Abstract
Background:
This study sought to identify Attention-Deficit Hyperactivity Disorder (ADHD) abnormalities in relationships between brain white matter structure and individual differences in several types of impulsive behavior.
Methods:
Sixty-seven ADHD, Combined subtype, and 68 non-ADHD adolescents were given neuropsychological tests and diffusion tensor imaging (DTI) scans. Principal component analysis (PCA) reduced test scores into factors representing different types of impulsive behavior. Tract-based spatial statistics (TBSS) quantified white matter integrity in relationship to components of impulsive behavior. ADHD versus non-ADHD differences in the strength and nature of linear relationships between regional white matter and three impulsivity components were examined using multiple regression.
Results:
PCA found three separate impulsivity-related factors that were interpreted as motor response inhibition, impulsive choice, and delay aversion. Relationships between regional fractional anisotropy and response inhibition or impulsive choice did not differ between ADHD and non-ADHD. There was a significant interaction between diagnostic group and delay aversion test performance relationships with regional fractional anisotropy. For non-ADHD youth, greater anisotropy in numerous tracts predicted better delay aversion test performance. In contrast, anisotropy in regions including the corpus callosum, corona radiata, internal capsule, and corticospinal tracts either had a negative or no relationship with delay aversion test performance in ADHD.
Conclusions:
The results provide additional support that different proposed etiological pathways to ADHD have discretely different neurobiological features. Large disorganization of white matter microstructure appears to contribute to reward-based ADHD pathways rather than motor inhibition.
Keywords: ADHD, DTI, response inhibition, delay aversion, impulsive choice, impulsivity
Approximately 5 to 11% of American children are affected by Attention-Deficit Hyperactivity Disorder (ADHD), a behaviorally-defined disorder typically diagnosed in childhood characterized by inattention, hyperactivity and impulsivity.(1, 2) Several neurocognitive theories of ADHD have been proposed. Some theories separate impulsive behavior into at least two domains that reflect Sonuga-Barke’s(3) seminal “dual-pathway” model: a) inability to withhold a behavioral response,(4) linked to frontostriatal neural system dysfunction(5) and b) intolerance for delay thought to arise from abnormalities in the brain’s reward system.(3, 6) These are separable constructs in non-ADHD and other clinical populations(7, 8) and have distinct neuropsychological and neuroimaging abnormalities in ADHD.(9, 10) Commonly-used paradigms to measure response inhibition include the Go/NoGo or Stop Signal task (SST), which quantify how well and efficiently participants can withhold a motor response (i.e., “inhibition”).(11) Although there are important differences between constructs of delay aversion(3) and preference for immediate reward, delay aversion often has been operationalized by performance on a Delayed Discounting Task/Questionnaire (DDQ). The DDQ asks participants to make choices between small rewards after short delays or larger rewards after longer delays. A preference for sooner, immediate rewards has been termed “impulsive choice.”(8) On these paradigms, adolescents and children with ADHD often make more errors of commission, are slower to inhibit responses, or fail to cancel a Go response on stop trials,(12-14)(“disinhibition” pathway), and/or prefer smaller but more immediate rewards to larger delayed rewards (“impulsive choice” pathway).(15, 16)
One purpose of neurocognitive models of ADHD is to link meaningful behavioral profiles to specific neurobiological influences – in this context, to explain how different forms of impulsivity could arise in ADHD. Many prior studies have asked if ADHD patients have abnormalities in the major white matter connections among brain regions. Although nearly all prior diffusion tensor imaging (DTI) studies find ADHD deficits, a hallmark of these studies is their inconsistency. At a broad level, ADHD deficits in white matter integrity tend to be reported most often in cingulum bundle (CB), corona radiata, corpus callosum (CC), inferior longitudinal fasciculus, internal capsule, middle cerebellar peduncle, and superior longitudinal fasciculus (SLF) (see review: (17)). Yet, positive and negative evidence for abnormality in each of these regions exists, and no specific white matter tract or region is found in every study. Associations between white matter abnormalities and inattentive and hyperactive/impulsive symptom dimensions of ADHD have likewise failed to provide consistent findings, with the most consistent association between lower white matter integrity in SLF with inattentive symptoms.(18-20)
This heterogeneity raises the question of how useful tests of group-level white matter abnormalities are in our efforts to understand ADHD etiology. If most white matter abnormalities are not reliably linked to the diagnostic phenotype, are they instead non-specific, variable phenomena that ultimately are unrelated to factors that underlie ADHD? Alternatively, perhaps abnormal white matter microstructure might not relate to the ADHD diagnostic phenotype itself, but rather to distinct proposed etiological pathways to ADHD (e.g., disinhibition, impulsive choice). This possibility has not yet been directly examined in ADHD. In recent years, DTI studies in non-clinical samples have linked performance on motor inhibition and delay discounting tasks to separate white matter tracts.(21) In ADHD, an increasing number of studies have attempted to link DTI-measured white matter abnormalities to specific cognitive abilities. Such studies provide some evidence that ADHD deficits in motor inhibition might be linked to the integrity of white matter tracts that connect frontal lobe and striatal brain regions,(22-24) intra-parietal connections,(25) or the cingulum bundle.(22) Only one study has examined delay discounting associations with regional white matter in ADHD, finding a modest association with fractional anisotropy (FA) in the splenium of the corpus callosum.(26) However, these approaches have been somewhat piecemeal in test selection as well as post hoc – neither testing hypotheses about expected white matter-impulsivity relationships, nor statistically evaluating whether any brain-behavior associations actually differ between diagnoses. No previous study has tested whether neurocognitive markers of theoretically different ADHD etiological pathways have different white matter characteristics than found in non-ADHD.
This study’s primary innovation was its a priori focus on abnormalities in the relationship between individual differences in DTI-measured white matter and neurocognitive markers of proposed etiological pathways in youth with ADHD. So rather than futilely seeking to identify neurocognitive or white matter deficits in the diagnostic phenotype, our goal was to target the proposed pathways by directly comparing the relationship of white matter to disinhibition- and impulsive choice-related conceptualizations of impulsivity. We wished to learn whether any differences between ADHD and non-ADHD in these associations could be found in the same white matter tracts, different tracts for different cognitive pathways, or even essentially normal relationships with white matter microstructure in one or both proposed pathways. We included several different tasks theoretically-linked to each neurocognitive pathway to produce a generalized index of the constructs that did not rely on a single test indicator. We hypothesized these different neurocognitive profiles would have distinct white matter correlates, observable as different strength or direction of associations between regional white matter and either disinhibition or impulsive choice test performance.
