Abstract
Although the spatial and temporal orchestration of early vertebrate embryogenesis is missing from cell culture systems, recent work suggests that many of the same signals affecting neural induction in vertebrate embryos also regulate embryonic stem (ES) cell neurogenesis. One key regulatory mechanism involved in both in vivo and in vitro neural induction is the inhibition of BMP signals. Wnts and FGFs represent additional regulatory influences which may affect the adoption of neural fates through both BMP-dependent and -independent mechanisms. Insights into neural induction in vivo help to guide paradigms for promoting neural differentiation by ES cells. Conversely, insights into the mechanisms by which ES cells adopt neural fates may provide an improved understanding of neural induction in the early embryo.
Keywords: BMP, TGFβ, Smad, FGF, MAPK, Wnt, Retinoic acid, Default model, Organizer
Introduction
It is a truism that ES cells hold tremendous promise as an inexhaustible source for novel cell-based therapies for diseases for which treatments are imperfect or nonexistent. Work over the past twenty-five years has aimed at understanding the properties of stem cells, especially the means to derive specific differentiated cell types from stem cells. One of the first lineages to be generated from ES cells was neural (1–4), and many subsequent studies have succeeded in promoting the differentiation of ES cells towards the neural lineage to create various kinds of neuronal and glial subtypes. This work reached an important milestone with the demonstration that neural cells derived from ES cells can be transplanted back into vertebrate embryos and integrate into many areas of the central nervous system (5, 6).
Beyond the therapeutic implications, neural differentiation by ES cells can also provide insights into the mechanisms of neural induction, the initial steps by which uncommitted cells begin differentiating along the neural lineage. Several recent reviews have focused on the properties of neural stem cells and neural differentiation along defined pathways (7–9). Here, we contrast neural induction in vertebrate model organisms with neural induction in ES cells. Similarities and differences between in vivo and in vitro neural induction may lend insights into the molecular signals that direct neural induction in the embryo. Additionally, a better understanding of the signaling and tissue dynamics that underlie neural induction in vivo may lead to more efficient ways of deriving neural subtypes in vitro.
Neural induction through BMP antagonism
Neural induction has fascinated scientists since Spemann and Mangold established the presence of an organizer in amphibians capable of conferring neural identity to ectoderm (10). The presence of similar organizers in other species, such as the embryonic shield in teleosts, Hensen’s node in birds, and the node in mice, suggests that the mechanisms by which the organizer imparts neural identity are at least partly conserved in vertebrates (11–13). The first clues to the molecular nature of the organizer’s inductive influence came from studies of a receptor for TGFβ superfamily members, and Noggin, a secreted factor expressed by the organizer (14–16). The ectodermal region of the early Xenopus embryo animal pole normally gives rise to both epidermis and neurectoerm (Fig. 1A). However, if this region is physically isolated as an animal cap, it does not form neural tissue, though expression of either a dominant negative form of a TGFβ receptor, or Noggin is sufficient to generate neural cells in Xenopus animal caps (14–16). Noggin, as well as other factors that can induce neural fates in animal caps such as Chordin and Follistatin, share the ability to bind to and inhibit the activity of Bone Morphogenetic Proteins (BMPs), TGFβ superfamily members (17–19). The finding that these factors specify neural identity by inhibiting the repressive influence of BMPs suggests that it is the absence of repressive influences, and not an active promoting influence, that may be essential for neural induction (20). This so-called default model of neural induction is further supported by the finding that dissociated Xenopus animal caps, deprived of extensive intercellular contacts and communication, adopt neural fates (21–24) and that isolated animal caps of some amphibians differentiate into neural tissue (25).
Figure 1. In vivo neural induction.
