Abstract
OBJECTIVE:
To estimate expulsion rates among women with postpartum intrauterine device (IUD) placement by timing of insertion, IUD type, and delivery method.
DATA SOURCES:
We searched PubMed, Cochrane Library, and ClinicalTrials.gov from 1974 to May 2018.
METHODS OF STUDY SELECTION:
We searched databases for any published studies that examined post-partum placement of a copper IUD or levonorgestrel intrauterine system and reported counts of expulsions. We assessed study quality using the U.S. Preventive Services Task Force evidence grading system. We calculated pooled absolute rates of IUD expulsion and estimated adjusted relative risks (RRs) for timing of postpartum placement, delivery method, and IUD type using log-binomial multivariable regression model.
TABULATION, INTEGRATION, AND RESULTS:
We identified 48 level I to II-3 studies of poor to good quality. Pooled rates of expulsion varied by timing of IUD placement, ranging from 1.9% with interval placements (4 weeks postpartum or greater), 10.0% for immediate placements (10 minutes or less after placental delivery), and 29.7% for early placements (greater than 10 minutes to less than 4 weeks postpartum). Immediate and early postpartum placements were associated with increased risk of expulsion compared with interval placement (adjusted RR 7.63, 95% CI 4.31–13.51; adjusted RR 6.17, 95% CI 3.19–11.93, respectively). Postpartum placement less than 4 weeks after vaginal delivery was associated with an increased risk of expulsion compared with cesarean delivery (adjusted RR 5.19, 95% CI 3.85–6.99). Analysis of expulsion rates at less than 4 weeks postpartum also indicated that the levonorgestrel intrauterine system was associated with a higher risk of expulsion (adjusted RR 1.91, 95% CI 1.50–2.43) compared with CuT380A.
CONCLUSION:
Postpartum IUD expulsion rates vary by timing of placement, delivery method, and IUD type. These results can aid in counseling women to make an informed choice about when to initiate their IUD and to help institutions implement postpartum contraception programs.
Postpartum intrauterine device (IUD) placement provides safe and highly effective contraception at a time when women are accessing medical care.1–4 Previously published systematic reviews of postpartum IUD placement generally report low rates of complications such as perforations and infections.2–4 The U.S. Medical Eligibility Criteria for Contraceptive Use (2016)5 and professional organizations including the American College of Obstetricians and Gynecologists support the safety of immediate post-partum IUD placement.6 However, previous reviews suggest that rates of IUD expulsions are higher when placed in the immediate or early postpartum period compared with placement later at a postpartum or interval visit.3 Expulsions may compromise effectiveness, especially when replacement IUDs are not easily accessible. However, because many postpartum women do not return for a postpartum visit and therefore never have an IUD placed, the benefit of placing an IUD immediately or soon after delivery may outweigh the risk of expulsion.3,7 Recent studies have shown high continuation rates among women receiving immediate postpartum IUDs as well as cost-effectiveness, despite higher expulsion rates.8–11 Although previous narrative systematic reviews have concluded that IUD expulsion rates are increased with postpartum placement compared with interval placement,2–4 absolute rates vary widely across studies and it has been difficult to quantify the magnitude of increased risk.1 With this review, our aim was to calculate pooled absolute rates of IUD expulsion and to estimate relative risk of expulsion for timing of placement in the postpartum period, delivery method, and IUD type.
SOURCES
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting this review.12 We searched PubMed, Cochrane Library, and ClinicalTrials.gov for all primary research studies of any study design, in any language, published from database inception through May 2018 that examined postpartum placement of IUDs. We searched PubMed using the following search strategy: ((((“Intrauterine Devices”[Mesh] OR “Intrauterine Devices, Copper”[Mesh] OR “Intrauterine Devices, Medicated”[Mesh] OR ((intrauterine OR intrauterine) AND (device OR system OR contracept*)) OR IUD OR IUC OR IUCD OR IUS OR mirena OR Skyla OR liletta OR paragard OR “Copper T380” OR CuT380 OR “Copper T380a” OR “Cu T380a”) AND (postpartum OR Puerperium*) NOT (“Animals”[Mesh] NOT “Humans”[Mesh])))). We searched Cochrane Library and ClinicalTrials.gov for any published reviews or additional studies including “Postpartum AND IUD.” We hand-searched relevant articles and reviews for additional references.
STUDY SELECTION
We included studies that examined immediate post-partum placement (10 minutes or less after placental delivery) or early postpartum placement (greater than 10 minutes to less than 4 weeks) and reported counts of expulsion and counts of women with follow-up. We included studies with any length of follow-up and any rate of follow-up. We excluded studies that did not report counts of women with IUD placement and occurrence of expulsion, which were required for calculation of pooled estimates of expulsions across studies. We included studies that examined copper-bearing IUDs that are currently available (CuT380A) or were previously available in the United States (Copper 7, TCu200) and the levonorgestrel intrauterine system (LNG-IUS). We excluded studies that evaluated IUDs that were modified from their standard structure and were not commercially available (eg, sutures added to the IUD to anchor the IUD to the endometrium). We included studies that examined IUD placement after vaginal delivery, cesarean delivery, or both.
Two coauthors (T.C.J. and M.K.W.) independently screened all titles and abstracts identified from the initial search to determine whether the studies met inclusion criteria. Full-text articles were reviewed as needed. Pertinent non-English articles were professionally translated by U.S. Centers for Disease Control and Prevention translation services. The lead author (T.C.J.) and one additional coauthor (M.K.W., E.B.-B., N.K.T.) reviewed each included study and abstracted the following information: study author, year of publication, country, funding source, study design, IUD type, timing of IUD placement, delivery method, length of study follow-up, number of women enrolled or randomized, number of IUDs initially placed, number of women with any follow-up, and counts of expulsion (overall, complete, and partial). To assess risk of bias, two coauthors independently reviewed each study according to the U.S. Preventive Services Task Force system to evaluate study design and methodologic features such as potential for selection bias (eg, groups not comparable at baseline for randomized controlled trials), mis-classification (eg, outcome of expulsion diagnosed inconsistently by nonblinded health care provider), and confounding (eg, parity and breastfeeding status not collected or adjusted for).13 Two coauthors (E.B.-B. and N.K.T.) assessed one study that was authored by the lead author of this review.14 Any discrepancies between authors for selection, abstraction, or risk of bias assessment were resolved through discussion.