Methods and Materials
Participants
ADHD Combined subtype youth ranging from ages 12–18 and an age-, gender- and IQ-matched group of non-ADHD youth were recruited through local advertisements and physician referral at the Olin Neuropsychiatry Research Center at Hartford Hospital. Informed consent and assent were obtained from a parent or legal guardian and youth as approved by Hartford Hospital’s Institutional Review Board. ADHD diagnoses were confirmed by experienced Master’s and Bachelor’s level staff who administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL)(27) to youth and a parent or legal guardian. Diagnoses were reached by collective consensus of the research team in a supervised discussion of videotaped interview content by MCS, a licensed psychologist with 16 years’ experience using the K-SADS-PL for ADHD research. Current comorbid Oppositional Defiant Disorder, Conduct Disorder and substance abuse (but not dependence) were allowed in ADHD youth. Potential non-ADHD participants were excluded for any current or lifetime psychiatric diagnoses. Other exclusion criteria included self-reported neurological conditions or evidence of gross brain structure abnormalities on structural MRI, reported psychotic illness in a first-degree relative, and IQ estimate<80 on the two-subtest Wechsler Abbreviated Scale of Intelligence.(28) The study only included unmedicated participants or ADHD participants who took “psychostimulant” medications with short half-lives ensuring full washout within 24 hours. All testing was done following psychostimulant washout. All youth were screened with a drug and pregnancy test prior to scanning. Several self- and parent-report measures of behavioral, attentive, and clinical problems were collected to fully characterize the sample, including the Brown Attention-Deficit Disorder Scales for Adolescents (BADDS),(29) Adolescent Alcohol and Drug Involvement Scale (AADIS),(30) and Disruptive Behavior Rating Scale – Parent Version (DBRS).(31)
In all, 135 youth completed neuropsychological evaluations and quality DTI scanning (see Supplemental Materials for additional details). These youth were an average of 15.42 years old, primarily male (77.8%), and right-handed (91.9%). ADHD and non-ADHD youth were comparable in age, gender, ethnicity/race, handedness, IQ, and alcohol and drug abuse. ADHD-diagnosed youth evidenced greater behavioral and inattentive problems, indexed by BADDS and DBRS (see Table 1). Group comparison of impulsivity-related raw test scores also are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics, and Neuropsychological Performance of ADHD and non-ADHD Adolescents.
| Characteristics | ADHD (n=67) |
Non-ADHD (n=68) |
Statistical Testa |
p- value |
||
|---|---|---|---|---|---|---|
| N/Mean | %/SD | N/Mean | %/SD | |||
| Age (years) | 15.41 | 1.78 | 15.43 | 1.73 | t133 = 0.07 | ns |
| Gender (female) | 12 | 18 | 18 | 27 | χ21 = 0.98 | ns |
| Race | χ22 = 2.06 | ns | ||||
| Caucasian | 52 | 85 | 48 | 75 | ||
| Black | 6 | 10 | 11 | 17 | ||
| Asian/Other | 3 | 5 | 5 | 8 | ||
| Handedness (right) | 58 | 87 | 66 | 97 | χ21 = 3.66 | ns |
| Comorbid Diagnosis | 29 | 43 | 0 | χ21 = 34.50 | .001 | |
| Oppositional Defiant Disorder | 14 | 21 | χ21 = 13.48 | <.001 | ||
| Conduct Disorder | 5 | 8 | χ21 = 3.32 | ns | ||
| Substance Abuse/Dependence | 3 | 5 | χ21 = 1.36 | ns | ||
| Past Mood/Anxiety Disorder | 6 | 9 | χ21 = 4.36 | .04 | ||
| Current Medications | 42 | 64 | 0 | - | χ21 = 60.11 | <.001 |
| (Dextro)Amphetamine | 11 | 17 | χ21 = 10.23 | <.001 | ||
| Methylphenidate | 20 | 30 | χ21 = 21.89 | <.001 | ||
| Lisdexamphetamine | 4 | 6 | χ21 = 2.41 | ns | ||
| Dexmethylphenidate | 5 | 8 | χ21 = 3.45 | ns | ||
| BADDS - Parent | 69.08 | 23.67 | 17.07 | 19.39 | t116 = 12.94 | <.001 |
| BADDS - Child | 57.82 | 24.52 | 29.87 | 20.76 | t130 = 7.08 | <.001 |
| DBRS Inattention | 19.41 | 5.80 | 3.16 | 4.04 | t111 = 17.94b | <.001 |
| DBRS Hyperactive/Impulsive | 15.76 | 5.84 | 1.37 | 2.88 | t93 = 17.37b | <.001 |
| WASI 2-Subscale IQ | 105.28 | 12.06 | 108.05 | 9.25 | t124 = 1.49b | ns |
| AADIS Total Score | 11.52 | 16.93 | 9.41 | 14.52 | t127 = 0.76 | ns |
| Neuropsychological Performance | ||||||
| Conner’s CPT-II, Commissions Errors | 21.96 | 7.86 | 21.28 | 6.16 | t125= −0.54 | ns |
| Stop Signal Reaction Time | 294.57 | 72.18 | 286.30 | 71.86 | t118= −0.63 | ns |
| Delayed Memory Test, Commission Error % | 0.52 | 0.18 | 0.43 | 0.18 | t131= −2.90 | .004 |
| Single Key Impulsivity Paradigm, Average IRT | 7.37 | 17.95 | 15.03 | 25.54 | t110= 1.92b | .06 |
| Experiential Delay Task | 0.61 | 0.15 | 0.62 | 0.15 | t116= 0.29 | ns |
| Delay Discounting Questionnaire | 0.34 | 0.22 | 0.41 | 0.31 | t108= 1.47b | ns |
Abbreviations: BADDS = Brown ADD Scales; DBRS = Disruptive Behavior Rating Scale, Parent Report; WASI = Wechsler Abbreviated Scale of Intelligence; AADIS = Adolescent Alcohol and Drug Involvement Scale; CPT = Continuous Performance Test; IRT = Inter-Response Time.
