Abstract
Introduction
Haemophilia A is a rare bleeding disorder caused by coagulation factor VIII (FVIII) deficiency. This is treated with factor VIII, conventionally using products with a half-life of 8–12 hours typically administered every 2–3 days. Recombinant FVIII Fc (rFVIIIFc) represents a new generation of products with an extended half-life allowing higher FVIII levels and longer dosing interval. The efficacy and safety of rFVIIIFc have been established in clinical studies and several years of postmarketing use. However, there remains a need to compare treatment outcome with conventional products in routine clinical use.
Methods and analysis
A-SURE is an ongoing, non-interventional European study with the primary objective to compare the clinical effectiveness of rFVIIIFc with conventional factor products used for haemophilia A prophylaxis. Data covering a 24-month prospective period and a 12-month retrospective period will be collected. Three primary endpoints: bleeding rate, injection frequency and factor consumption will be used to evaluate treatment outcomes. Enrolment of 175 patients on rFVIIIFc and 175 on conventional products is planned. All eligible patients from participating centres will be invited to participate. Visits and treatments follow routine clinical practice. Bias will be reduced by patient matching for age at baseline and the last weekly prophylaxis dose of a conventional product prior to baseline. Propensity scores will be calculated based on prognostic factors and potential confounders assessed at baseline and adjusted for in the estimation of the treatment effect.
Ethics and dissemination
Study approval was obtained by local independent ethics committees and/or authorities, and informed consent from patients or their legal representative is a requirement for participation. Names of ethical committees and approval numbers are provided as supplementary information. The study results will be submitted for publication in a peer-reviewed scientific journal and presented at scientific conferences.
Trial registration number
NCT02976753, Pre-results.
Keywords: haemophilia A, non-interventional study, recombinant factor VIII Fc
Strengths and limitations of this study.
The observational design allows the study to capture real-life experience.
The prospective design allows the study to capture prespecified data.
Innovative study design for comparing conventional and recombinant FVIII Fc prophylactic regimens in haemophilia A.
The risk of bias due to the observational prospective design with a control group is a limitation.
Introduction
Haemophilia A is a rare genetic disorder estimated to occur in 1 in 10 000 births.1 The disease is characterised by a deficiency in coagulation factor VIII (FVIII) causing impaired haemostasis and prolonged bleeding episodes. Bleeding into joints can cause acute pain and swelling and may result in reduced joint range of motion, long-term cartilage damage and debilitating haemophilic arthropathy.2 As a result of bleeding episodes and consequent progressive joint damage, patients experience decreased physical functioning, pain and poor health-related quality of life.3
Treatment for haemophilia A aims to prevent occurrence of bleeding episodes and to prevent sequelae including joint degradation and pain. While on-demand treatment is used for acute bleeding episodes, prophylaxis aim to prevent or reduce future bleeds. Primary prophylaxis (preventive treatment initiated prior to joint damage) and secondary prophylaxis (prophylaxis initiated after the onset of joint damage) are recommended for patients with moderate to severe haemophilia A.4–7 Conventional factor products are either plasma derived or recombinant FVIII replacement products that have a circulating half-life of approximately 8–12 hours with a typical dosing regimen of every 2–3 days.1
Recombinant factor VIII Fc (rFVIIIFc) therapy was approved for the treatment of children, adolescents and adults with haemophilia A in Europe in 2015.8 Unlike conventional factor products, rFVIIIFc has an extended half-life, which allows for higher FVIII levels and longer intervals between injections or more flexible treatment schedules and therefore has the potential to improve both adherence and treatment outcomes.9 10
The efficacy and safety of rFVIIIFc have been established in the phase III studies: A-LONG (NCT01181128)9 and Kids A-LONG (NCT01458106),10 and confirmed by the phase III extension study, ASPIRE (NCT01454739), investigating the long-term efficacy and safety of rFVIIIFc.11 12 Results from ASPIRE have demonstrated sustained safety and efficacy of rFVIIIFc, with low annualised bleeding rates (ABRs), over a period of up to 4 years.11 12
The lower clearance of rFVIIIFc compared with conventional FVIII products has the potential to improve bleed protection without increasing the overall factor consumption. At the same time, rFVIIIFc offers less frequent injections and increased flexibility to tailor treatment to the individual patient.13 14 Any differences between rFVIIIFc and conventional FVIII regarding bleed prevention, injection frequency and factor consumption should be further evaluated with real-world evidence and used to inform the haemophilia community (eg, health authorities, payers, physicians and patients) to promote optimal patient care. Moreover, observational studies complement strictly controlled, phase III studies performed on highly selected patient populations since they reflect real-world treatment effectiveness. For these reasons, the A-SURE study was designed to evaluate the effectiveness of rFVIIIFc compared with conventional FVIII products in the prophylactic treatment of patients with haemophilia A over a 24-month prospective period.