DATA SYNTHESIS
We calculated expulsion rates by pooling the number of women with IUD placements and the number of IUD expulsions reported for each individual study for women with any follow-up. This calculation of the proportion of a pooled number of expulsions over a pooled number of IUD placements is equivalent to a sum of individual expulsion rates weighed by their corresponding study size. We assumed expulsions were complete expulsions if not otherwise defined, and we excluded partial expulsions from our primary analysis, because not all studies reported them, definitions varied or were absent from studies, or malpositioned IUDs were reported as partial expulsions. Because many of the included randomized controlled trials reported follow-up by intent-to-treat analyses, we were unable to determine how many women received IUDs at the assigned time period per protocol and returned for follow-up; therefore, for those studies that reported only intent-to-treat results, we used the number of women randomized who had follow-up as our denominator.
We calculated pooled expulsion rates within strata defined by factors including: timing of post-partum placement (immediate, early, either immediate or early [mixed], or interval placement at 4 weeks postpartum or greater), delivery method (cesarean, vaginal, either cesarean or vaginal [mixed], or unknown), IUD type (CuT380A, LNG-IUS, either CuT380A or LNG-IUS [mixed], Copper 7, or TCu200), length of study follow-up, study region (defined by the World Health Organization, www.who.int/about/regions/en/), and study quality (defined by U.S. Preventive Services Task Force13). The calculations were weighted by number of women in each individual study with respect to different factors listed. We included interval placements only when describing expulsions by placement timing because we wanted to compare the various postpartum periods with interval placements; for other comparisons, we focused only on immediate or early postpartum placements.
Using a log-binomial regression model, we estimated adjusted relative risks (RRs) of IUD expulsion and their associated 95% CIs with inclusion of potential risk factors (timing of IUD placement, delivery method, and IUD type) while adjusting for other covariates including World Health Organization study region, study quality, and length of study follow-up. Finally, we performed a number of sensitivity analyses related to our exclusions based on losses to follow-up and partial expulsion reporting. Women who had an IUD placement and were lost to follow-up were excluded from the primary analysis. To assess the possible effect of loss to follow-up, we included studies with a follow-up rate of at least 70% for additional analyses by assuming that all women lost to follow-up either continued their IUD or experienced an expulsion.15 In separate analyses, we also estimated adjusted RRs of overall expulsions (complete and partial expulsions together) for timing of placement, delivery method, and IUD type. All analyses were conducted using SAS 9.4.
RESULTS
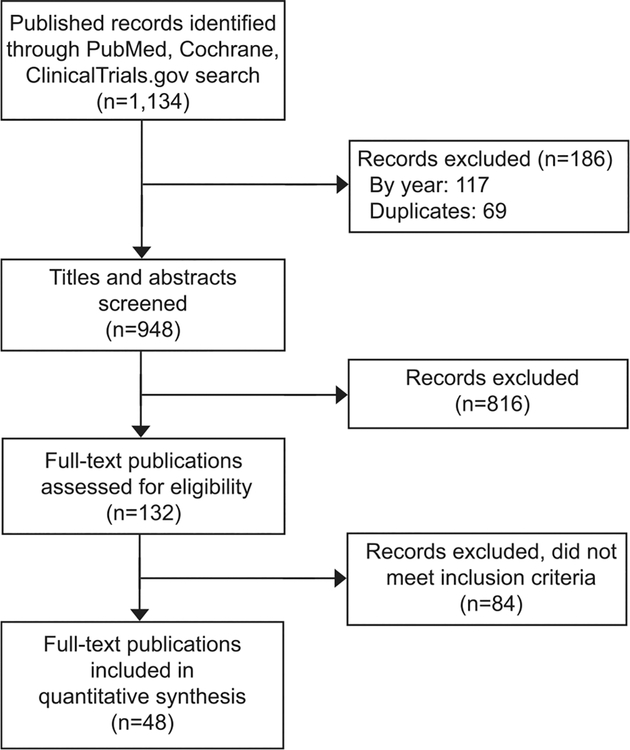
We identified 1,112 articles in PubMed, 19 studies in ClinicalTrials.gov, and three Cochrane reviews, for a total of 1,134 records (Fig. 1). After removing 69 duplicates and excluding 117 articles published before 1974, the first year when IUDs of interest were initially studied and available, we screened the title and abstract of the 948 articles. We excluded 816 not relevant to our search and then reviewed the full text of 132 articles. Based on full-text review, we excluded 84 articles that did not include IUDs that met inclusion criteria, did not include postpartum IUD placements, or did not provide individual counts for expulsions. We did not identify any additional completed studies with published data from ClinicalTrials.gov. From our Cochrane search, we identified one updated Cochrane review that included 15 trials, of which five met inclusion criteria.3 A total of 48 studies met our inclusion criteria. Two of the articles that met our inclusion criteria were translated from Spanish or French16,17 and the remaining were published in English.
Fig. 1.
Flow diagram of publication selection for inclusion into the review.
We describe study characteristics in Table 1. The body of evidence included studies largely published in the past 10 years, from all World Health Organization regions, with levels of evidence of I, II-2, and II-3 and with quality ratings assessed most frequently as fair or poor (compared with good for three studies).7,18,19 Twenty-two studies included only CuT380A IUDs,8,16–18,20–37 13 included only LNG-IUS,19,38–49 and nine included a mix of the two7,9,10,14,50–54; the remaining four studies included CuT20055–57 or Cu7 IUDs.58 Twenty-eight studied women with IUDs placed only in the immediate postpartum period,9,10,14,17,18,21,23,24,26–30,32–37,40–43,52–54,57,58 four studied only early postpartum placements,44,47,55,56 and the remaining 16 studied more than one placement period.7,8,16,19,20,22,25,31,38,39,45,46,48–50,51 Similar numbers of studies evaluated vaginal and cesarean deliveries, with 14 including only vaginal deliveries,14,16,19,20,22,31,34,39,42,44,45,48,53,58,16 including only cesarean deliveries,7,8,17,18,27–30,35,37,38,40,41,43,46,54 16 including both delivery methods,9,10,21,23–26,32,33,36,47,49,50,51,52,57 and two not reporting delivery method.55,56 Sample sizes ranged from 7–2,733 women; follow-up duration ranged from 4 weeks–5 years, and follow-up rates ranged from 29–100%.
Table 1.