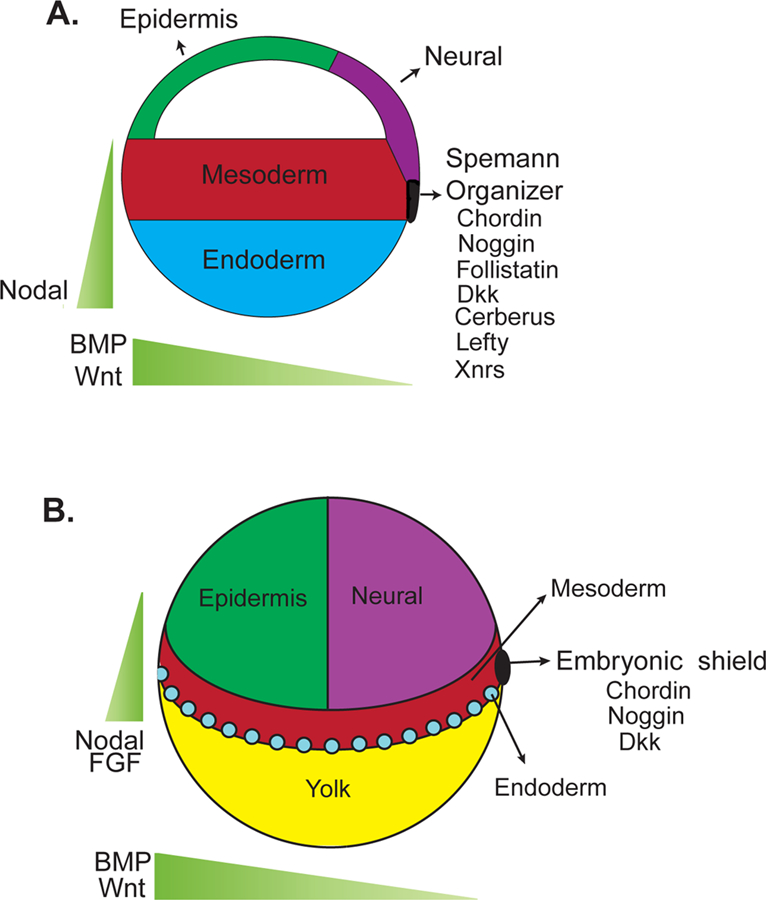
The dorsal organizer in Xenopus (A.) and similarly the shield in zebrafish (B.) express many factors that protect the ectodermal domain that will give rise to neural fates from activators of the BMP, Nodal, and Wnt pathways. The combined functions of secreted agonists of these pathways and the inhibitors produced by the dorsal organizer give rise to gradients that both pattern the embryo and allow for neural induction.
Given the central role that inhibition of BMP plays in neural induction, it is somewhat surprising that mouse embryos mutant for Chordin, Noggin, or Follistatin do not display gross defects in neural plate formation (26–28). Indeed, individual loss of these BMP inhibitors, as well as others including Twisted gastrulation, Gremlin and Cerberus-like, does not dramatically affect neural allocation in mice (29–31). However, loss of multiple BMP antagonists, such as both Chordin and Noggin, results in neural deficits, indicating functional overlap among BMP inhibitors (30). Consistent with this interpretation, Xenopus or zebrafish embryos depleted of multiple BMPs, or mouse embryos lacking BMPR1a, a component of the principle BMP receptor during early embryonic development, display increased and early neural differentiation (32, 33). The similarity of these phenotypes suggests that inhibition of BMP activity through the overlapping functions of several BMP antagonists and the resulting BMP activity gradient play a critical role in vertebrate neural induction (Fig. 1).
Beyond the Default Model
The default model may be too simple to completely describe neural induction (34, 35). For example, additional influences, such as Wnt signals, have complex roles in promoting, inhibiting, and patterning neural tissue at discrete times during embryogenesis. Xenopus and zebrafish both require an initial activation of the β-catenin-dependent Wnt signaling pathway during the blastula stage for specification of the organizer, and therefore for subsequent BMP antagonism (36–39). In Xenopus, this early Wnt signaling depends on Wnt11 (40).
While this initial Wnt signaling is required early in embryo development for subsequent neural induction, both the Wnt and BMP pathways must be inhibited later in development for neural induction to occur, consistent with the central tenant of the default model. At gastrulation stages, Wnt signals collaborate with BMPs to ventralize frog and fish embryos and inhibit neural fates (Fig. 1) (41–44). Similarly, Wnt signaling inhibits neural fates in the chick epiblast, and inhibition of Wnt signaling is sufficient to induce neural differentiation in regions normally fated to become epidermis (45).
This inhibitory role for Wnts may be conserved in mammals, as genetic studies support an inhibitory function for Wnt signaling in mouse neural induction. Mouse mutants lacking effectors of β-catenin-dependent Wnt signaling, such as Wnt3a, display increased neural tissue and even ectopic neural tubes (46). Similarly, mutation of the Wnt co-receptors, Lrp5 and Lrp6, results in an expansion of anterior neuroectoderm (47). Consistent with this negative regulatory role, loss of Dickkopf (Dkk), a Wnt inhibitor, prevents forebrain development (48).