Methods
Study design
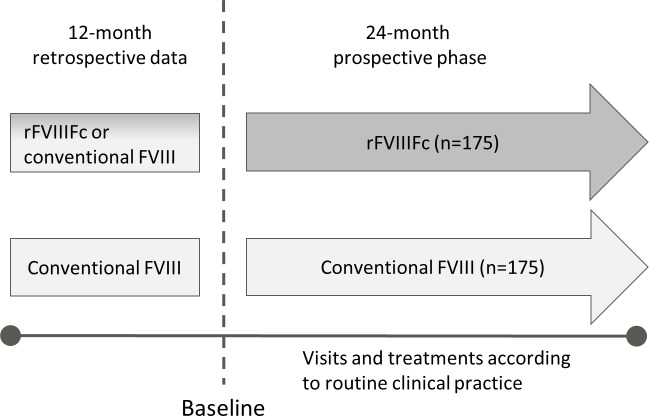
A-SURE is a 24-month prospective, comparative, non-interventional, ongoing phase IV study in males with haemophilia A who are receiving FVIII prophylaxis (figure 1). Patients from approximately 50 centres across Europe will participate in the study. The choice of treatment is made by the patient and the investigator before, and independently from, the decision to include the patient in the study. Patients prescribed rFVIIIFc are enrolled in the rFVIIIFc treatment arm and matched with patients with similar baseline characteristics receiving conventional FVIII therapy. The treatment is prescribed according to usual clinical practice and not dictated by the study protocol. Prebaseline data covering a 12-month period will be collected retrospectively.
Figure 1.
A-SURE study design. A-SURE is a 24-month prospective observational study also including the collection of 12 months prebaseline data. The prophylactic factor treatment will be prescribed according to usual clinical practice and patients prescribed recombinant factor VIII Fc (rFVIIIFc) will be enrolled in the rFVIIIFc treatment arm and matched with patients with similar baseline characteristics receiving conventional coagulation factor VIII (FVIII) therapy.
Study objectives and endpoints
The primary objective of the A-SURE study is to evaluate the effectiveness of rFVIIIFc compared with conventional factor products in the prophylactic treatment of patients with haemophilia A over a 24-month prospective period (box 1). To assess the primary objective, three primary endpoints were established: (1) ABR, as assessed by local practice and defined as any bleeding episode during the trial, which requires treatment with a FVIII product. This can be a patient reported bleed and/or a bleed confirmed by the physician; (2) annualised injection frequencies, as assessed by prescription and (3) annualised factor consumption as assessed by dispensed factor product (box 2). The primary endpoints will be calculated for the 24-month prospective period. Secondary objectives include, for example, evaluation of the effectiveness of rFVIIIFc on patient-reported outcomes (PROs) and health economic parameters compared with conventional factor during the prospective period.
Box 1. A-SURE study objectives.
Primary objective
To evaluate the effectiveness of recombinant FVIII Fc (rFVIIIFc) compared with conventional factor products in the prophylactic treatment of patients with haemophilia A over a 24-month prospective period.
Secondary objectives
To evaluate the effectiveness of rFVIIIFc on patient-reported outcomes (PROs), compared with conventional factor product(s) over a 24-month prospective period.
To evaluate the effectiveness of rFVIIIFc on health economic parameters, compared with conventional factor product(s) over a 24-month prospective period.
To evaluate the effectiveness of rFVIIIFc on bleeding rate, injection frequency and factor consumption over a 24-month prospective period compared with conventional factor product(s) assessed retrospectively for patients initiating rFVIIIFc at baseline.
