the progression of pulmonary fibrosis and serve as therapeutic targets and early diagnostic and prognostic biomarkers for patients with pulmonary fibrosis. For example, epigenetic regulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis, miR-29 inhibits BLM-induced pulmonary fibrosis in mice, miR-21 promotes fibrogenic activation of pulmonary fibroblasts and lung fibrosis, miR-154 promotes fibroblast migration and proliferation, miR-140 protects against radiation-induced lung fibrosis through inhibiting myofibroblast differentiation and inflammation, and miR-200 can reverse the fibrogenic activity of pulmonary fibroblasts from mice with BLM-induced pulmonary fibrosis and patients with pulmonary fibrosis (8–13).
lncRNAs have attracted increasing attention in recent years due to their crucial effects in the life cycle of genes from transcription to mRNA splicing and translation and, when dysfunctional, in the presence of various diseases, including pulmonary fibrosis. For example, Cao et al. (22) have identified 210 upregulated and 358 downregulated lncRNAs in rats with lung fibrosis by performing a microarray profiling study. Song et al. (23) reported that lncRNA MRAK088388 and MRAK081523 are significantly upregulated in fibrotic rats and are highly correlated with the expression levels of N4bp2 and Plxna4, respectively. The molecular mechanisms by which lncRNAs function are complicated, and the interaction of lncRNAs and microRNAs is one of the most important molecular mechanisms. This molecular mechanism has been confirmed by numerous studies. For example, lncRNA UCA1 exerts oncogenic functions in non-small-cell lung cancer by targeting miR-193a-3p, lncRNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer, lncRNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing miR-99a/miR-449a, lncRNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21, and H19 contributes to gallbladder cancer cell proliferation by modulated miR-194-5p targeting AKT2 (24–28). In addition, H19 is widely reported to be associated with fibrosis, including pulmonary fibrosis (19–21). Song et al. reported that H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of EpCAM (20). Tang et al. reported that H19 could affect a bleomycin-induced mouse model of idiopathic pulmonary fibrosis through interaction with miR-29b (21). All of the studies have suggested that H19 is involved in the occurrence and development of pulmonary fibrosis, and H19 could be a new target for the treatment of pulmonary fibrosis. Thus, a further investigation on the roles of H19 in pulmonary fibrosis and the probable molecular mechanism behind it is very significant.
In our study, we revealed a new working mechanism for H19 in pulmonary fibrosis. We employed starBase v2.0 to predict miRNA recognition elements on H19, and miR-140 was predicted to be a potential miRNA target on H19. The expression of H19 and miR-140 was detected, and the results showed that pulmonary fibrotic tissues from IPF patients had higher H19 levels and lower miR-140 levels than normal lung tissues, and H19 expression was inversely related to miR-140 expression in the lung tissues of IPF patients. In addition, TGF-β1 treatment significantly increased H19 expression and decreased miR-140 expression. Both H19 knockdown and the increase of miR-140 inhibited TGF-β1-induced pulmonary fibrosis, while H19 upregulation diminished the inhibitory effects of miR-140 on TGF-β1-induced pulmonary fibrosis in vitro. Luciferase reporter assays further confirmed that H19 could directly target miR-140. These results suggested that H19 exerted its effects in pulmonary fibrosis by suppressing miR-140 expression.
TGF-β/Smad3 signaling is one of the key pathways responsible for pulmonary fibrosis. Wang et al. (14) have reported that low-dose paclitaxel ameliorates pulmonary fibrosis by upregulating miR-140 and suppressing the TGF-β/Smad3 pathway. We further explored whether H19 exerted regulatory effects in pulmonary fibrosis by targeting miR-140 and regulating the TGF-β/Smad3 pathway. We conducted a series of experiments and found that TGF-β1 treatment significantly increased TGF-β signaling activator p-Smad3 expression and miR-140 repressed TGF-β1 and p-Smad3 expression in HBE and A549 cells, while H19 upregulation relieved the inhibitory effects of miR-140 on TGF-β1 and p-Smad3 expression. These data confirm that H19 targets miR-140 and regulates the TGF-β/Smad3 pathway in vitro. To further characterize the roles and molecular mechanism of H19 in pulmonary fibrosis, BLM was used to produce pulmonary fibrosis mouse models. BLM is known to produce pulmonary fibrosis in experimental animals and humans, and a BLM-induced pulmonary fibrosis animal model has been widely used to explore the pathogenesis of pulmonary fibrosis (29–33). The established pulmonary fibrosis mouse models were verified by H&E staining assays. The treatment of sh-H19 attenuated BLM-induced pulmonary fibrosis. In addition, sh-H19 treatment decreased TGF-β1, p-Smad3, collagen I, collagen III, and α-SMA expression and increased miR-140 expression in BLM-induced mouse models of pulmonary fibrosis. Overall, these results indicate that H19 knockdown represses pulmonary fibrosis by regulating miR-140 expression and inhibiting the TGF-β/Smad3 pathway in vivo and in vitro.