However, mammalian Wnt signaling is involved in both anteroposterior patterning and cell movement in the primitive streak, raising the possibility that the neural phenotypes of Wnt pathway mutants may reflect indirect consequences of disregulated Wnt signaling. Indeed, ectopic neural tubes similar to those caused by loss of Wnt3a can result from defective cell movement in the primitive streak (49).
In addition to BMP inhibitors such as Chordin, Noggin, and Follistatin, the Xenopus organizer expresses Frzb-1, Crescent, SFRP2, Dkk1, Cerberus, and Lefty (Fig. 1), inhibitors of the Wnt and Nodal pathways (50). The production of so many signaling pathway inhibitors by the organizer substantiates the underlying supposition of the default model. So, is there no role for positive signaling in neural induction?
At least in non-mammalian vertebrates, there is substantial evidence that activation of the MAPK pathway promotes neural induction. For example, neural induction in isolated salamander animal caps depends on the MAPK pathway (51), and the members of one class of MAPK activators, Fibroblast Growth Factors (FGFs), induce early neural plate markers, such as Sox3 and ERNI (52–55). In support of the involvement of FGFs in neural induction, FGFs can act cooperatively with BMP inhibition to promote Xenopus neural induction (32). One possible molecular mechanism for this functional cooperation is through MAPK pathway convergence on BMP signaling through the differential phosphorylation of Smad1, an important BMP effector (56). GSK3, a component and inhibitor of the Wnt pathway, promotes additional phosphorylation and degradation of Smad1 after a priming phosphorylation by MAPK, which may similarly explain the anti-neuralizing properties of Wnts (57). However, mutation of the MAPK phosphorylation site of Smad1 does not overtly abrogate neural induction in mice, suggesting that this interaction is not essential for the effects of FGFs on neural induction (58). Another possibility is that early FGF signals downregulate expression of BMPs in the prospective neural domain, allowing neural differentiation to proceed (59, 60). A third possibility is that early FGF signaling promotes neural fate through a parallel, BMP-independent mechanism (61, 62). One BMP-independent mechanism may involve Wnts which, as described above, can inhibit neural induction during gastrulation stages (45). However, addition of multiple FGFs, BMP antagonists, and Wnt antagonists to the chick embryonic epiblast is not sufficient to induce the expression of the neural marker Sox2, suggesting that still other pathways regulate neural induction (62).
Although these data from amphibians and birds suggest that FGFs are positive effectors of vertebrate neural induction, to date there has not been genetic confirmation in fish or mice. For example, mouse epiblast cells lacking the FGF receptor, Fgfr1, can adopt neural fates (49). Also, inhibition of FGF signaling in the pre-gastrula mouse embryo does not block neural induction (33). It is possible that the involvement of an early FGF signal in neural induction does not pertain to mammals, or may be redundant with other inputs into the MAPK pathway.
ES cells: “All cases are unique, and very similar to others”
Partially due to ease of manipulation, ES cells represent an attractive model for studying early embryonic differentiation in general, and neural induction in particular. ES cells can be differentiated spontaneously to embryoid bodies or along specific lineages by addition of extrinsic factors or by genetic manipulation.
The relevance of ES cell differentiation to in vivo events is often unclear. For example, retinoic acid is one of the most potent inducers of neural differentiation in both mouse and human embryonic stem cells (63, 64), but there is little evidence for a role for retinoic acid in embryonic neural induction. Nevertheless, in ES cells, retinoic acid has dosage dependent effects whereby high concentrations drive ES cells towards a neural lineage and low retinoic acid concentrations cause cardiac differentiation (65).
In contrast, other neural influences are conserved between ES cells and in vivo systems (Fig. 2). Given that mouse and human ES cells have markedly different growth factor requirements for maintaining pluripotency, it is interesting that they both respond relatively similarly to BMP and BMP inhibitors with regards to neural induction. In both mouse and human ES cells, high level BMP signaling blocks neural differentiation (66–70). For these reasons, most neural differentiation protocols require that ES cells be grown in low or no serum conditions to mitigate the effect of BMPs found in serum. Further evidence that BMPs inhibit ES cell neural induction comes from a recent study showing that mouse ES cells lacking Smad4 preferentially differentiate down the neural lineage (71). As Smad4 is required for all signaling by TGFβ superfamily members, and not just by BMPs, it is possible that this finding reflects inhibitory influences by Nodal or other TGFβ superfamily members in addition to BMPs. Indeed, Nodal signaling may inhibit neural differentiation as mouse ES cells mutant for Cripto, a Nodal co-receptor, exhibit enhanced neural differentiation, and human ES cells overexpressing Lefty or a truncated version of Cerberus, Nodal pathway inhibitors, display increased neural induction (71–73). Also, mouse Nodal mutants show early and ectopic adoption of neural fates (74).