Characteristics of Included Studies Reporting Counts of Expulsions Among Postpartum Women
| Study Author, Year | Region* | Level of Evidence† | Study Quality‡ | IUD Type§ | Placement Timingǁ | Delivery Method¶ | Length of Follow-up | No. Enrolled or Randomized | No. of Women With lUDs Placed | No. of Women With IUDs Placed With Follow-up (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Agarwal, 201 735 | Southeast Asia | II-3 | Fair | CuT380A | Immediate | Cesarean | 3 mo | 50 | 50 | 50 (100) |
| Baldwin, 201650 | North America | I | Fair | Mixed | Mixed | Mixed | 6 mo | 201 | 139 | Unknown (66#) |
| Bonilla Rosales, 200516 | South America | II-2 | Fair | CuT380A | Mixed | Vaginal | 3 mo | 250 | 250 | 239 (96) |
| Braniff, 201538 | Western Pacific | I | Fair | LNG-IUS | Mixed | Cesarean | 6 mo | 48 | 42 | Unknown (84#) |
| Bryant, 201320 | Africa | I | Poor | CuT380A | Mixed | Vaginal | 12 wk | 49 | 28 | 28 (100) |
| Celen, 201118 | Eastern Mediterranean | II-3 | Good | CuT380A | Immediate | Cesarean | 12 mo | 245 | 245 | 245 (100) |
| Chen, 201019 | North America | I | Good | LNG-IUS | Mixed | Vaginal | 6 mo | 124 | 96 | 84 (88) |
| Chen, 201751 | North America | II-2 | Poor | Mixed | Mixed | Mixed | 6 mo | 74 | 74 | 59 (80) |
| Cohen, 20169 | North America | II-3 | Poor | Mixed | Immediate | Mixed | 12 mo | 82 | 82 | 67 (82) |
| Colwill, 201836 | North America | II-2 | Fair | CuT380A | Immediate | Mixed | 6 wk | 210 | 210 | 169 (81) |
| Dahlke, 201139 | North America | I | Poor | LNG-IUS | Mixed | Vaginal | 6 mo | 53 | 46 | 45 (98) |
| Dias, 201521 | Southeast Asia | II-2 | Poor | CuT380A | Immediate | Mixed | 6 wk | 91 | 91 | 91 (100) |
| Eggebroten, 201752 | North America | II-2 | Poor | Mixed | Immediate | Mixed | 6 mo | 211 | 211 | 186 (88) |
| Elsedeek, 201241 | Eastern Mediterranean | II-3 | Fair | LNG-IUS | Immediate | Cesarean | 2y | 65 | 65 | 62 (95) |
| Elsedeek, 201540 | Eastern Mediterranean | II-3 | Fair | LNG-IUS | Immediate | Cesarean | 5y | 80 | 80 | 80 (100) |
| Eroglu, 20 0622 | Eastern Mediterranean | II-2 | Fair | CuT380A | Mixed | Vaginal | 12 mo | 268 | 268 | 257 (96) |
| Goldthwaite, 201753 | North America | II-2 | Fair | Mixed | Immediate | Vaginal | 12 wk | 123 | 123 | 96 (78) |
| Gueye, 201317 | Africa | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 mo | 46 | 46 | 39 (85) |
| Gupta, 201 423 | Southeast Asia | II-3 | Fair | CuT380A | Immediate | Mixed | 6 mo | 100 | 100 | 92 (92) |
| Hayes, 200742 | North America | II-3 | Fair | LNG-IUS | Immediate | Vaginal | 10 wk | 20 | 20 | 16 (80) |
| Heller, 201754 | Europe | II-3 | Fair | Mixed | Immediate | Cesarean | 12 mo | 120 | 114 | 99 (87) |
| Hooda, 201 624 | Southeast Asia | II-2 | Poor | CuT380A | Immediate | Mixed | 6 wk | 593 | 593 | 171 (29) |
| Jatlaoui, 201414 | North America | II-3 | Fair | Mixed | Immediate | Vaginal | 6 mo | 99 | 99 | 88 (89) |
| Kumar, 201 425 | Southeast Asia | II-3 | Poor | CuT380A | Mixed | Mixed | 6 wk | 2,733 | 2,733 | 1,730 (63) |
| Laes, 197555 | Europe | II-3 | Poor | CuT200 | Early | Unknown | 12 mo | 197 | 197 | 197 (100) |
| Lavin, 198356 | South America | II-3 | Poor | CuT200 | Early | Unknown | 12 mo | 1,142 | 1,142 | 945 (83) |
| Lester, 20158 | Africa | I | Poor | CuT380A | Mixed | Cesarean | 6 mo | 68 | 52 | Unknown (90#) |
| Letti Muller, 200526 | South America | II-2 | Fair | CuT380A | Immediate | Mixed | 1 mo | 38 | 38 | 37 (97) |
| Levi, 201227 | North America | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 mo | 90 | 90 | 42 (47) |
| Levi, 20157 | North America | I | Good | Mixed | Mixed | Cesarean | 6 mo | 112 | 87 | Unknown (88#) |
| Mishra, 201433 | Southeast Asia | II-3 | Poor | CuT380A | Immediate | Mixed | 4–6 wk | 564 | 564 | 434 (77) |
| Nelson, 200928 | North America | II-3 | Fair | CuT380A | Immediate | Cesarean | 6 wk | 7 | 7 | 7 (100) |
| Newton, 197758 | Europe | II-3 | Poor | Cu7 | Immediate | Vaginal | 6 wk | 123 | 123 | 123 (100) |
| Puzey, 200543 | Africa | II-3 | Poor | LNG-IUS | Immediate | Cesarean | 6 mo | 33 | 33 | 20 (61) |
| Ragab, 201529 | Eastern Mediterranean | II-3 | Fair | CuT380A | Immediate | Cesarean | 12 mo | 40 | 40 | 40 (100) |
| Shukla, 201257 | Southeast Asia | II-3 | Poor | CuT200 | Immediate | Mixed | 6 wk | 1,317 | 1,317 | 1,037 (79) |
| Singal, 201430 | Southeast Asia | II-3 | Fair | CuT380A | Immediate | Cesarean | 12 mo | 300 | 300 | 300 (100) |
| Singh, 20 1 631 | Southeast Asia | II-3 | Fair | CuT380A | Mixed | Vaginal | 8 wk | 80 | 80 | 80 (100) |
| Soon, 201848 | North America | I | Poor | LNG-IUS | Mixed | Vaginal | 6 mo | 11 | 8 | 7 (88) |
| Stuart, 201244 | North America | II-3 | Fair | LNG-IUS | Early | Vaginal | 6 mo | 40 | 29 | 27 (93) |
| Stuart, 201545 | North America | I | Poor | LNG-IUS | Mixed | Vaginal | 6 mo | 35 | 31 | Unknown (80#) |
| Sucak, 201532 | Eastern Mediterranean | II-2 | Fair | CuT380A | Immediate | Mixed | 12 mo | 160 | 160 | 153 (96) |
| Turok, 201749 | North America | I | Poor | LNG-IUS | Mixed | Mixed | 8 wk | 285 | 228 | 214 (94) |
| Unal, 201837 | Eastern Mediterranean | II-3 | Fair | CuT380A | Immediate | Cesarean | 3 mo | 70 | 70 | 68 (97) |
| Whitaker, 201446 | North America | I | Poor | LNG-IUS | Mixed | Cesarean | 12 mo | 42 | 37 | Unknown (81#) |
| Woo, 201510 | North America | II-3 | Poor | Mixed | Immediate | Mixed | 12 mo | 76 | 76 | 43 (57) |
| Xu, 199934 | Western Pacific | II-3 | Fair | CuT380A | Immediate | Vaginal | 36 mo | 384 | 384 | 381 (99) |
| Zerden, 201747 | North America | II-3 | Fair | LNG-IUS | Early | Mixed | 6 mo | 50 | 50 | 43 (86) |
IUD, intrauterine device; Cu, copper; LNG-IUS, levonorgestrel intrauterine system.