All chi-square tests use a continuity correction if a 2×2 variable table (e.g., χ21).
Due to significantly different variances determined by Levene’s test, ADHD and non-ADHD were compared using an equality of variances degrees of freedom adjustment.
Neuropsychological Assessments and Data Preparation
A fixed-order battery of computerized tests assessed impulsive behavior in all participants. Tests selected to measure the proposed “disinhibition” factor included the Connors’ Continuous Performance Test II (CPT-II), SST, and Immediate and Delayed Memory Task (IMT/DMT). The CPT-II is a 14-minute computerized task with 360 trials over a total of 18 blocks.(32, 33) Participants press a key when any letter except “X” appears on the screen (10%). The dependent measure from this task was number of responses to a non-target (commissions). CPT-II inhibition failures typically are interpreted as deficits in the ability to withhold a prepotent response. The SST is similar, with the exception that auditory stop signals are presented shortly after “go” signals on 25% of trials, requiring participants to withhold pre-potent responses that have already started.(34) Stop signal delays were adjusted until responses were correctly inhibited on approximately 50% of trials. Elapsed time from “go” presentation to stop signal determines the stop signal reaction time (SSRT), or the amount of time participants require to correctly inhibit half of their “go” responses. Task duration varied across participants but was never longer than 12.1 minutes. SSRT is typically interpreted as one’s efficiency in countermanding active responses. The IMT/DMT is another CPT variant that assesses impulsive responding with or without cognitive demands.(35-37) Sequential 5-digit stimuli are presented and participants are tasked to respond to identical stimuli consecutively (IMT) or three trials later (DMT). Using default settings, stimuli have variable presentation rates and inter-trial intervals, lasting a total of 21.5 minutes. The dependent measure from this task was percent commission errors to “catch” stimuli on the DMT, which differ in one digit from target sequences and require longer than other non-targets to accurately process. This measure differs from other inhibition tests by adding greater information processing demands to capture inhibition failures that might not occur in simpler contexts.
Tests of “impulsive choice” were the DDQ, Experiential Delay Task (EDT), and Single Key Impulsivity Paradigm (SKIP). The DDQ randomly presents questions in which participants are asked to choose between a varying amount of hypothetical money now or after a varying delay.(38) Hyperbolic equations were fitted to each participant’s indifference point. The area under the curve (AUC) from the hyperbolic model was used as the primary measure. The EDT is a behaviorally-based measure of temporal discounting that requires participants to experience choice consequences (waiting) during the task itself.(39) It is similar to the DDQ, but presents participants with immediate consequences of their decisions, which has shown differing performance from hypothetical versions of the paradigm.(40) Similar to DDQ, AUC was used as the primary measure. The SKIP measures the ability to endure long delays between reward-directed responses in a free operant procedure.(37) In a 20-minute session, participants clicked a button whenever desired to add monetary reward to a counter, briefly displayed to enable participants to detect a delay contingency.(41, 42) The average inter-response time interval (IRT) was used as the primary measure. Although the SKIP was constructed based on free operant conditioning principles, average IRT behaviorally operationalizes one’s preference for immediate reward instead of relying on self-report or forced-choice methods.
Confirmatory factor analyses examined if test choices fit our proposed two-factor model of Disinhibition and Impulsive Choice. These analyses were conducted in R (version 3.5.0), using the lavaan package and ML estimation with the first dependent variable as the standard indicator. Inhibition was indicated by CPT, SST and DMT; reward by EDT, DDQ and SKIP. However, poor representation of the data instead led to using exploratory principal component analysis (PCA) with Varimax rotation to depict more than two orthogonal impulsivity components. Eigenvalues and scree plots were used to determine number of components (see Supplemental Materials for details).
Diffusion Tensor Imaging
Diffusion data were acquired on a Siemens 3T Allegra head-only scanner at the Olin Neuropsychiatry Research Center. The pulse sequence was a single-shot spin echo EPI sequence (repetition time/echo time=6300/85msec, FOV=220mm, matrix 100, diffusion-sensitizing orientations=32, b=0 and 1000s/mm2) that covered the whole brain in 45 slices with 1.7×1.7×3.0 mm3 resolution. Three sequences were acquired for a total scanning lasting approximately 11 minutes. Visual inspection ensured that all DTI data were artifact-free (see Supplemental Materials for details). Diffusion-weighted images were distortion-corrected using gradient echo fieldmaps, then registered to a common non-diffusion-weighted image using a mutual information cost function as employed in FSL’s FLIRT toolbox(43, 44) with eddy current corrections.(45) To avoid possible head movement-related signal contamination, any image with a scan-to-scan displacement >2 mm was discarded. The remaining images were concatenated and tensor calculated. A sample-specific mean FA image was calculated from all datasets, then tract-based spatial statistics (TBSS)(46) calculated the scalar FA measures for subject-specific skeletons. Data were spatially normalized to a common Montreal Neurological Institute (MNI) space template using FNIRT toolbox.(47)
Association of Performance with White Matter Integrity
Three multivariate general linear models (GLMs) using FSL’s(48) randomise program examined the interaction of diagnostic study group by each impulsivity component on FA across the whole brain. Age, gender and IQ were included in the model as nuisance covariates.(49, 50) Statistical significance was evaluated using non-parametric permutation-based inference (5000 samples) and thresholded for contiguous voxels surpassing threshold-free cluster enhancement (TFCE), corr p<.05.(51) Tract labels were applied from the John Hopkins University atlas within FSL combined with visual inspection in reference to published stereotactic atlases.(52) Individual FA values were extracted to depict these interaction patterns at the locations of peak effect for each distinct region as determined by visual inspection. Cohen’s d effect sizes were computed for these peak values for added comprehension of effect magnitude.(53) To clarify the nature of the basic associations identified by GLM, simple SPSS v24.0 Pearson correlations determined direction and magnitude of interaction patterns.