Figure 2. In vitro neural induction.
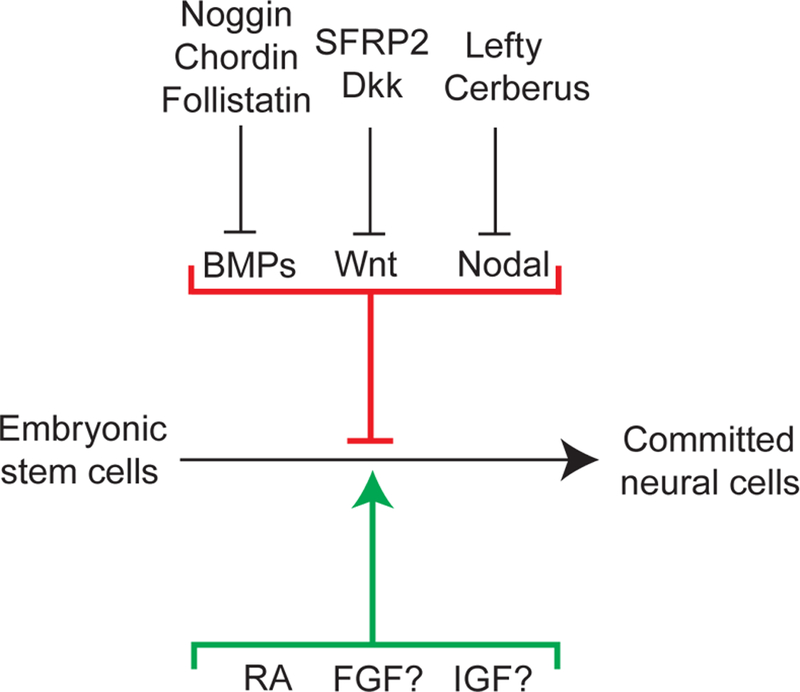
Many in vivo neural inducers that act as inhibitors of BMPs, Nodal, and Wnt signaling also promote ES cell differentiation to committed neural cells. In contrast, RA, which promotes neural induction in ES cells, is not known to be important for neural induction in vivo.
While it is clear that BMPs inhibit neural differentiation in vitro, it is ambiguous whether adding BMP inhibitors increases neural induction beyond that caused by serum starvation. For example, treatment of mouse ES cells with Noggin does not affect neural induction in ES cells differentiated as embryoid bodies or as an adherent monolayer (66, 68, 75, 76). Moreover, neither addition of Chordin nor Follistatin significantly affects ES cell neural differentiation (67, 77). On the other hand, Noggin increases the differentiation of mouse ES cells to neurospheres (69) and can enhance neural differentiation by mouse and human ES cells (77–80).
The requirement for BMPs in maintaining mouse ES cell pluripotency is not shared by human ES cells, for which even low level BMP signaling promotes trophoblast differentiation (70). These different requirements may reflect different cells of origin for mouse and human ES cells; most mouse ES cell lines are derived at the late inner cell mass stage, whereas human ES cell lines are derived at the blastocyst stage. Indeed, mouse ES cells that, like human ES cells, are derived from blastocysts require growth factors and display gene expression profiles similar to human ES cells (81, 82).
Like the BMPs, Wnts appear to affect neural induction by ES cells in ways similar to those observed in vivo. For example, activating the Wnt pathway in differentiating mouse ES cells, either with Wnt1 or with an inhibitor of GSK3, inhibits neural differentiation (83). Consistent with a negative role for Wnts in neural induction, genetic manipulations that increase Wnt signaling, such as loss of the extracellular Wnt inhibitor Dkk1, or both GSK3α and GSK3β, abrogate neural differentiation (84, 85). Conversely, treatment with Dkk1, or overexpression of another Wnt inhibitor, SFRP2, enhances mouse ES cell neural differentiation (83, 86). These data suggest that high level Wnt signaling prevents neural differentiation whereas the inhibition of Wnt signaling enhances neural differentiation, closely paralleling in vivo mouse models.