Based on World Health Organization regions with region of the Americas separated into North and South American regions.
Level of evidence: I, a randomized, controlled trial; II-2, a cohort or case-controlled study that includes a comparison group; III, an uncontrolled descriptive study including case series.
Defined by U.S. Preventive Services Task Force (Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the U.S. Preventive Services Task Force: a review of the process.
Mixed=CuT380A and LNG-IUS combined.
Immediate=10 min or less after placental delivery; Early=greater than 10 min to less than 4 wk postpartum; Mixed=Immediate and early placements or, immediate or early placements and interval placements (4 wk or greater postpartum).
Mixed=vaginal or cesarean delivery.
Number of women with IUDs placed having follow-up not reported; therefore, percentage represents number of women with IUD placements among all women randomized.
We describe the crude pooled rates of expulsion by placement timing, delivery method, and IUD type among women with any follow-up in Table 2. Expulsion rates from individual studies ranged from 0% to 46.7% and generally increased with increasing length of study follow-up, ranging from 7.3% (range 0.0–21.4) for studies with follow-up less than 3 months to 18.4% (range 0.0–39.4) for studies with follow-up greater than 6 months. Although the most frequent study follow-up time period was 3–6 months, those with follow-up less than 3 months contributed the largest total number of women to our primary analysis.
Table 2.
Pooled Expulsion Rates by Study Follow-up Length
| Study Follow-up (mo) | Study Follow-up (mo) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Studies | Longer Than 6 | 3–6 | Less Than 3 | |||||||||
| No. of Studies | No. of Women With IUDs Placed With Follow-up | Complete Expulsion Rate* | No. of Studies | No. of Women With lUDs Placed With Follow-up | Complete Expulsion Rate* | No. of Studies | No. of Women With IUDs Placed With Follow-up | Complete Expulsion Rate* | No. of Studies | No. of Women With IUDs Placed With Follow-up | Complete Expulsion Rate* | |
| Placement timing†‡ | ||||||||||||
| Total | 48 | 8,569 | 11.3 (0.0–46.7) | 14 | 2,906 | 18.4 (0.0–39.4) | 20 | 1,430 | 8.6 (0.0–46.7) | 14 | 4,233 | 7.3 (0.0–21.4) |
| Immediate | 39 | 4,754 | 10.0 (0.0–26.7) | 12 | 1,573 | 9.2 (0.0–25.4) | 15 | 876 | 11.1 (0.0–26.7) | 12 | 2,305 | 10.2 (0.0–21.4) |
| Early | 9 | 1,372 | 29.7 (0.0–46.7) | 3 | 1,185 | 32.7 (3.6–39.4) | 6 | 187 | 11.2 (0.0–46.7) | 0 | NA | NA |
| Interval | 14 | 633 | 1.9 (0.0–3.9) | 2 | 148 | 3.4 (0.0–3.9) | 10 | 367 | 1.4 (0.0–2.9) | 2 | 118 | 1.7 (0.0–2.0) |
| Early or immediate | 2 | 1,810 | 3.8 (3.6–7.5) | 0 | NA | NA | 0 | NA | NA | 2 | 1,810 | 3.8 (3.6–7.5) |
| Delivery method†§ | ||||||||||||
| Total | 48 | 7,936 | 12.0 (0.0–46.7) | 14 | 2,758 | 19.3 (0.0–39.4) | 20 | 1,063 | 11.1 (0.0–46.7) | 14 | 4,115 | 7.4 (0.0–22.2) |
| Cesarean | 22 | 1,512 | 3.6 (0.0–21.1) | 8 | 936 | 3.9 (0.0–21.1) | 9 | 369 | 4.3 (0.0–11.8) | 5 | 207 | 1.5 (0.0–3.2) |
| Vaginal | 20 | 1,543 | 14.9 (3.3–46.7) | 3 | 570 | 15.8 (4.8–7.6) | 8 | 378 | 21.4 (16.0–46.7) | 9 | 595 | 9.9 (3.3–22.2) |
| Cesarean or vaginal | 10 | 3,739 | 7.8 (0.0–25.4) | 2 | 110 | 23.6 (20.9–25.4) | 4 | 316 | 6.7 (0.0–11.3) | 4 | 3,313 | 7.3 (3.6–21.4) |
| Unknown | 2 | 1,142 | 33.2 (3.6–39.4) | 2 | 1,142 | 33.2 (3.6–39.4) | 0 | NA | NA | 0 | NA | NA |
| IUD type†§ | ||||||||||||
| Total | 48 | 7,936 | 12.0 (0.0–46.7) | 14 | 2,758 | 19.3 (0.0–39.4) | 20 | 1,063 | 11.1 (0.0–46.7) | 14 | 4,115 | 7.4 (0.0–27.3) |
| CuT380A | 25 | 4,567 | 6.7 (0.0–19.2) | 6 | 1,246 | 9.4 (2.0–17.6) | 9 | 549 | 8.9 (0.0–19.2) | 10 | 2,772 | 5.1 (0.0–10.8) |
| LNG | 16 | 718 | 15.5 (0.0–46.7) | 3 | 161 | 2.5 (0.0–21.1) | 10 | 374 | 17.4 (0.0–46.7) | 3 | 183 | 22.9 (18.8–27.3) |
| CuT380A or LNG | 6 | 349 | 10.0 (0.0–25.4) | 3 | 209 | 14.8 (5.1–25.4) | 3 | 140 | 2.9 (0.0–7.6) | 0 | NA | NA |
| Cu7 | 1 | 123 | 3.3 (NA) | 0 | NA | NA | 0 | NA | NA | 1 | 123 | 3.3 (NA) |
| CuT200 | 3 | 2,179 | 22.8 (3.6–39.4) | 2 | 1,142 | 33.2 (3.6–39.4) | 0 | NA | NA | 1 | 1,037 | 11.3 (NA) |
IUDs, intrauterine devices; NA, not applicable; Cu, copper; LNG, levonorgestrel
Among women with IUDs placed with any follow-up; data are % (range among studies).