In conclusion, our findings showed that H19 was significantly upregulated and miR-140 was markedly reduced in pulmonary fibrotic tissues from IPF- and TGF-β1-induced HBE and A549 cells. H19 knockdown attenuated pulmonary fibrosis via the regulatory network of lncRNA H19–miR-140–TGF-β/Smad3 signaling in vivo and in vitro. These findings provide great insights into the molecular mechanisms involved in the pathogenesis of pulmonary fibrosis and novel potential therapeutic targets and early diagnostic and prognostic biomarkers for pulmonary fibrosis.
MATERIALS AND METHODS
Primary tissue samples.
Lung tissues from 15 IPF patients and 15 normal lung histology samples were obtained from the Lung Tissue Research Consortium. Written informed consent was obtained from all the participants. The Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved this patient study.
Cell culture and transfection.
A549 human type II alveolar epithelial cells and human bronchial epithelium (HBE) cells were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured according to instructions. Plasmid cDNA-H19 cDNA (pcDNA-H19) was obtained by introducing H19 cDNA sequence into the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA). The short hairpin RNA (shRNA) and short interfering RNA (siRNA) targeting H19, miR-140 mimic, and their respective controls were synthesized by Genepharma Company (Shanghai, China). Oligonucleotide and plasmid transfection was performed by using Lipofectamine 2000 reagent (Invitrogen). For induction of fibrosis, the cells were treated with TGF-β1 (10 ng/ml; Peprotech, Rocky Hill, NJ) for 48 h.
RNA extraction and RT-PCR.
Total RNA was extracted using TRIzol reagent and was reverse transcribed with reverse transcriptase (ReverTra Ace; Toyobo, Osaka, Japan). The expression levels of H19, α-SMA, and TGF-β1 were quantified using SYBR green RT-PCR and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The expression level of miR-140 was quantified using stem-loop reverse transcription-PCR on a CFX96 RT-PCR detection system (Bio-Rad, Richmond, CA) and normalized to that of U6.
Animals and BLM administration.
All animal procedures were performed in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (34). The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Male C57BL/6 mice aged 6 to 8 weeks were obtained from the Branch of National Breeder Center of Rodents (Shanghai, China) and maintained under strict specific-pathogen-free conditions. The animals were randomly divided into four groups: the saline group, where the mice were given a single intratracheal instillation of 0.05 ml of sterile saline, the BLM group, where the mice were given a single intratracheal instillation of 0.05 ml of sterile saline containing BLM (1.5 U/kg of body weight; Nippon Kayaku, Tokyo, Japan), and BLM+sh-NC and BLM+sh-H19 groups, where sh-NC or sh-H19 was administered after BLM treatment. At 4 weeks, the mice were sacrificed and blood and lung tissue were collected. Serum was separated from blood by centrifugation, and the serum TGF-β1 level was determined.
ELISA.
The protein level of α-SMA in cell culture supernatants and TGF-β1 protein level in serum were measured by using an ELISA kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
Histology.
Paraformaldehyde-fixed lung tissues were embedded in paraffin, cut into 4-μm sections, and then stained with hematoxylin and eosin (H&E). The slides were viewed by light microscope (model BX40; Olympus, Japan), and the images were captured and analyzed.
Western blotting.