Supplemental analyses were also performed to test group-level whole-brain differences in FA, while covarying for age, gender and IQ, using the same thresholds for statistical significance (for results, see Supplemental Materials).
Results
Neuropsychological Measures Data Reduction
CFA found evidence contrary to our two-factor Disinhibition and Impulsive Choice model. Although model fit was adequate (TLI=1.37), DDQ scores loaded opposite to other reward-test indicators on the impulsive choice factor, there was poor latent variable consistency (Cronbach’s alpha 0.47 and 0.15), and variance between DMT and SKIP was not well-accounted for. Exploratory PCA helped to clarify that a three-factor model best fit the data (see Table 2), explaining 61% of test variance. All six tests showed significant communalities with all extractions >.4, indicating good model fit. Loading profiles for two factors offered straightforward interpretations. As expected, three tests of motor inhibition (CPT, SSRT, and DMT) loaded significantly on the first component (eigenvalue=1.50; 25% of the variance) comprising the ability to accurately withhold a response to non-target stimuli (Disinhibition). Also as expected, two tests (DDQ and EDT) loaded significantly on another component (eigenvalue=1.02; 17% of the variance), reflecting planned willful choices about waiting for rewards (Impulsive Choice). In addition, the EDT, SKIP and DMT scores loaded on a final component (eigenvalue=1.13; 19% of the variance), thus labeled “Delay Aversion,” in part to differentiate it from Impulsive Choice, which has been well-established in prior published reports to reflect DDQ scores.(54) It also represented the clearest behavioral manifestation of a characteristic aversion to waiting rather than a planned willful choice. Although EDT loaded onto both this factor and Impulsive Choice, the factor structure indicates a clear dissociation between neuropsychological measures in real-world performance-based contexts (SKIP and EDT) versus those emphasizing decision-making (DDQ and EDT). DMT commission error percent negatively loaded onto Delay Aversion, and positively loaded onto Disinhibition along with CPT commission errors. Given the additional separation of DMT from CPT commission errors, it is possible that ADHD behavioral manifestations of delay aversion may somehow be linked to relative weaknesses holding pertinent information in mind to direct behavior, though other interpretations also are possible.
Table 2.
Factor Loadings of Neuropsychological Measures Using Principle Component Analysis with Varimax Rotation and Group Differences on These Factors.
| Disinhibition | Delay Aversion |
Impulsive Choice |
|
|---|---|---|---|
| Neuropsychological Test | |||
| Conners’ CPT-II, Commission Errors | .73 | .06 | −.01 |
| Stop Signal Reaction Time (RT) | .71 | .28 | −.12 |
| Delayed Memory Test, Commission Error Percent | .64 | −.49 | .17 |
| Single Key Impulsivity Paradigm, Average IRT | .12 | .80 | .08 |
| Experiential Delay Task | .11 | .37 | .52 |
| Delayed Discounting Questionnaire | −.12 | −.10 | .86 |
| Group Characteristics | M (SD) | M (SD) | M (SD) |
| ADHD | −0.16 (0.94) | −0.03 (0.89) | −0.17 (0.82) |
| Non-ADHD | 0.16 (1.04) | 0.03 (1.11) | 0.17 (1.13) |
| T-test (df = 133) | 1.90 | 0.31 | 2.03* |
Factor loadings > .30 are in boldface. Abbreviations: CPT = Continuous Performance Task; RT = Reaction Time; IRT = Inter-Response Time.
p < .05
Relationships Between Impulsivity and White Matter Tracts in ADHD and non-ADHD
No significant interaction between ADHD and non-ADHD youth was found between Disinhibition or Impulsive Choice components and white matter integrity at TFCE-corrected significance levels. However, numerous widespread tracts, including CC, bilateral anterior and posterior corona radiata, right superior and left inferior longitudinal fasciculus, bilateral internal capsules, left external capsule, and corticospinal tracts, were associated with a differing relationship between ADHD and non-ADHD youth in performance on Delay Aversion measures, showing a range of small to medium effect. Table 3 lists tracts, peak t coordinates, TFCE-corrected significance levels, and Cohen’s d effect size for all findings; Figure 1 depicts these findings. Pearson correlations for significant tracts within ADHD and non-ADHD youth are reported and presented in Figure S1. In general, associations at the location of peak effects indicate non-ADHD youth have a significant positive relationship between FA and Delay Aversion performance. Across different tracts, ADHD youth showed either a negative or insignificant relationship between FA and performance on delay aversion measures.
Table 3.
List of white matter regions from tract-based spatial statistics (TBSS) analysis where fractional anisotropy was significantly differently related to Delay Aversion in ADHD compared to non-ADHD adolescents. All locations represent distinct peak regions within three clusters found after statistical corrections for searching the TBSS-derived white matter skeleton using threshold-free cluster enhancement (TFCE), corr p < .05.