However, others have found that inducing Wnt signaling can increase neural differentiation in mouse ES cells (87). The discrepant effects of both Wnts and BMP inhibitors among various protocols may be attributable to differences in culture media, which can contain BMPs, Wnts and/or additional signaling inhibitors. Another possible explanation is that BMP inhibitors or Wnts may influence neural differentiation only under specific conditions, such as the presence of FGF signaling or the absence of Nodal signaling. A third possibility may reflect the complex involvement of these factors in both ES maintenance and differentiation. For example, in addition to inhibiting neural induction, intermediate levels of BMP signaling maintain mouse ES cell pluripotency (88). Similarly, Wnt signaling may promote ES cell pluripotency (89). Because some of the factors required for ES cell pluripotency and survival are the same factors that promote neural fates, it is difficult to discern if these factors act primarily to induce neural differentiation or to simply enhance progenitor survival or proliferation. This uncertainty reflects the artificiality inherent in ES cell culture systems, as maintenance of pluripotency does not occur outside of the germline in vivo.
Given the diverse roles that FGFs play in regulating proliferation, self-renewal, survival, and differentiation, it is perhaps unsurprising that FGFs similarly have complex effects on ES cell neural induction. FGF2 and FGF4 are used in several neural differentiation protocols (68, 69), (90), but it is unclear whether they promote differentiation, proliferation and/or survival of neural precursor cells. This ambiguity is especially relevant to human ES cells, as FGF2 is used to maintain human ES cell pluripotency.
Consistent with a role for FGF signaling in promoting neural induction, treatment of differentiating mouse ES cells with a pharmacological inhibitor of FGF signaling, PD184352, arrests neural differentiation at an early stage (75). In contrast, treatment with a different pharmacological inhibitor of FGF signaling, SU5402, does not affect early neural differentiation (but may affect survival of neural precursors), and mouse ES cells lacking Fgfr1 are able to differentiate down the neural lineage similarly to wild-type cells (78). PD184352 inhibits the activity of MEK, a component of the MAPK cascade, whereas SU5402 inhibits the tyrosine kinase activity of Fgfr1, raising the possibility that MAPK pathway activation through an Fgfr1-independent mechanism may be important for ES cell neural induction. Insulin-like growth factors (IGFs) can also activate the MAPK pathway and promote neural fates in Xenopus (91), raising the possibility that IGFs are the activators of the MAPK pathway relevant to neurogenesis in mammals.
Conclusions and perspectives
Two of the remarkable properties of ES cells are pluripotency and a capacity for genetic modification. The potential to use ES cells to create cell-based therapies has received much attention, and neural and glial derivatives of ES cells are likely to be important for treating traumatic injuries and degenerative diseases affecting the nervous system. The same remarkable properties are also valuable for creating cellular models of human diseases critical both for elucidating disease pathogenesis and for testing potential therapies. Neural and glial derivatives of ES cells are already providing insights into the molecular and cellular bases of neurodegenerative disorders such as amyotrophic lateral sclerosis (92). ES cells can also be used to create genetic models of human disease and to test the linkage between genes and associated disorders. For example, expression of alleles associated with Huntington’s and Parkinson’s diseases in ES cells has provided insights into underlying molecular pathogenesis (93, 94).
One important step toward these potential biomedical advances is the development of a better understanding of neural induction by ES cells. The study of neural induction has a long history that provides extensive knowledge of early neural differentiation in several different vertebrate embryos. Common themes have emerged from these developmental model organisms, suggesting that early low-level FGF signals, combined with protective influences from the dorsal organizer shielding the ectoderm from Wnt and BMP signals, are critical for neural induction. Several of these influences are reflected in the requirements of ES cells for neural differentiation, although important differences between neural induction in vivo and in vitro are demonstrable. Better understanding of the regulatory influences that control neural induction, especially in mammals, may provide additional means of promoting neural induction and differentiation in ES cells. Conversely, better understanding of the molecular mechanisms by which ES cells adopt neural fates may provide new insights into the regulation of neural induction in the early vertebrate embryo.
Acknowledgments
We thank members of the Reiter lab for helpful discussions. This work was supported by funding from the Sandler Family Supporting Foundation, the Burroughs Wellcome Fund, and the National Institutes of Health (R01AR054396).