Some studies included and reported more than one category.
Immediates=10 min or less after placental delivery; early=greater than 10 min to less than 4 wk postpartum; early or immediate=immediate and early combined; interval=4 wk or greater postpartum.
Among immediate and early postpartum placements.
By IUD placement timing (n58,569 women with immediate, early, or interval placements), immediate placement had a pooled expulsion rate of 10.0% (range 0.0–26.7%; n54,754), whereas early placement had a pooled expulsion rate of 29.7% (range 0.0–46.7%; n51,372) compared with 1.9% (range 0.0–3.9%; n5633) for interval placement when including all lengths of follow-up (Table 2). By delivery method (n57,936 women with immediate or early placements), the pooled expulsion rate for vaginal deliveries was 14.9% (range 3.3–46.7%; n51,543) and for cesarean deliveries was 3.6% (range 0.0–21.1%; n51,512) with higher rates for vaginal deliveries compared with cesarean delivery for all follow-up intervals. By IUD type including immediate and early placements (n57,936 women with immediate or early placements), pooled expulsion rates were highest for CuT200 IUDs (22.8%, range 3.6–39.4%; n52,179), and rates were higher for LNG-IUS (15.5%, range 0.0–46.7%; n5718) compared with CuT380A (6.7%, range 0.0–19.2%; n54,567). Although CuT380A pooled expulsion rates varied from 5.1%, 8.9%, and 9.4% among studies with less than 3 months, 3–6 months, and longer than 6 months follow-up, respectively, pooled rates of LNG-IUS expulsion decreased among studies with longer follow-up (22.9% during less than 3 months follow-up, 17.4% during 3–6 months follow-up, and 2.5% for follow-up longer than 6 months).
Studies from Africa (n54), Europe (n53), eastern Mediterranean (n57), and Southeast Asia (n59) had pooled postpartum expulsion rates of 2.2%, 3.8%,6.3%, and 6.4%, respectively, whereas three studies pooled from South America had rates of 32.7%. The expulsion rate from 20 studies in North America was 11.3% in comparison with 15.8% from two studies in the Western Pacific. Expulsion rates did not differ by study quality (data not shown).
In multivariable analysis, examining complete expulsions among the sample of women with any follow-up, placement timing, delivery method, and IUD type were associated with risk of expulsion (Table 3). Compared with interval placement, both immediate and early placement had a more than sixfold higher risk of expulsion (adjusted RR 7.63, 95% CI 4.31–13.51; adjusted RR 6.17, 95% CI 3.19–11.93, respectively). Compared with cesarean delivery, IUD placement after vaginal delivery had a fivefold higher risk of expulsion (adjusted RR 5.19, 95% CI 3.85–6.99). Compared with CuT380A, risk of expulsion was higher for the LNG-IUS (adjusted RR 1.91, 95% CI 1.50–2.43) and CuT200 (adjusted RR 1.42, 95% CI 1.06–1.90); however, risk was lower for Cu7 (adjusted RR 0.21, 95% CI 0.08–0.56).
Table 3.
Risk Ratio for Intrauterine Device Expulsion Among Postpartum Women by Placement Timing, Delivery Method, and Intrauterine Device Type
| Sensitivity Analyses | ||||
|---|---|---|---|---|
| Complete Expulsions* | Complete Expulsions LTFU, Expulsions† | Complete Expulsions LTFU, No Expulsion† | ||
| RR (95% CI) | aRR (95% CI)‡ | aRR (95% CI)‡ | aRR (95% CI)‡ | |
| Placement timing§ | ||||
| Interval | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Immediate | 5.29 (3.00–9.33) | 7.63 (4.31–13.51) | 3.70 (2.73–5.01) | 4.75 (2.98–7.56) |
| Early | 15.69 (8.9–27.64) | 6.17 (3.19–11.93) | 2.67 (1.79–3.97) | 4.03 (2.27–7.14) |
| Early or immediate | 2.01 (1.10–3.69) | 3.57 (1.86–6.84) | 1.04 (0.45–2.40) | 3.39 (1.30–8.83) |
| Delivery methodǁ | ||||
| Cesarean | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Vaginal | 4.10 (3.08–5.45) | 5.19 (3.85–6.99) | 2.56 (2.07–3.18) | 4.70 (3.46–6.37) |
| Cesarean or vaginal | 2.13 (1.61–2.83) | 2.60 (1.76–3.84) | 2.02 (1.52–2.69) | 2.78 (1.94–3.99) |
| Unknown | 9.12 (6.95–11.98) | 6.47 (3.43–12.19) | 6.16 (3.72–10.20) | 7.85 (4.42–13.94) |
| IUD typeǁ | ||||
| CuT380A | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| LNG | 2.29 (1.87–2.81) | 1.91 (1.50–2.43) | 1.35 (1.16–1.58) | 1.81 (1.42–2.29) |
| CuT380A or LNG | 1.49 (1.07–2.07) | 1.59 (1.07–2.35) | 1.71 (1.28–2.27) | 1.38 (0.89–2.14) |
| Cu7 | 0.48 (0.18–1.27) | 0.21 (0.08–0.56) | 0.10 (0.04–0.26) | 0.28 (0.11–0.77) |
| CuT200 | 3.38 (2.96–3.85) | 1.42 (1.06–1.90) | 1.14 (0.98–1.32) | 1.31 (0.98–1.76) |
LTFU, lost to follow-up; RR, unadjusted risk ratios; aRR, adjusted risk ratios; IUD, intrauterine device; Cu, copper; LNG, levonorgestrel.