Western blotting was performed as previously described (35). Primary antibodies against p-Smad3, collagen I, collagen II, TGF-β1, and β-actin and a horseradish peroxidase-conjugated secondary antibody were employed in these assays.
Luciferase reporter assay.
Cells cultured in 24-well plates were cotransfected with miR-140 mimic or miRNA negative control and luciferase reporter plasmids containing the H19 fragments with WT or MUT binding sites of miR-140 by Lipofectamine 2000. At 48 h posttransfection, cells were lysed and luciferase activity was monitored by using a GloMax 20/20 luminometer (Promega, Madison, WI).
Statistical analysis.
Data were expressed as means ± standard deviations. The comparisons between groups were performed via Student's t test or analysis of variance. P values of <0.05 were considered statistically significant. All experiments were performed in at least three replicates.
ACKNOWLEDGMENT
We have no potential conflicts of interest to disclose.
REFERENCES
- 1.Ley B, Collard HR, King TE Jr. 2011. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 2.Bartram U, Speer CP. 2004. The role of transforming growth factor β in lung development and disease. Chest J 125:754–765. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 3.Derynck R, Zhang YE. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 4.Qin W, Chung AC, Huang XR, Meng X-M, Hui DS, Yu C-M, Sung JJ, Lan HY. 2011. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakos G, Takagawa S, Chen S-J, Ferreira AM, Han G, Masuda K, Wang X-J, DiPietro LA, Varga J. 2004. Targeted disruption of TGF-β/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol 165:203–217. doi: 10.1016/S0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Königshoff M, Jayachandran A, Handley D, Seeger W, Kaminski N, Eickelberg O. 2008. Transgelin is a direct target of TGF-β/Smad3-dependent epithelial cell migration in lung fibrosis. FASEB J 22:1778–1789. doi: 10.1096/fj.07-083857. [DOI] [PubMed] [Google Scholar]
- 7.Warburton D, Shi W, Xu B. 2013. TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol 304:L83–L85. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, Mikhail A, Hitchcock CL, Wright VP, Nana-Sinkam SP, Piper MG, Marsh CB. 2013. Epigenetic regulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 187:397–405. doi: 10.1164/rccm.201205-0888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao J, Meng X-M, Huang XR, Chung AC, Feng Y-L, Hui DS, Yu C-M, Sung JJ, Lan HY. 2012. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther 20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. 2010. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, McMillan M, Kahn M, Kaminski N. 2012. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol 47:879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duru N, Zhang Y, Gernapudi R, Wolfson B, Lo P-K, Yao Y, Zhou Q. 2016. Loss of miR-140 is a key risk factor for radiation-induced lung fibrosis through reprogramming fibroblasts and macrophages. Sci Rep 6:39572. doi: 10.1038/srep39572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G. 2012. Participation of miR-200 in pulmonary fibrosis. Am J Pathol 180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Song X, Li Y, Han F, Gao S, Wang X, Xie S, Lv C. 2013. Low-dose paclitaxel ameliorates pulmonary fibrosis by suppressing TGF-β1/Smad3 pathway via miR-140 upregulation. PLoS One 8:e70725. doi: 10.1371/journal.pone.0070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu F, Zheng J, Mao Y, Dong P, Li G, Lu Z, Guo C, Liu Z, Fan X. 2015. Long non-coding RNA APTR promotes the activation of hepatic stellate cells and the progression of liver fibrosis. Biochem Biophys Res Commun 463:679–685. doi: 10.1016/j.bbrc.2015.05.124. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Wang S, Yao D, Yan Q, Lu W. 2016. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol 426:136–145. doi: 10.1016/j.mce.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Yao D, Wang S, Yan Q, Lu W. 2016. Long non-coding RNA ENSMUST00000147869 protects mesangial cells from proliferation and fibrosis induced by diabetic nephropathy. Endocrine 54:81–92. doi: 10.1007/s12020-016-0950-5. [DOI] [PubMed] [Google Scholar]