References
- 1.Martin GR and Evans MJ (1975) Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A, 72, 1441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ and Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature, 292, 154–6. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A, 78, 7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–7. [DOI] [PubMed] [Google Scholar]
- 5.Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S and McKay RD (1997) In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci U S A, 94, 14809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wichterle H, Garcia-Verdugo JM, Herrera DG and Alvarez-Buylla A (1999) Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci, 2, 461–6. [DOI] [PubMed] [Google Scholar]
- 7.Temple S (2001) The development of neural stem cells. Nature, 414, 112–7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SC (2006) Neural subtype specification from embryonic stem cells. Brain Pathol, 16, 132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merkle FT and Alvarez-Buylla A (2006) Neural stem cells in mammalian development. Curr Opin Cell Biol, 18, 704–9. [DOI] [PubMed] [Google Scholar]
- 10.Spemann HM (1924) Über Induktion von Embryonanlagen durch Implantation artfremder Organisatoren. Wilhelm Roux Arch. Entw. Mech. Organ, 100, 599–638. [Google Scholar]
- 11.Waddington CH (1932) Experiments on the Development of Chick and Duck Embryos, Cultivated in vitro. Phil. Trans. Roy. Soc. Lond. B, 179–230.
- 12.Oppenheimer J (1936) Structures developed in amphibians by implantation of living fish organizer. Structures developed in amphibians by implantation of living fish organizer
- 13.Beddington RS (1994) Induction of a second neural axis by the mouse node. Development, 120, 613–20. [DOI] [PubMed] [Google Scholar]
- 14.Hemmati-Brivanlou A and Melton DA (1992) A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature, 359, 609–14. [DOI] [PubMed] [Google Scholar]
- 15.Smith WC and Harland RM (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell, 70, 829–40. [DOI] [PubMed] [Google Scholar]
- 16.Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD and Harland RM (1993) Neural induction by the secreted polypeptide noggin. Science, 262, 713–8. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman LB, De Jesus-Escobar JM and Harland RM (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell, 86, 599–606. [DOI] [PubMed] [Google Scholar]
- 18.Hemmati-Brivanlou A, Kelly OG and Melton DA (1994) Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell, 77, 283–95. [DOI] [PubMed] [Google Scholar]
- 19.Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK and De Robertis EM (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell, 79, 779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmati-Brivanlou A and Melton D (1997) Vertebrate neural induction. Annu Rev Neurosci, 20, 43–60. [DOI] [PubMed] [Google Scholar]
- 21.Born J, Janeczek J, Schwarz W, Tiedemann H and Tiedemann H (1989) Activation of masked neural determinants in amphibian eggs and embryos and their release from the inducing tissue. Cell Differ Dev, 27, 1–7. [DOI] [PubMed] [Google Scholar]
- 22.Godsave SF and Slack JM (1989) Clonal analysis of mesoderm induction in Xenopus laevis. Dev Biol, 134, 486–90. [DOI] [PubMed] [Google Scholar]
- 23.Grunz H and Tacke L (1989) Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev, 28, 211–7. [DOI] [PubMed] [Google Scholar]
- 24.Sato SM and Sargent TD (1989) Development of neural inducing capacity in dissociated Xenopus embryos. Dev Biol, 134, 263–6. [DOI] [PubMed] [Google Scholar]
- 25.Barth LG (1941) Neural differentiation without organizer. J. Exp. Zool, 371–384.
- 26.Brunet LJ, McMahon JA, McMahon AP and Harland RM (1998) Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science, 280, 1455–7. [DOI] [PubMed] [Google Scholar]
- 27.Bachiller D, Klingensmith J, Shneyder N, Tran U, Anderson R, Rossant J and De Robertis EM (2003) The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development, 130, 3567–78. [DOI] [PubMed] [Google Scholar]
- 28.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR and Bradley A (1995) Multiple defects and perinatal death in mice deficient in follistatin. Nature, 374, 360–3. [DOI] [PubMed] [Google Scholar]
- 29.Gazzerro E and Canalis E (2006) Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord, 7, 51–65. [DOI] [PubMed] [Google Scholar]
- 30.Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J et al. (2000) The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature, 403, 658–61. [DOI] [PubMed] [Google Scholar]
- 31.Khokha MK, Hsu D, Brunet LJ, Dionne MS and Harland RM (2003) Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat Genet, 34, 303–7. [DOI] [PubMed] [Google Scholar]
- 32.Reversade B, Kuroda H, Lee H, Mays A and De Robertis EM (2005) Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development, 132, 3381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y and Rodriguez TA (2007) BMP signalling inhibits premature neural differentiation in the mouse embryo. Development, 134, 3359–69. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda H, Fuentealba L, Ikeda A, Reversade B and De Robertis EM (2005) Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev, 19, 1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern CD (2006) Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol, 18, 692–7. [DOI] [PubMed] [Google Scholar]