To evaluate the effectiveness of rFVIIIFc on health economic parameters over a 24-month prospective period compared with conventional factor product(s) assessed retrospectively for patients initiating rFVIIIFc at baseline.
To evaluate the effectiveness of rFVIIIFc on PROs, by comparing PROs on rFVIIIFc with conventional factor treatment at baseline in patients initiating rFVIIIFc at baseline over a 24-month prospective period.
Box 2. A-SURE study endpoints.
Primary endpoints
Annualised bleeding rate (ABR).*
Annualised injection frequency.†
Annualised factor VIII (FVIII) product consumption (IU).‡
Secondary endpoints supporting the primary objective
Joint ABR (as available based on bleeding episode assessed by local practice).
Target joint ABR (as available and based on bleeding episode assessed by local practice).
Secondary endpoints
Change (prospectively assessed–retrospectively assessed) in ABR, annualised injection frequency and annualised FVIII consumption.
-
Patient-reported outcomes:
Euro-QoL-5D-5L.
Haemophilia Activities List (HAL)/Paediatric HAL; children’s and parents’ version.
Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis (Veritas-PRO).
Treatment Satisfaction Questionnaire for Medication.
-
Health economics endpoints:
Inpatient visits: number, length of stay and principal diagnosis of hospitalisations and any surgical procedures.
Outpatient visits: number of emergency room visits or number of outpatient clinic visits (including physical therapy, etc) and reason for outpatient clinic visits.
Missed planned activity and productivity due to haemophilia, that is, the number of times and hours a patient or caregiver missed work or school.
Bleeding episode: a bleed that requires treatment with a FVIII product. Target joint: a joint in which three or more spontaneous bleeds have occurred within a consecutive 6-month period in the last year. Euro-QoL-5D-5L: standardised instrument for patient self-reported quality of life.
*Based on bleeding episodes assessed by local practice,
†Assessed by prescription.
‡Assessed by dispensed factor product.
Patient eligibility and recruitment
To limit bias in the selection of patients, the investigators at the participating centres are encouraged to enrol all eligible patients with prescribed rFVIIIFc treatment. Patients eligible for study participation must meet all inclusion criteria and none of the exclusion criteria (table 1). The first patient was enrolled in December 2016 and the study is expected to end in 2020.
Table 1.
Patient eligibility criteria
| Inclusion criteria | Exclusion criteria |
| Male patients with a diagnosis of haemophilia A. | Enrolment in a concurrent clinical interventional study involving intake of an investigational medicinal product within 1 year prior to enrolment. |
| Received prophylactic treatment with a factor product for the management of haemophilia A in the 12 months prior to enrolment. | Previously treated with commercially available extended half-life FVIII products other than rFVIIIFc. |
| At enrolment, prescribed prophylactic treatment with rFVIIIFc or conventional factor product regardless of participation in the study. | Presence of FVIII inhibitors (≥0.60 Bethesda unit/mL) at the latest available inhibitor test using the Nijmegen modified Bethesda assay. |
| Having ≥12 months’ documented prestudy treatment data regarding prophylactic treatment prescriptions and bleeding episodes prior to the baseline visit. | |
| Signed and dated informed consent provided by the patient or the patient’s legally acceptable representative. |
FVIII, coagulation factor VIII; rFVIIIFc, recombinant FVIII Fc.
Statistical methods
Sample size calculations for this study were based on estimating the power of the study to reject null hypotheses of no difference between treatment groups related to the first two primary effectiveness outcomes, that is, ABR and annualised injection frequency.
For evaluation of effect on bleeding episodes between the two treatments, simulations were used for power calculations. The simulations were based on the number of bleeding episodes calculated from a Poisson distribution. Assuming an average annual bleeding rate of four bleedings per year for conventional factor products,15 and an average annual bleeding rate of 3.38 for rFVIIIFc, 300 patients (150 on each treatment) will have approximately 85% power to reject the null hypothesis of no difference when using a two-sided test at 5% significance level.
With 150 patients per group, the study will also have 85% power to reject the null hypothesis of no difference between treatment groups at the 5% level on annual injection frequency assuming a standard deviation of 35 and a reduction of at least 12 injections per year.
To allow for dropouts, patients switching between groups and possible difficulties to find a matched control for each rFVIIIFc patient, 175 patients will be enrolled in the study per treatment group and thus aim for a total of 350 patients in total.