| Location Description | Interaction Effect |
Pearson Correlation |
||||
|---|---|---|---|---|---|---|
| MNI x, y, z | t | TFCE p | Cohen’s d | ADHD | HC | |
| FRONTAL | ||||||
| Body of Corpus Callosum | 11, 19, 21 | 1.63 | .047 | 0.29 | −0.09 | 0.12 |
| Genu of Corpus Callosum (Forceps Minor) | 14, 31, 11 | 3.00 | .042 | 0.53 | −0.29* | 0.07 |
| Left Anterior Corona Radiata | −25, 12, 19 | 2.41 | .015 | 0.42 | −0.08 | 0.24 |
| Right Anterior Corona Radiata | 26, 20, 13 | 3.46 | .019 | 0.61 | −0.30* | 0.21 |
| Right Anterior Corona Radiata (Forceps Minor) | 17, 35, 7 | 2.55 | .043 | 0.45 | −0.12 | 0.31* |
| Right Tapetum | 33, −43, 10 | 2.51 | .027 | 0.44 | −0.16 | 0.24* |
| OCCIPITAL | ||||||
| Splenium of Corpus Callosum (Forceps Major) | 28, −59, 11 | 3.05 | .028 | 0.54 | −0.13 | 0.34* |
| TEMPORAL | ||||||
| Left Inferior Longitudinal Fasciculus | −27, −19, −4 | 2.98 | .027 | 0.52 | −0.16 | 0.33* |
| Left Uncinate Fasciculus/Inferior Fronto-Occipital Fasciculus | −37, −7, −15 | 2.69 | .030 | 0.47 | −0.21 | 0.26* |
| Right Superior Longitudinal Fasciculus | 42, −52, 4 | 2.15 | .030 | 0.38 | −0.19 | 0.15 |
| PARIETAL | ||||||
| Left Posterior Corona Radiata (Corticospinal Tract) | −26, −28, 22 | 2.46 | .016 | 0.43 | −0.13 | 0.26* |
| Right Posterior Corona Radiata | 26, −39, 26 | 2.64 | .027 | 0.46 | −0.10 | 0.23 |
| Left Superior Corona Radiata | −26, 7, 22 | 3.43 | .013 | 0.60 | −0.25* | 0.27* |
| SUBCORTICAL | ||||||
| Left Anterior Limb of Internal Capsule (Ant Thalamic Radiation) | −19, 16, 6 | 2.66 | .018 | 0.47 | −0.21 | 0.15 |
| Right Anterior Limb of Internal Capsule (Ant Thalamic Radiation) | 17, 4, 10 | 2.45 | .020 | 0.43 | −0.24 | 0.05 |
| Left Posterior Limb of Internal Capsule (Corticospinal Tract) | −20, −16, −4 | 2.85 | .015 | 0.50 | −0.28* | 0.15 |
| Right Posterior Limb of Internal Capsule (Corticospinal Tract) | 21, −16, −4 | 3.92 | .017 | 0.69 | −0.34* | 0.25* |
| Left Retrolenticular Part of Internal Capsule | −27, −24, −2 | 2.86 | .015 | 0.50 | −0.10 | 0.40* |
| Right Retrolenticular Part of Internal Capsule | 26, −20, 1 | 3.36 | .019 | 0.59 | −0.30* | 0.23 |
| Right Posterior Thalamic Radiation | 35, −55, 15 | 1.94 | .028 | 0.34 | −0.08 | 0.20 |
| Left External Capsule | −27, 14, 10 | 2.00 | .020 | 0.35 | −0.13 | 0.17 |
| BRAINSTEM | ||||||
| Left Cerebral Peduncle (Corticospinal Tract) | −17, −14, −9 | 2.02 | .017 | 0.35 | −0.22 | 0.08 |
| Right Cerebral Peduncle (Corticospinal Tract) | 18, −17, −9 | 2.41 | .019 | 0.42 | −0.20 | 0.17 |
| Left Superior Cerebellar Peduncle | −5, −39, −20 | 2.27 | .020 | 0.40 | −0.12 | 0.18 |
| Left Corticospinal Tract | −9, −27, −28 | 3.41 | .019 | 0.60 | −0.20 | 0.31* |
| Left Medial Lemniscus | −5, −36, −29 | 2.49 | .020 | 0.44 | −0.24* | 0.08 |
relationship with delay aversion significant, p < .05.
Figure 1.
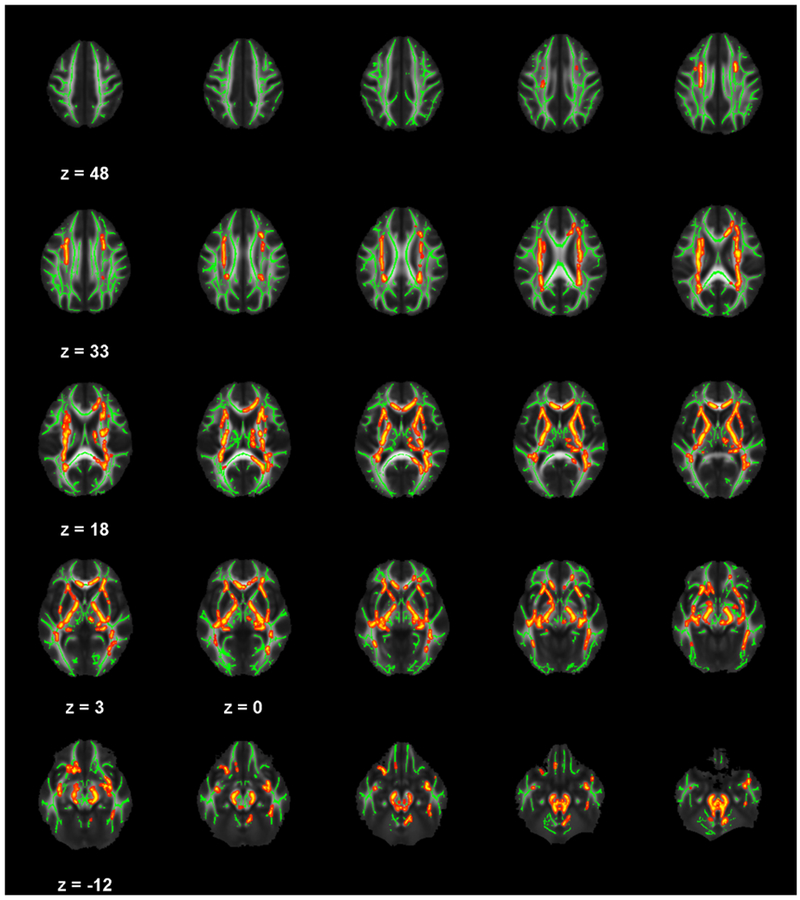
White matter regions from TBSS analysis where fractional anisotropy was significantly differently related to delay aversion in ADHD compared to non-ADHD adolescents, as indicated in red-yellow (p < .05, corrected for searching the TBSS-derived white matter skeleton using TFCE).