- 36.Pelegri F and Maischein HM (1998) Function of zebrafish beta-catenin and TCF-3 in dorsoventral patterning. Mech Dev, 77, 63–74. [DOI] [PubMed] [Google Scholar]
- 37.Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ and Weinberg ES (2000) Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development, 127, 3899–911. [DOI] [PubMed] [Google Scholar]
- 38.Nojima H, Shimizu T, Kim CH, Yabe T, Bae YK, Muraoka O, Hirata T, Chitnis A, Hirano T and Hibi M (2004) Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev, 121, 371–86. [DOI] [PubMed] [Google Scholar]
- 39.Wessely O, Agius E, Oelgeschlager M, Pera EM and De Robertis EM (2001) Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol, 234, 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X and Heasman J (2005) Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell, 120, 857–71. [DOI] [PubMed] [Google Scholar]
- 41.Erter CE, Wilm TP, Basler N, Wright CV and Solnica-Krezel L (2001) Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development, 128, 3571–83. [DOI] [PubMed] [Google Scholar]
- 42.Lekven AC, Thorpe CJ, Waxman JS and Moon RT (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell, 1, 103–14. [DOI] [PubMed] [Google Scholar]
- 43.Baker JC, Beddington RS and Harland RM (1999) Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev, 13, 3149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heeg-Truesdell E and LaBonne C (2006) Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev Biol, 298, 71–86. [DOI] [PubMed] [Google Scholar]
- 45.Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM and Edlund T (2001) The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature, 411, 325–30. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa Y, Fujimori T, McMahon AP and Takada S (1997) Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol, 183, 234–42. [DOI] [PubMed] [Google Scholar]
- 47.Kelly OG, Pinson KI and Skarnes WC (2004) The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development, 131, 2803–15. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP et al. (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell, 1, 423–34. [DOI] [PubMed] [Google Scholar]
- 49.Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP and Rossant J (1997) Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development, 124, 2829–41. [DOI] [PubMed] [Google Scholar]
- 50.De Robertis EM and Kuroda H (2004) Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol, 20, 285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurtado C and De Robertis EM (2007) Neural induction in the absence of organizer in salamanders is mediated by MAPK. Dev Biol, 307, 282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamb TM and Harland RM (1995) Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development, 121, 3627–36. [DOI] [PubMed] [Google Scholar]
- 53.Storey KG, Goriely A, Sargent CM, Brown JM, Burns HD, Abud HM and Heath JK (1998) Early posterior neural tissue is induced by FGF in the chick embryo. Development, 125, 473–84. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez IS, Araujo M and Nieto MA (1998) Neural induction in whole chick embryo cultures by FGF. Dev Biol, 199, 42–54. [DOI] [PubMed] [Google Scholar]
- 55.Kengaku M and Okamoto H (1993) Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development, 119, 1067–78. [DOI] [PubMed] [Google Scholar]
- 56.Pera EM, Ikeda A, Eivers E and De Robertis EM (2003) Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev, 17, 3023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM and De Robertis EM (2007) Integrating Patterning Signals: Wnt/GSK3 Regulates the Duration of the BMP/Smad1 Signal. Cell, 131, 980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aubin J, Davy A and Soriano P (2004) In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev, 18, 1482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson SI, Graziano E, Harland R, Jessell TM and Edlund T (2000) An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol, 10, 421–9. [DOI] [PubMed] [Google Scholar]
- 60.Furthauer M, Van Celst J, Thisse C and Thisse B (2004) Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development, 131, 2853–64. [DOI] [PubMed] [Google Scholar]
- 61.Delaune E, Lemaire P and Kodjabachian L (2005) Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development, 132, 299–310. [DOI] [PubMed] [Google Scholar]
- 62.Linker C and Stern CD (2004) Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development, 131, 5671–81. [DOI] [PubMed] [Google Scholar]
- 63.Bain G, Kitchens D, Yao M, Huettner JE and Gottlieb DI (1995) Embryonic stem cells express neuronal properties in vitro. Dev Biol, 168, 342–57. [DOI] [PubMed] [Google Scholar]
- 64.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA and Benvenisty N (2000) Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A, 97, 11307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rohwedel J, Guan K and Wobus AM (1999) Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs, 165, 190–202. [DOI] [PubMed] [Google Scholar]