Among women with IUDs placed with any follow-up.
Including 43 studies with a follow-up rate of at least 70%.
Adjusted for IUD type, delivery method, placement timing, study region, study quality, and length of follow-up.
Immediate=10 min or less after placental delivery; early=greater than 10 min to less than 4 wk postpartum; early or immediate=immediate and early combined; interval=4 wk or greater postpartum.
Among immediate and early postpartum placements.
For our sensitivity analyses that included women lost to follow-up (n510,948) (Table 3), we only included 43 studies with a follow-up rate of at least 70%. We first assumed all women lost to follow-up (n52,379) experienced an expulsion and then assumed all women lost to follow-up retained their IUD. Although some variations of estimated risks of complete expulsions existed for both assumptions in comparison with the primary analysis, in general, results were similar with regard to placement timing, delivery methods, and IUD type.
For our sensitivity analysis that examined all expulsions (complete and partial expulsions), the adjusted RR of overall expulsion for early compared with interval placement was nearly twofold higher compared with our primary analysis (adjusted RR 11.17, 95% CI 6.74–18.52) (data not shown). The significantly higher risk of expulsion for immediate placement compared with interval placement remained when examining risk for overall expulsions as did the higher risk of expulsion for vaginal delivery compared with cesarean delivery (data not shown). When examining overall expulsions by IUD type, the higher risk of expulsion for LNG-IUS and CuT200 compared with CuT380A and lower risk for Cu7 remained (data not shown).
DISCUSSION
This analysis describes pooled postpartum expulsion rates of Cu-IUDs and LNG-IUS and adjusted RRs of expulsion by timing of placement, delivery method, and IUD type. In adjusted multivariable analyses, RRs of expulsion were higher for immediate placement and early placement compared with interval placement, for vaginal compared with cesarean delivery, and for LNG-IUS and CuT200 compared with CuT380A.
These results generally confirm previous findings from narrative systematic reviews2–4 and provide new information regarding absolute expulsion rates for IUDs placed in the postpartum period. Although several studies have been published recently, most have been pilots or small studies designed to look at continuation rates; sample sizes were too small to detect differences in expulsion rates between groups by timing of placement, delivery method, or IUD type—key clinical factors that may influence expulsion rates. High attrition rates also have been common, and most studies have follow-up periods of 3–6 months. By pooling these data, we had a sample of approximately 8,000 women with follow-up data who had an IUD placed in the postpartum period from study locations across the world, with more than 90% receiving a copper IUD. In addition to our factors of interest, we adjusted for study quality, which varied from poor to good, and length of follow-up. We also included study region because certain areas (eg, Southeast Asia) have had more experience with postpartum IUD placements and thus may have lower expulsion rates.
This analysis is subject to several limitations. First, expulsions were not always clearly defined in the studies, diagnostic criteria were rarely reported, and expulsions may have been ascertained by varied methods, including clinic visit, patient report, or chart review. We assumed when studies reported expulsions not further characterized as complete or partial that these were complete expulsions, which may overestimate our primary outcome. Even for those reporting complete and partial expulsions, definitions distinguishing the two types were rarely reported. The ranges of pooled expulsion rates highlight the lack of data precision even when stratified by placement timing, delivery method, IUD type, and length of follow-up. We were unable to examine potential factors for IUD expulsion such as health care provider experience, insertion technique, or ultrasound use because studies did not consistently report these variables. Because our analysis focused on factors at the study level, we were not able to examine potential confounders at the patient level such as age, parity, and breastfeeding status, which may also influence expulsion. Additionally, we were not able to pinpoint when expulsions most commonly occur because studies did not consistently report timing of expulsions after placement.
To improve this body of evidence and inform a future pooled expulsion analysis, we recommend studies include clear diagnostic criteria for complete and partial expulsions, timing of expulsion, IUD type, insertion technique (eg, manual, ring forceps, or inserter), level of health care provider experience, and breastfeeding status.
Although complete expulsions may be less prone to misclassification, partial expulsions may or may not include malpositioned IUDs, whose clinical significance remains unknown. Our sensitivity analysis examining complete and partial expulsions confirms findings from our primary analysis of complete expulsions and suggests women with early postpartum IUD placements may be at much higher risk for complete and partial expulsions compared with women with interval placements. One recent case–control study found more pregnancies among women with malpositioned IUDs than those with IUDs in normal position as a result of high rates of IUD removal and lack of subsequent highly effective contraception.59 None of 28 women with malpositioned IUDs who kept the IUD experienced a pregnancy within 2 years.