Discussion
This study’s primary question was not whether ADHD white matter itself is abnormal, but rather if the relationship between white matter microstructure and cognitive ability was different in ADHD than what is typically found in non-ADHD. On the neurocognitive level, we replicated two well-established domains of impulsivity – motor response inhibition and preference for smaller, immediate rewards over later, larger rewards. However, the relationship between white matter microstructure and those specific ADHD neurocognitive pathway markers did not differ in our sample of ADHD and non-ADHD youth. Hypotheses were supported by different white matter-neurocognitive pathway associations for a factor that reflected a behavioral index of ADHD delay aversion. Non-ADHD youth evidenced a positive relationship between delay aversion performance and FA in CC, bilateral corona radiata, internal capsules, and corticospinal tracts, suggesting that typical development of these tracts, whether increased myelination, increased collinearity of fibers or pruning of neuronal branches,(55-59) or another specific microstructural feature linked to FA, reflects a greater ability to tolerate delayed reward delivery. In contrast, ADHD youth demonstrated negative (or no) relationship in different tracts. This clearly does not reflect a simple explanation such as diminished normal associations. Instead, it most likely reflects disorganization of brain-behavior relationships, but the possibility that these abnormal associations arise because of atypical development or even compensation for deficits cannot be ruled out. Importantly, these results statistically controlled for known influences on test performance and white matter from differences in participant maturation, intelligence or gender. Similar tracts to these have been implicated in ADHD broadly in prior meta-analyses and even our supplemental group analysis (e.g., right anterior corona radiata, genu of the CC and bilateral internal capsules,(17) and splenium and tapetum of the CC and left inferior fronto-occipital fasciculus).(60) The specific findings here – abnormal association between white matter and specific neurocognitive test performance –consistent with the idea that different neurocognitive pathways have unique neural correlates. These results suggest ADHD white matter microstructure may be linked to the delay aversion proposed pathway,(61) rather than microstructural differences in the diagnosis of ADHD more broadly. Future studies that attempt to replicate this delay aversion-white matter relationship and possibly extend it to other putative etiological pathways might ultimately explain inconsistencies seen across prior ADHD studies of white matter ((e.g., 17)).
Delay aversion has been conceptualized as a motivational style thought to result from mesolimbic reward circuit dysfunction.(3) The current findings link white matter microstructure in projection fibers connecting thalamus and putamen with widespread prefrontal cortex (corticospinal, corona radiata, thalamic radiation and internal capsule) to delay aversion tendencies in ADHD. In addition, brain-behavior relationships in ADHD differed in projection fibers passing through thalamus, basal ganglia, and medulla to spinal cord (e.g., left superior cerebellar peduncle, corticospinal tracts), commissural fibers connecting cerebellar hemispheres (e.g., CC), and a few long association fibers connecting distal portions of the cortex (e.g., superior and inferior longitudinal fasciculus). These findings not only implicate mesolimbic circuitry and its’ integration with higher-order cognition in ADHD delay aversion behavior, but also suggest dopamine-sensitive sensory-motor circuits that have been implicated in ADHD pathology.(62) For instance, previous studies have demonstrated a relationship between motor control, reward performance and specific white matter tracts. Faster delay discounting reaction times were associated with left inferior longitudinal fasciculus microstructure in adolescents at risk for alcoholism,(63) steeper delay discounting with splenium of the CC in adult ADHD,(26) and steeper delay discounting with frontostriatal tracts in healthy adults.(64) Considerable literature has also begun to expand upon the role of faulty time estimation in impulsiveness,(65-67) ADHD dysfunction,(61) and its’ potential mediating role in the relationship between ADHD and reward sensitivity.(68-70) Further research disentangling potential effects of time estimation difficulties, behavioral delay aversion, and other forms of impulsivity are needed to differentiate and determine the relationship of these tracts to these cognitive constructs.
In Sonuga-Barke’s(3) dual-pathway ADHD conceptual model, delay aversion arises as a secondary consequence of deficits in dopaminergic reward system inefficiencies producing impulsive drive to immediate reward, frequent conflict with others, frustration, and ultimately a tendency to escape from delay when possible. Although the current study links delay aversion to differing brain-behavior relationships, it is unclear if they reflect the proposed ADHD primary deficit, or the proposed behavioral consequent. It seems less likely that widespread differences in brain-behavior relationships would be associated with what is believed to be an acquired behavioral tendency. The latter cannot be ruled out, as evidence exists for experience-dependent change in DTI-measured white matter.(71, 72) Because we statistically controlled for maturational differences that typically approach adult levels by mid- to late-adolescence for both behavioral performance and white matter, we likely have observed a differing relationship not bound to one period of development. However, ADHD children have shown a delay in myelin development in tracts involved in limbic system function such as the internal capsule.(17, 19, 72) Thus, additional research is needed to ascertain exactly when in adolescent maturation this relationship between delay aversion tendencies and white matter is first observable, when the relationship is most different in ADHD relative to non-ADHD peers, and whether or not this difference eventually resolves if white matter microstructural differences “catch up.”(73) Both cross-sectional and longitudinal comparisons of brain-behavior relationships in younger ADHD-diagnosed youth will be needed to tease out these possibilities.
In contrast to the findings with delay aversion, brain-behavior relationship differences were not found between ADHD and non-ADHD for the other impulsivity-related components. These other neurocognitive pathways may not involve white matter differences, might have smaller effects requiring even larger sample sizes for detection, or might be more prominent in other clinical presentations of ADHD (e.g., Inattentive subtype). Our lack of findings with response inhibition may also be due to specific examination of this construct rather than executive functioning more broadly, since some other studies show a negative relationship between executive functioning and FA.(74, 75) Despite this, the current findings are consistent with theories of multiple neurocognitive pathways in ADHD. They highlight the significance of behavioral manifestations of delay aversion in white matter integrity.(76)
The current study has several limitations to take into consideration. First, DTI methodology cannot determine the underlying mechanism of reduced FA, which can be lower myelination, loss of axons, cell-packing density, gliosis, edema/hydration or fiber orientation.(55-58) If future research determines the current findings ultimately reflect differences in FA development during adolescence, the mechanism of reduced FA might be myelination or axonal diameter growth.(59, 77) While it remains unclear due to under-examination whether ADHD psychostimulants have long-term effects on brain structure, such effects may exist and should be considered in future experiments and meta-analyses. Importantly, the current study did not find ADHD differences in white matter, several cognitive tasks, and PCA-derived disinhibition and delay aversion. While these findings raise questions about diagnostic certainty or test selection, these issues were carefully addressed. Instead, the lack of simple case-control differences at rigorous thresholds likely reflects heterogeneity of ADHD neuropathophysiology. We previously have demonstrated neurocognitive differences on these tests in the larger sample that these ADHD cases are drawn from.(78) Here, although the DTI×Cognition interaction effects were strong enough to survive rigorous statistical control, study group association r values between DTI and test performance were modest. This suggests these white matter characteristics might be but one of several influences on ADHD delay aversion behavior. Indeed, we also recently reported strong evidence for non-overlapping fMRI-measured brain dysfunction in subgroups identified using similar methods.(78) The current study employed an exploratory PCA data reduction approach, which found a component of impulsivity that could be variously interpreted. Finally, some prior research has suggested important differences between temporal estimation/discounting and other forms of reward-related impulsivity.(61) Our CFA and PCA results support this distinction, but future studies will need to confirm this latent structure of reward-related impulsivity both in ADHD and non-ADHD adolescents.