- 66.Finley MF, Devata S and Huettner JE (1999) BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol, 40, 271–87. [PubMed] [Google Scholar]
- 67.Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI and Sasai Y (2000) Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron, 28, 31–40. [DOI] [PubMed] [Google Scholar]
- 68.Ying QL, Stavridis M, Griffiths D, Li M and Smith A (2003) Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol, 21, 183–6. [DOI] [PubMed] [Google Scholar]
- 69.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J and van der Kooy D (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron, 30, 65–78. [DOI] [PubMed] [Google Scholar]
- 70.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP and Thomson JA (2002) BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol, 20, 1261–4. [DOI] [PubMed] [Google Scholar]
- 71.Sonntag KC, Simantov R, Bjorklund L, Cooper O, Pruszak J, Kowalke F, Gilmartin J, Ding J, Hu YP, Shen MM et al. (2005) Context-dependent neuronal differentiation and germ layer induction of Smad4−/− and Cripto−/− embryonic stem cells. Mol Cell Neurosci, 28, 417–29. [DOI] [PubMed] [Google Scholar]
- 72.Smith JR, Vallier L, Lupo G, Alexander M, Harris WA and Pedersen RA (2007) Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol [DOI] [PubMed]
- 73.Vallier L, Reynolds D and Pedersen RA (2004) Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol, 275, 403–21. [DOI] [PubMed] [Google Scholar]
- 74.Camus A, Perea-Gomez A, Moreau A and Collignon J (2006) Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol, 295, 743–55. [DOI] [PubMed] [Google Scholar]
- 75.Stavridis MP, Lunn JS, Collins BJ and Storey KG (2007) A discrete period of FGF-induced Erk½ signalling is required for vertebrate neural specification. Development, 134, 2889–94. [DOI] [PubMed] [Google Scholar]
- 76.Conley BJ, Ellis S, Gulluyan L and Mollard R (2007) BMPs regulate differentiation of a putative visceral endoderm layer within human embryonic stem-cell-derived embryoid bodies. Biochem Cell Biol, 85, 121–32. [DOI] [PubMed] [Google Scholar]
- 77.Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D and Mummery C (2004) Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci, 117, 1269–80. [DOI] [PubMed] [Google Scholar]
- 78.Smukler SR, Runciman SB, Xu S and van der Kooy D (2006) Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol, 172, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R and Isacson O (2007) Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells, 25, 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou JM, Chu JX and Chen XJ (2007) An improved protocol that induces human embryonic stem cells to differentiate into neural cells in vitro. Cell Biol Int [DOI] [PubMed]
- 81.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA et al. (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 448, 191–5. [DOI] [PubMed] [Google Scholar]
- 82.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL and McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature, 448, 196–9. [DOI] [PubMed] [Google Scholar]
- 83.Aubert J, Dunstan H, Chambers I and Smith A (2002) Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol, 20, 1240–5. [DOI] [PubMed] [Google Scholar]
- 84.Verani R, Cappuccio I, Spinsanti P, Gradini R, Caruso A, Magnotti MC, Motolese M, Nicoletti F and Melchiorri D (2007) Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem, 100, 242–50. [DOI] [PubMed] [Google Scholar]
- 85.Doble BW, Patel S, Wood GA, Kockeritz LK and Woodgett JR (2007) Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell, 12, 957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindsley RC, Gill JG, Kyba M, Murphy TL and Murphy KM (2006) Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development, 133, 3787–96. [DOI] [PubMed] [Google Scholar]
- 87.Otero JJ, Fu W, Kan L, Cuadra AE and Kessler JA (2004) Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development, 131, 3545–57. [DOI] [PubMed] [Google Scholar]
- 88.Ying QL, Nichols J, Chambers I and Smith A (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell, 115, 281–92. [DOI] [PubMed] [Google Scholar]
- 89.Sato N, Meijer L, Skaltsounis L, Greengard P and Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med, 10, 55–63. [DOI] [PubMed] [Google Scholar]
- 90.Zhang SC, Wernig M, Duncan ID, Brustle O and Thomson JA (2001) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol, 19, 1129–33. [DOI] [PubMed] [Google Scholar]
- 91.Pera EM, Wessely O, Li SY and De Robertis EM (2001) Neural and head induction by insulin-like growth factor signals. Dev Cell, 1, 655–65. [DOI] [PubMed] [Google Scholar]
- 92.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T and Eggan K (2007) Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci, 10, 608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T and Abeliovich A (2004) Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol, 2, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lorincz MT, Detloff PJ, Albin RL and O’Shea KS (2004) Embryonic stem cells expressing expanded CAG repeats undergo aberrant neuronal differentiation and have persistent Oct-4 and REST/NRSF expression. Mol Cell Neurosci, 26, 135–43. [DOI] [PubMed] [Google Scholar]