- 18.Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, Yang T, Zhan L, Yuan Y, Chu W. 2017. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep 7:42657. doi: 10.1038/srep42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao H, Cao W, Yang J-J, Shi K-H, Zhou X, Liu L-P, Li J. 2016. Long noncoding RNA H19 controls DUSP5/ERK1/2 axis in cardiac fibroblast proliferation and fibrosis. Cardiovasc Pathol 25:381–389. doi: 10.1016/j.carpath.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Liu C, Liu X, Trottier J, Beaudoin M, Zhang L, Pope C, Peng G, Barbier O, Zhong X. 2017. H19 promotes cholestatic liver fibrosis by preventing ZEB1–mediated inhibition of EpCAM. Hepatology 66:1183–1196. doi: 10.1002/hep.29209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, He R, An J, Deng P, Huang L, Yang W. 2016. The effect of H19-miR-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem Biophys Res Commun 479:417–423. doi: 10.1016/j.bbrc.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Cao G, Zhang J, Wang M, Song X, Liu W, Mao C, Lv C. 2013. Differential expression of long non-coding RNAs in bleomycin-induced lung fibrosis. Int J Mol Med 32:355–364. doi: 10.3892/ijmm.2013.1404. [DOI] [PubMed] [Google Scholar]
- 23.Song X, Cao G, Jing L, Lin S, Wang X, Zhang J, Wang M, Liu W, Lv C. 2014. Analysing the relationship between lncRNA and protein–coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J Cell Mol Med 18:991–1003. doi: 10.1111/jcmm.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nie W, Ge H-J, Yang X-Q, Sun X, Huang H, Tao X, Chen W-S, Li B. 2016. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett 371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Liu X-H, Sun M, Nie F-Q, Ge Y-B, Zhang E-B, Yin D-D, Kong R, Xia R, Lu K-H, Li J-H, De W, Wang K-M, Wang Z-X. 2014. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer 13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang E-B, Kong R, Yin D-D, You L-H, Sun M, Han L, Xu T-P, Xia R, Yang J-S, De W, Chen JF. 2014. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 5:2276–2292. doi: 10.18632/oncotarget.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y, Yang X, Shen J, Liu Q, Zhang J. 2016. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol 37:2691–2702. doi: 10.1007/s13277-015-4111-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang S-H, Wu X-C, Zhang M-D, Weng M-Z, Zhou D, Quan Z-W. 2016. Long noncoding RNA H19 contributes to gallbladder cancer cell proliferation by modulated miR-194-5p targeting AKT2. Tumour Biol 37:9721–9730. doi: 10.1007/s13277-016-4852-1. [DOI] [PubMed] [Google Scholar]
- 29.Sharma SK, MacLean JA, Pinto C, Kradin RL. 1996. The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. Am J Respir Crit Care Med 154:193–200. doi: 10.1164/ajrccm.154.1.8680680. [DOI] [PubMed] [Google Scholar]
- 30.Adamson IY, Bowden DH. 1974. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 77:185. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H-D, Yamaya M, Okinaga S, Jia Y-X, Kamanaka M, Takahashi H, Guo L-Y, Ohrui T, Sasaki H. 2002. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med 165:406–411. doi: 10.1164/ajrccm.165.3.2003149. [DOI] [PubMed] [Google Scholar]
- 32.Sato N, Suzuki Y, Nishio K, Suzuki K, Naoki K, Takeshita K, Kudo H, Miyao N, Tsumura H, Serizawa H, Suematsu M, Yamaguchi K. 2000. Roles of ICAM-1 for abnormal leukocyte recruitment in the microcirculation of bleomycin-induced fibrotic lung injury. Am J Respir Crit Care Med 161:1681–1688. doi: 10.1164/ajrccm.161.5.9907104. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuhashi H, Asano S, Nonaka T, Hamamura I, Masuda K-I, Kiyoki M. 1996. Administration of truncated secretory leukoprotease inhibitor ameliorates bleomycin-induced pulmonary fibrosis in hamsters. Am J Respir Crit Care Med 153:369–374. doi: 10.1164/ajrccm.153.1.8542145. [DOI] [PubMed] [Google Scholar]
- 34.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 35.Xie H, Xue J-D, Chao F, Jin Y-F, Fu Q. 2016. Long non-coding RNA-H19 antagonism protects against renal fibrosis. Oncotarget 7:51473. doi: 10.18632/oncotarget.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]