Our results, along with safety data and other recommendations, can be used to support essential postpartum contraception initiatives1–5,60 and aid in counseling women to make an informed choice as to when to initiate their IUD. Although expulsion risk is increased with IUD placement in the immediate and early postpartum periods compared with interval placement, providing contraceptive access to women may outweigh expulsion risk for women who prefer the convenience and other beneficial factors of postpartum IUD placement or who may face additional barriers to placement at a later time. When feasible, provision of IUDs immediately postpartum (within 10 minutes after placental delivery) may result in a lower expulsion risk than early postpartum placement.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Whitaker AK, Chen BA. Society of Family Planning Guidelines: postplacental insertion of intrauterine devices. Contraception 2018;97:2–13. [DOI] [PubMed] [Google Scholar]
- 2.Kapp N, Curtis KM. Intrauterine device insertion during the postpartum period: a systematic review. Contraception 2009; 80:327–36. [DOI] [PubMed] [Google Scholar]
- 3.Lopez LM, Bernholc A, Hubacher D, Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. The Cochrane Database of Systematic Reviews 2015, Issue 6 Art. No.: CD003036 DOI: 10.1002/14651858. CD003036.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonalkar S, Kapp N. Intrauterine device insertion in the post-partum period: a systematic review. Eur J Contracept Reprod Health Care 2015;20:4–18. [DOI] [PubMed] [Google Scholar]
- 5.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. medical eligibility criteria for contraceptive use. MMWR Recomm Rep 2016;65:1–103. [DOI] [PubMed] [Google Scholar]
- 6.Immediate postpartum long-acting reversible contraception. Committee Opinion No. 670. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016;128:e32–7. [DOI] [PubMed] [Google Scholar]
- 7.Levi EE, Stuart GS, Zerden ML, Garrett JM, Bryant AG. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol 2015;126:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lester F, Kakaire O, Byamugisha J, Averbach S, Fortin J, Maurer R, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception 2015;91:198–203. [DOI] [PubMed] [Google Scholar]
- 9.Cohen R, Sheeder J, Arango N, Teal SB, Tocce K. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception 2016;93:178–83. [DOI] [PubMed] [Google Scholar]
- 10.Woo I, Seifert S, Hendricks D, Jamshidi RM, Burke AE, Fox MC. Six-month and 1-year continuation rates following post-partum insertion of implants and intrauterine devices. Contraception 2015;92:532–5. [DOI] [PubMed] [Google Scholar]
- 11.Washington CI, Jamshidi R, Thung SF, Nayeri UA, Caughey AB, Werner EF. Timing of postpartum intrauterine device placement: a cost-effectiveness analysis. Fertil Steril 2015;103: 131–7. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001; 20(suppl):21–35. [DOI] [PubMed] [Google Scholar]
- 14.Jatlaoui TC, Marcus M, Jamieson DJ, Goedken P, Cwiak C. Postplacental intrauterine device insertion at a teaching hospital. Contraception 2014;89:528–33. [DOI] [PubMed] [Google Scholar]
- 15.Diedrich JT, Klein DA, Peipert JF. Long-acting reversible contraception in adolescents: a systematic review and meta-analysis. Am J Obstet Gynecol 2017;216:364.e1–12. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla Rosales F, Aguilar Zamudio ME, Cázares Montero Mde L, Hernández Ortiz ME, Luna Ruiz MA. Factors for expulsion of intrauterine device Tcu380A applied immediately postpartum and after a delayed period [in Spanish]. Rev Med Inst Mex Seguro Soc 2005;43:5–10. [PubMed] [Google Scholar]
- 17.Gueye M, Gaye YF, Diouf AA, Mbaye M, Niang MM, Gueye SM, et al. Trancesarean intra-uterine device. Pilot study performed at Dakar teaching hospital [in French]. J Gynecol Obstet Biol Reprod (Paris) 2013;42:585–90. [DOI] [PubMed] [Google Scholar]
- 18.Çelen, Sucak A, Yildiz Y, Danis man N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception 2011;84:240–3. [DOI] [PubMed] [Google Scholar]
- 19.Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol 2010;116:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant AG, Kamanga G, Stuart GS, Haddad LB, Meguid T, Mhango C. Immediate postpartum versus 6-week postpartum intrauterine device insertion: a feasibility study of a randomized controlled trial. Afr J Reprod Health 2013;17:72–9. [PubMed] [Google Scholar]
- 21.Dias T, Abeykoon S, Kumarasiri S, Gunawardena C, Padeniya T, D’Antonio F. Use of ultrasound in predicting the success of intrauterine contraceptive device insertion immediately after delivery. Ultrasound Obstet Gynecol 2015;46:104–8. [DOI] [PubMed] [Google Scholar]
- 22.Eroğlu [notdef] K, Akkuzu G, Vural G, Dilbaz B, Akin A, Tas kin L, et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early postpartum period with interval period: 1 year follow-up. Contraception 2006;74:376–81. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Malik S, Sinha R, Shyamsunder S, Mittal MK. Association of the position of the copper T 380A as determined by the ultrasonography following its insertion in the immediate postpartum period with the subsequent complications: an observational study. J Obstet Gynaecol India 2014;64:349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooda R, Mann S, Nanda S, Gupta A, More H, Bhutani J. Immediate postpartum intrauterine contraceptive device insertions in caesarean and vaginal deliveries: a comparative study of follow-up outcomes. Int J Reprod Med 2016;2016:7695847 [Epub Aug 17]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Sethi R, Balasubramaniam S, Charurat E, Lalchandani K, Semba R, et al. Women’s experience with postpartum intrauterine contraceptive device use in India. Reprod Health 2014;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letti Müller AL, Lopes Ramos JG, Martins-Costa SH, Palma Dias RS, Valerio EG, Hammes LS, et al. Transvaginal ultrasonographic assessment of the expulsion rate of intrauterine devices inserted in the immediate postpartum period: a pilot study. Contraception 2005;72:192–5. [DOI] [PubMed] [Google Scholar]
- 27.Levi E, Cantillo E, Ades V, Banks E, Murthy A. Immediate postplacental IUD insertion at cesarean delivery: a prospective cohort study. Contraception 2012;86:102–5. [DOI] [PubMed] [Google Scholar]
- 28.Nelson AL, Chen S, Eden R. Intraoperative placement of the Copper T-380 intrauterine devices in women undergoing elective cesarean delivery: a pilot study. Contraception 2009;80: 81–3. [DOI] [PubMed] [Google Scholar]
- 29.Ragab A, Hamed HO, Alsammani MA, Shalaby H, Nabeil H, Barakat R, et al. Expulsion of Nova-T380, Multiload 375, and Copper-T380A contraceptive devices inserted during cesarean delivery. Int J Gynaecol Obstet 2015;130:174–8. [DOI] [PubMed] [Google Scholar]