Data collection and primary analyses
Baseline data, as well as retrospective data from the past 12 months relating to bleeds, surgeries, prescribed and dispensed factor product and health economics parameters will be collected at the time of enrolment. Clinical data will be collected in an electronic case report form and PROs will be collected using paper questionnaires at routine visits throughout the 24-month prospective period.
The primary analyses aim to estimate the effectiveness of rFVIIIFc by comparing ABR, annualised injection frequency and factor consumption to conventional factor products during the 24-month prospective period. The number and percentage of patients who had a bleeding episode as well as the total number of bleeding episodes will be described per treatment group. Comparisons between the two treatment groups will be based on ABR estimates from a negative binomial regression model adjusted for the propensity score, using observed time on each treatment as an offset term, and including treatment during the 24-month prospective period as a time-dependent exposure. Bleeding rate prior to baseline will be included as a covariate to adjust for potential residual confounding by indication. A term for centre/region will also be included in the model. A negative binomial model will be preferred over a Poisson regression model due to expected overdispersion of the data. The same approach will be used for the supportive analyses of joint ABR and target joint ABR.
The annualised injection frequency and annualised factor product consumption (IU) will also be described per treatment group and comparisons will be based on estimates from generalised linear models (analyses of covariance) adjusted for the propensity score, centre/region and including treatment during the 24-month prospective period as a time-dependent exposure. The factor consumption will be assessed based on the amount of dispensed product (from pharmacy, home delivery, etc). Thus, the overall consumption will include treatment for bleeds. Treatment during surgery will, however, be collected separately in the electronic case report form.
Bias and bias limitation
Several approaches have been taken to minimise bias in the A-SURE study. To mitigate selection bias, broad coverage of large centres are included. Approximately 50 recognised treatment centres across Europe have been invited to participate, with each centre being encouraged to enrol all eligible patients prescribed rFVIIIFc. Because each centre comprise a population of volunteers, a non-response selection bias is possible, and the final sample may not be perfectly representative. To describe this potential bias, certain centre information will be collected (ie, type of centre according to European Heamophilia Network, European Haemophilia Treatment Centres or European Haemophilia Comprehensive Care Centres and country) for all contacted centres, and whether or not they responded and if they participated in the study. These data will allow comparison of participating and non-participating centres. To identify elevated background risk factors for bias such as physicians selectively prescribing a certain treatment to a particular patient group for example, to more severely affected patients (confounding by indication), or that the presence of another factor (eg, previous inhibitors influence the treatment effect (effect modifiers), retrospective and baseline information will be collected). These factors will be used to calculate a propensity score for each patient which in turn will be used to adjust the negative binomial regression model used for estimating treatment effectiveness. Thus, the propensity score is the estimated probability of receiving a particular treatment based on measured relevant covariates included in the model. The baseline covariates of interest in the A-SURE study are: history of prophylactic treatment, age, weight, length, ABO blood type, severity of haemophilia, bleeding rate in the past 12 months, presence of target joints, presence of HIV or hepatitis C virus, history of FVIII inhibitors, factor VIII genotype and endogenous von Willebrand factor levels.
Moreover, to reduce potential differences in characteristics between patients on different treatments, patients in the two treatment groups will be matched. Matching is based on age at baseline and the last prescribed weekly dose of prophylactic conventional FVIII product prior to baseline. The age matching uses an age range of ±3 years for patients <16 years and of ±5 years for patients aged ≥16 years. However, patients aged 16 years or older should not be matched with patients younger than 16 years old. The dose matching is based on a similar prescribed weekly factor dose (IU) of conventional factor product for prophylactic treatment defined as ±20%. If a matching control fulfilling age and dose is not available, a patient with the closest age should be invited.