In conclusion, this study found differing widespread corticospinal FA in relationship to delay aversion performance across ADHD and non-ADHD youth, suggesting this brain-behavior relationship may be one (of many) multifactorial pathway to observed ADHD behavioral outcomes. A concerted effort from large-scale studies using careful case-control approaches could confirm relationships between these proposed etiological pathways and various forms of neurobiological dysfunction in ADHD.
Supplementary Material
Acknowledgements
This study was funded by NIMH grant R01MH080956. Manuscript preparation was also funded by NIMH grant T32-MH067631 (KLB). Many thanks go to research staff on the project, including Danielle Francois, Nicole Pompay, Ethan Rosenfeld, and Christina Wong. This work was previously presented in shortened form at the Society of Biological Psychiatry Conference.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.McKeown RE, Holbrook JR, Danielson ML, Cuffe SP, Wolraich ML, Visser SN (2015): The impact of case definition on attention-deficit/hyperactivity disorder prevalence estimates in community-based samples of school-aged children. J Am Acad Child Adolesc Psychiatry. 54:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- 3.Sonuga-Barke EJ (2003): The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 27:593–604. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA (1997): Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 121:65–94. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD (2005): Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 162:1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagvolden T, Johansen EB, Aase H, Russell VA (2005): A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 28:397–419; discussion 419–368. [DOI] [PubMed] [Google Scholar]
- 7.Solanto MV (2002): Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 130:65–71. [DOI] [PubMed] [Google Scholar]
- 8.Bari A, Robbins TW (2013): Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 108:44–79. [DOI] [PubMed] [Google Scholar]
- 9.Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, et al. (2012): The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 7:e36781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solanto MV, Abikoff H, Sonuga-Barke EJ, Schachar R, Logan GD, Wigal T, et al. (2001): The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of abnormal child psychology. 29:215–228. [DOI] [PubMed] [Google Scholar]
- 11.Verbruggen F, Logan GD (2008): Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 12:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schachar R, Tannock R, Marriott M, Logan G (1995): Deficient inhibitory control in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 23:411–437. [DOI] [PubMed] [Google Scholar]
- 13.Nigg JT (1999): The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM–IV combined type, extension, and qualification. Journal of abnormal child psychology. 27:393–402. [DOI] [PubMed] [Google Scholar]
- 14.Purvis KL, Tannock R (2000): Phonological processing, not inhibitory control, differentiates ADHD and reading disability. J Am Acad Child Adolesc Psychiatry. 39:485–494. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer JB, Sulzer-Azaroff B (1995): Self-control in boys with attention deficit hyperactivity disorder: effects of added stimulation and time. J Child Psychol Psychiatry. 36:671–686. [DOI] [PubMed] [Google Scholar]
- 16.Sonuga-Barke EJ, Williams E, Hall M, Saxton T (1996): Hyperactivity and delay aversion. III: The effect on cognitive style of imposing delay after errors. J Child Psychol Psychiatry. 37:189–194. [DOI] [PubMed] [Google Scholar]
- 17.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J (2012): Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- 18.Witt ST, Stevens MC (2015): Relationship between white matter microstructure abnormalities and ADHD symptomatology in adolescents. Psychiatry Research: Neuroimaging. 232:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, et al. (2011): Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 50:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Phillips OR, et al. (2008): Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 19:1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hampton WH, Alm KH, Venkatraman V, Nugiel T, Olson IR (2017): Dissociable frontostriatal white matter connectivity underlies reward and motor impulsivity. Neuroimage. 150:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang HL, Chen YJ, Lo YC, Tseng WY, Gau SS (2015): Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention-deficit/ hyperactivity disorder. J Psychiatry Neurosci. 40:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YH, Gau SS, Lo YC, Tseng WY (2014): White matter tract integrity of frontostriatal circuit in attention deficit hyperactivity disorder: association with attention performance and symptoms. Hum Brain Mapp. 35:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu ZM, Bralten J, Cao QJ, Hoogman M, Zwiers MP, An L, et al. (2017): White Matter Microstructural Alterations in Children with ADHD: Categorical and Dimensional Perspectives. Neuropsychopharmacology. 42:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong SB, Zalesky A, Fornito A, Park S, Yang YH, Park MH, et al. (2014): Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biol Psychiatry. 76:656–663. [DOI] [PubMed] [Google Scholar]
- 26.Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, et al. (2015): Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuropsychopharmacol Biol Psychiatry. 63:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D (1999): Wechsler abbreviated scale of intelligence. Psychological Corporation. [Google Scholar]
- 29.Brown TE (2001): Attention-Deficit Disorder Scales for Children and Adolescents. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 30.Moberg DP (2003): Screening for alcohol and other drug problems using the Adolescent Alcohol and Drug Involvement Scale (AADIS). Madison, WI: Center for Health Policy and Program Evaluation, University of Wisconsin-Madison. [Google Scholar]
- 31.Barkley RA (1998): Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. [DOI] [PubMed] [Google Scholar]
- 32.Conners CK, Staff M, Connelly V, Campbell S, MacLean M, Barnes J (2000): Conners’ continuous performance Test II (CPT II v. 5). Multi-Health Syst Inc. 29:175–196. [Google Scholar]
- 33.Conners CK, Epstein JN, Angold A, Klaric J (2003): Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 31:555–562. [DOI] [PubMed] [Google Scholar]
- 34.Schachar R, Mota VL, Logan GD, Tannock R, Klim P (2000): Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 28:227–235. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty DM, Marsh DM, Mathias CW (2002): Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comput. 34:391–398. [DOI] [PubMed] [Google Scholar]