- 30.Singal S, Bharti R, Dewan R, Divya, Dabral A, Batra A, et al. Clinical outcome of postplacental Copper T 380A insertion in women delivering by caesarean section. J Clin Diagn Res 2014; 8:OC01–4 [Epub 2014 Sep 20]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Das V, Agarwal A, Dewan R, Mittal P, Bhamrah R, et al. A dedicated postpartum intrauterine device inserter: pilot experience and proof of concept. Glob Health Sci Pract 2016;4: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sucak A, Ozcan S, Çelen S¸, Çaglar[notdef] T, Göksu G, Danis man N.Immediate postplacental insertion of a copper intrauterine device: a pilot study to evaluate expulsion rate by mode of delivery. BMC Pregnancy Childbirth 2015;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra S Evaluation of safety, efficacy, and expulsion of post-placental and intra-cesarean insertion of intrauterine contraceptive devices (PPIUCD). J Obstet Gynaecol India 2014;64:337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Yang X, Gu X, Xu S, Zhou X, Chen Y, et al. Comparison between two techniques used in immediate postplacental insertion of TCu 380A intrauterine device: 36-month follow-up. Reprod Contracept 1999;10:156–62. [PubMed] [Google Scholar]
- 35.Agarwal K, Dewan R, Mittal P, Aggarwal A. Visibility of strings after postplacental intracesarean insertion of CuT380A and Cu375 intrauterine contraceptive device: a randomized comparative study. J Obstet Gynaecol India 2017;67:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colwill AC, Schreiber CA, Sammel MD, Sonalkar S. Six-week retention after postplacental copper intrauterine device placement. Contraception 2018;97:215–8. [DOI] [PubMed] [Google Scholar]
- 37.Unal C, Eser A, Tozkir E, Wildermeerach D. Comparison of expulsions following intracesarean placement of an innovative frameless copper-releasing IUD (Gyn-CSÒ) versus the TCu380A: a randomized trial. Contraception 2018. April 17 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Braniff K, Gomez E, Muller R. A randomised clinical trial to assess satisfaction with the levonorgestrel-releasing intrauterine system inserted at caesarean section compared to postpartum placement. Aust N Z J Obstet Gynaecol 2015;55:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahlke JD, Terpstra ER, Ramseyer AM, Busch JM, Rieg T, Magann EF. Postpartum insertion of levonorgestrel- intrauterine system at three time periods: a prospective randomized pilot study. Contraception 2011;84:244–8. [DOI] [PubMed] [Google Scholar]
- 40.Elsedeek MS. Five-year follow-up of two types of contraceptive device fitted during elective cesarean delivery. Int J Gynaecol Obstet 2015;130:179–82. [DOI] [PubMed] [Google Scholar]
- 41.Elsedeek MS. Puerperal and menstrual bleeding patterns with different types of contraceptive device fitted during elective cesarean delivery. Int J Gynaecol Obstet 2012;116:31–4. [DOI] [PubMed] [Google Scholar]
- 42.Hayes JL, Cwiak C, Goedken P, Zieman M. A pilot clinical trial of ultrasound-guided postplacental insertion of a levonorgestrel intrauterine device. Contraception 2007;76:292–6. [DOI] [PubMed] [Google Scholar]
- 43.Puzey M Mirena at caesarean section. Eur J Contracept Re-prod Health Care 2005;10:164–7. [DOI] [PubMed] [Google Scholar]
- 44.Stuart GS, Bryant AG, O’Neill E, Doherty IA. Feasibility of postpartum placement of the levonorgestrel intrauterine system more than 6 h after vaginal birth. Contraception 2012;85:359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart GS, Lesko CR, Stuebe AM, Bryant AG, Levi EE, Danvers AI. A randomized trial of levonorgestrel intrauterine system insertion 6 to 48h compared to 6weeks after vaginal delivery; lessons learned. Contraception 2015;91:284–8. [DOI] [PubMed] [Google Scholar]
- 46.Whitaker AK, Endres LK, Mistretta SQ, Gilliam ML. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception 2014;89:534–9. [DOI] [PubMed] [Google Scholar]
- 47.Zerden ML, Stuart GS, Charm S, Bryant A, Garrett J, Morse J. Two-week postpartum intrauterine contraception insertion: a study of feasibility, patient acceptability and short-term outcomes. Contraception 2017;95:65–70. [DOI] [PubMed] [Google Scholar]
- 48.Soon R, McGuire K, Salcedo J, Kaneshiro B. Immediate versus delayed insertion of the levonorgestrel intrauterine device in postpartum adolescents: a randomized pilot study. Hawaii J Med Public Health 2018;77:60–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Turok DK, Leeman L, Sanders JN, Thaxton L, Eggebroten JL, Yonke N, et al. Immediate postpartum levonorgestrel intrauterine device insertion and breast-feeding outcomes: a noninferiority randomized controlled trial. Am J Obstet Gynecol 2017; 217:665.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldwin MK, Edelman AB, Lim JY, Nichols MD, Bednarek PH, Jensen JT. Intrauterine device placement at 3 versus 6 weeks postpartum: a randomized trial. Contraception 2016;93: 356–63. [DOI] [PubMed] [Google Scholar]
- 51.Chen MJ, Hou MY, Hsia JK, Cansino CD, Melo J, Creinin MD. Long-acting reversible contraception initiation with a 2-to 3-week compared with a 6-week postpartum visit. Obstet Gynecol 2017;130:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggebroten JL, Sanders JN, Turok DK. Immediate postpartum intrauterine device and implant program outcomes: a prospective analysis. Am J Obstet Gynecol 2017;217:51.e1–7. [DOI] [PubMed] [Google Scholar]
- 53.Goldthwaite LM, Sheeder J, Hyer J, Tocce K, Teal SB. Post-placental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol 2017;217:674. e1–8. [DOI] [PubMed] [Google Scholar]
- 54.Heller R, Johnstone A, Cameron ST. Routine provision of intrauterine contraception at elective cesarean section in a national public health service: a service evaluation. Acta Obstet Gynecol Scand 2017;96:1144–51. [DOI] [PubMed] [Google Scholar]
- 55.Laes E, Lehtovirta P, Weintraub D, Pyorala T, Luukkainen T. Early puerperal insertions of copper-T-200. Contraception 1975;11:289–95. [DOI] [PubMed] [Google Scholar]
- 56.Lavin P, Waszak C, Bravo C. Preliminary report on a postpartum CuT 200 study, Santiago, Chile. Int J Gynaecol Obstet 1983;21:71–5. [DOI] [PubMed] [Google Scholar]
- 57.Shukla M, Qureshi S; Chandrawati. Post-placental intrauterine device insertion—a five year experience at a tertiary care centre in north India. Indian J Med Res 2012;136:432–5. [PMC free article] [PubMed] [Google Scholar]
- 58.Newton J, Harper M, Chan KK. Immediate post-placental insertion of intrauterine contraceptive devices. Lancet 1977;2:272–4. [DOI] [PubMed] [Google Scholar]
- 59.Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol 2011;118:1014–20. [DOI] [PubMed] [Google Scholar]
- 60.Long-acting reversible contraception: implants and uterine devices. Practice Bulletin No. 186. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;130:e251–69. [DOI] [PubMed] [Google Scholar]