Safety
This study is not designed to answer any safety questions. Therefore, only serious adverse events during rFVIIIFc treatment from first dose to 14 days following end of rFVIIIFc treatment or 14 days after the patients’ last study visit, whichever comes first, and non-serious adverse events leading to permanent discontinuation of rFVIIIFc will be captured. The adverse events will be reported to authorities in accordance with guideline on good pharmacovigilance practices, Module VI.16
Ethics and dissemination
Where appropriate according to national regulation, the protocol and the informed consent form were reviewed and approved by local independent ethics committee and/or authorities as applicable before the study was initiated. A complete list of ethical approvals are provided as online supplementary material. The study results will be disseminated by presentation at scientific congresses and by publication in a peer reviewed journal.
bmjopen-2018-028012supp001.pdf (218.1KB, pdf)
Patient and public involvememt
There has been no public or patient involvement in the design of this study. The study results will be disseminated to the participating patients via the investigators who will receive the study report.
Discussion
This will be the first study to provide prospective data on the effectiveness of prophylaxis with rFVIIIFc compared with conventional FVIII concentrates in patients with haemophilia A in routine clinical practice.
The various sources of potential bias are inherent limitations of observational studies. For the A-SURE study, one of the risks is that more severely affected patients or less well-controlled patients are prescribed with a particular treatment to a higher extent than others (confounding by indication). To minimise this risk, the matching on age and prescribed dose were implemented in addition to adjustment for propensity scores. While matching based on these parameters may be considered quite crude there are very limited options to define relevant matching criteria in a rare disease population with haemophilia A. Selection bias caused by the choice of the participating centres is another relevant risk of bias. To minimise this, haemophilia treatment centres across Europe were invited to participate in the study. Larger centres were chosen to the highest extent possible to ensure a sufficient patient cohort at each centre to increase the likelihood of finding an appropriate matched control patient for each rFVIIIFc patient enrolled.
The A-SURE study is designed to have three primary endpoints: ABR, injection frequency and factor consumption. This is to reflect the interrelation between these factors and to illustrate how the treatment and treatment outcome may be influenced by the extended half-life of rFVIIIFc. For a patient who is suboptimally treated with conventional FVIII, the treatment outcome if continuing conventional FVIII therapy may only be improved by increasing the injection frequency or the amount of FVIII, or both. Pharmacokinetics simulations on the other hand illustrate that if switching to rFVIIIFc, the treatment outcome may be improved without increasing the overall factor consumption or increasing the injection frequency or even allow less frequent injections.13 This is due to the reduced clearance of rFVIIIFc which allows for increased trough levels and improved bleed protection. A reduced injection frequency may also positively impact the patient’s treatment compliance and quality of life.14 17 Overall, the extended circulating half-life of rFVIIIFc improves treatment flexibility and has the potential to raise the standard of care for individuals with haemophilia A.
Supplementary Material
Acknowledgments
We thank Frits Rosendaal (Leiden University Medical Center, The Netherlands) for his intellectual contribution to the study design. We also thank Kristina Lindsten (Sobi, Sweden) for medical writing support in accordance with good publication practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Footnotes
Contributors: JO, CRMH, VJ-Y, FP, J-FS intellectually contributed during protocol development and participated in drafting and revising of the manuscript and read and approved the final version. JS was instrumental for all statistical aspects of the study design and participated in drafting and revising of the manuscript and read and approved the final version. BW was instrumental for protocol developments and overall study design and participated in drafting and revising of the manuscript and read and approved the final version. SL was responsible for the study concept and the overall study design and participated in drafting and revising of the manuscript and read and approved the final version.
Funding: This work was fully funded by Swedish Orphan Biovitrum AB (publ).
Competing interests: JO received grant/research support from Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma and Shire. Personal fees has been received for travel support, participation in advisory boards and participating in symposia as chair or speaker from Bayer, Biotest, CSL Behring, Novo Nordisk, Octapharma, Shire, Chugai, Grifols, Pfizer, Roche and Sobi. CRMH involved in clinical trials or observational research with Sobi, Bayer, Novo, Roche, Alnylam, Biomarin, Pfizer and Shire; speaker’s bureaus for Shire, Biotest, Sobi, Pfizer and Roche. VJ-Y received grant/research support from Bayer, Biotest, Grifols, Novo Nordisk, Octapharma, Pfizer, Shire and Sobi; Speaker Bureau with Bayer, Biogen, Novo Nordisk, Pfizer, Roche, Shire and Sobi. FP received honoraria for participating as a speaker with Bayer, Grifols, Sobi, Shire, F. Hoffmann-La Roche, Alnylam; received consulting fees from Kedrion and LFB; has been the member of scientific advisory boards of F. Hoffmann-La Roche and Shire. J-FS received grants from Sobi and Behring. JS, BW and SL are the employees of Sobi and holders of Sobi shares.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. World Federation of Hemophilia (WFH). Guidelines for the management of hemophilia. 2012. https://www.wfh.org/en/resources/wfh-treatment-guidelines (accessed 12 Nov 2018).