- 36.Mathias CW, Marsh DM, Dougherty DM (2002): Reliability estimates for the immediate and delayed memory tasks. Percept Mot Skills. 95:559–569. [DOI] [PubMed] [Google Scholar]
- 37.Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, et al. (2003): Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry. 44:1145–1157. [DOI] [PubMed] [Google Scholar]
- 38.Kirby KN, Santiesteban M (2003): Concave Utility, Transaction Costs, and Risk in Measuring Discounting of Delayed Rewards. Journal of Experimental Psychology Learning, Memory & Cognition. 29:66–79. [PubMed] [Google Scholar]
- 39.Reynolds B, Schiffbauer R (2004): Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 67:343–356. [DOI] [PubMed] [Google Scholar]
- 40.Lane SD, Cherek DR, Pietras CJ, Tcheremissine OV (2003): Measurement of delay discounting using trial-by-trial consequences. Behavioural Processes. 64:287–303. [DOI] [PubMed] [Google Scholar]
- 41.Marsh DM, Dougherty DM, Mathias CW, Moeller FG, Hicks LR (2002): Comparisons of women with high and low trait impulsivity using behavioral models of response-disinhibition and reward-choice. Personality and Individual Differences. 33:1291–1310. [Google Scholar]
- 42.Mathias CW, Dougherty DM, Marsh DM, Moeller FG (2002): Laboratory measures of impulsivity: A comparison of women with or without childhood aggression. The Psychological Record. 52:289. [Google Scholar]
- 43.Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal. 5:143–156. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- 46.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. (2006): Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 47.Andersson JL, Jenkinson M, Smith S (2007): Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford. 2. [Google Scholar]
- 48.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF (2010): Longitudinal characterization of white matter maturation during adolescence. Brain Res. 1327:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmithorst VJ, Yuan W (2010): White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 72:16–25. [DOI] [PubMed] [Google Scholar]
- 51.Smith SM, Nichols TE (2009): Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. [DOI] [PubMed] [Google Scholar]
- 52.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM (2005): MRI Atlas of Human White Matter. Amsterdam, The Netherlands: Elsevier, B.V. [Google Scholar]
- 53.Cohen J (1992): Statistical power analysis. Current directions in psychological science. 1:98–101. [Google Scholar]
- 54.Reynolds B (2006): A review of delay-discounting research with humans: relations to drug use and gambling. Behavioural pharmacology. 17:651–667. [DOI] [PubMed] [Google Scholar]
- 55.Barkovich AJ (2000): Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 21:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- 56.Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL (2008): Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 26:874–888. [DOI] [PubMed] [Google Scholar]
- 57.Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, et al. (1999): Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 212:770–784. [DOI] [PubMed] [Google Scholar]
- 58.Virta A, Barnett A, Pierpaoli C (1999): Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 17:1121–1133. [DOI] [PubMed] [Google Scholar]
- 59.Paus T (2010): Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 72:26–35. [DOI] [PubMed] [Google Scholar]
- 60.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, et al. (2016): A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 68:838–847. [DOI] [PubMed] [Google Scholar]
- 61.Sonuga-Barke EJ, Bitsakou P, Thompson M (2010): Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 49:345–355. [DOI] [PubMed] [Google Scholar]
- 62.Davis AS, Pass LA, Finch WH, Dean RS, Woodcock RW (2009): The canonical relationship between sensory-motor functioning and cognitive processing in children with attention-deficit/hyperactivity disorder. Archives of clinical neuropsychology. 24:273–286. [DOI] [PubMed] [Google Scholar]
- 63.Herting MM, Schwartz D, Mitchell SH, Nagel BJ (2010): Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 34:1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peper JS, Mandl RC, Braams BR, de Water E, Heijboer AC, Koolschijn PC, et al. (2013): Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cereb Cortex. 23:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evenden J, Ko T (2005): The psychopharmacology of impulsive behaviour in rats VIII: effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology (Berl). 180:294–305. [DOI] [PubMed] [Google Scholar]
- 66.Rivalan M, Gregoire S, Dellu-Hagedorn F (2007): Reduction of impulsivity with amphetamine in an appetitive fixed consecutive number schedule with cue for optimal performance in rats. Psychopharmacology (Berl). 192:171–182. [DOI] [PubMed] [Google Scholar]
- 67.Toplak ME, Dockstader C, Tannock R (2006): Temporal information processing in ADHD: findings to date and new methods. J Neurosci Methods. 151:15–29. [DOI] [PubMed] [Google Scholar]
- 68.Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, et al. (2009): Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 65:7–14. [DOI] [PubMed] [Google Scholar]
- 69.Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. (1999): Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. The Journal of Neuroscience. 19:9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valko L, Schneider G, Doehnert M, Muller U, Brandeis D, Steinhausen HC, et al. (2010): Time processing in children and adults with ADHD. J Neural Transm (Vienna). 117:1213–1228. [DOI] [PubMed] [Google Scholar]
- 71.Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics. 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R (2009a): White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 30:2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R (2009b): Structural development of the basal ganglia in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Psychiatry Res. 172:220–225. [DOI] [PubMed] [Google Scholar]
- 74.Svatkova A, Nestrasil I, Rudser K, Goldenring Fine J, Bledsoe J, Semrud-Clikeman M (2016): Unique white matter microstructural patterns in ADHD presentations-a diffusion tensor imaging study. Hum Brain Mapp. 37:3323–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiang HL, Chen YJ, Shang CY, Tseng WY, Gau SS (2016): Different neural substrates for executive functions in youths with ADHD: a diffusion spectrum imaging tractography study. Psychol Med. 46:1225–1238. [DOI] [PubMed] [Google Scholar]
- 76.Sonuga-Barke EJ (2005): Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 57:1231–1238. [DOI] [PubMed] [Google Scholar]
- 77.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. (2010): Longitudinal changes in grey and white matter during adolescence. Neuroimage. 49:94–103. [DOI] [PubMed] [Google Scholar]
- 78.Stevens MC, Pearlson GD, Calhoun VD, Bessette KL (2017): Functional Neuroimaging Evidence for Distinct Neurobiological Pathways in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