- 2. Roosendaal G, Lafeber FP. Blood-induced joint damage in hemophilia. Semin Thromb Hemost 2003;29:037–42. 10.1055/s-2003-37938 [DOI] [PubMed] [Google Scholar]
- 3. van Genderen FR, Westers P, Heijnen L, et al. Measuring patients' perceptions on their functional abilities: validation of the Haemophilia Activities List. Haemophilia 2006;12:36–46. 10.1111/j.1365-2516.2006.01186.x [DOI] [PubMed] [Google Scholar]
- 4. National Hemophilia Foundation (NHF). Medical and Scientific Advisory Council (MASAC) Recommendation Concerning Prophylaxis. 2016. https://www.hemophilia.org/sites/default/files/document/files/241Prophylaxis.pdf (accessed 12 Nov 2018).
- 5. Blanchette VS. Prophylaxis in the haemophilia population. Haemophilia 2010;16(Suppl 5):181–8. 10.1111/j.1365-2516.2010.02318.x [DOI] [PubMed] [Google Scholar]
- 6. Collins P, Faradji A, Morfini M, et al. Efficacy and safety of secondary prophylactic vs. on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-month crossover study. J Thromb Haemost 2010;8:83–9. 10.1111/j.1538-7836.2009.03650.x [DOI] [PubMed] [Google Scholar]
- 7. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007;357:535–44. 10.1056/NEJMoa067659 [DOI] [PubMed] [Google Scholar]
- 8. European Medicines Agency (EMA). Summary of Product Charateristics: Elocta. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/003964/WC500198642.pdf (accessed 12 Nov 2018).
- 9. Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood 2014;123:317–25. 10.1182/blood-2013-10-529974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost 2015;13:967–77. 10.1111/jth.12911 [DOI] [PubMed] [Google Scholar]
- 11. Nolan B, Mahlangu J, Perry D, et al. Long-term safety and efficacy of recombinant factor VIII Fc fusion protein (rFVIIIFc) in subjects with haemophilia A. Haemophilia 2016;22:72–80. 10.1111/hae.12766 [DOI] [PubMed] [Google Scholar]
- 12. Nolan B, Mahlangu J, Young G, et al. ASPIRE Final Results Confirm Established Safety and Sustained Efficacy for Up to 4 Years of Treatment With rFVIIIFc in Previously Treated Subjects With Severe Hemophilia A. ASH annual Meeting 2018, abstract 1192. https://ash.confex.com/ash/2018/webprogram/Paper118586.html (accessed 13 Nov 2018).
- 13. Berntorp E, Negrier C, Gozzi P, et al. Dosing regimens, FVIII levels and estimated haemostatic protection with special focus on rFVIIIFc. Haemophilia 2016;22:389–96. 10.1111/hae.12887 [DOI] [PubMed] [Google Scholar]
- 14. Iorio A, Krishnan S, Myrén KJ, et al. Indirect comparisons of efficacy and weekly factor consumption during continuous prophylaxis with recombinant factor VIII Fc fusion protein and conventional recombinant factor VIII products. Haemophilia 2017;23:408–16. 10.1111/hae.13160 [DOI] [PubMed] [Google Scholar]
- 15. Berntorp E, Dolan G, Hay C, et al. European retrospective study of real-life haemophilia treatment. Haemophilia 2017;23:105–14. 10.1111/hae.13111 [DOI] [PubMed] [Google Scholar]
- 16. Agency EM. EMA), Guideline on good pharmacovigilance practices (GVP). https://www.ema.europa.eu/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (Accessed 12 Nov 2018).
- 17. Steen Carlsson K, Andersson E, Berntorp E. Preference-based valuation of treatment attributes in haemophilia A using web survey. Haemophilia 2017;23:894–903. 10.1111/hae.13322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028012supp001.pdf (218.1KB, pdf)