Abstract
Objective: To assess the effectiveness, safety, and treatment burden in eyes with persistent diabetic macular edema (DME) for up to 1 year after administration of 0.19 mg fluocinolone acetonide (FAc) implant (Iluvien®).
Methods: This retrospective study at one private practice in the US included 40 eyes from 33 patients treated with an FAc implant. Eyes had previously been treated with VEGF antagonists, dexamethasone, or focal laser. The primary outcome was change in central foveal thickness from baseline. Data were also collected on demographics, visual acuity, intraocular pressure (IOP), use of IOP-lowering drugs for elevated IOP, lens clarity, and treatment burden before and after the implant.
Results: Average duration of diabetes and DME at baseline was 19 and 5 years, respectively, and average glycated hemoglobin was 7.21%. Severity of diabetic retinopathy before the implant had a slight bimodal distribution: moderate nonproliferative diabetic retinopathy (35%) and proliferative diabetic retinopathy (40%). Improvements in central foveal thickness from baseline were evident at 3 months (mean change –74.2 µm, P<0.001) and sustained through 12 months (–55.3 µm; P=0.005) for most eyes. Mean visual acuity remained stable (66.2 letters at baseline versus 67.2 letters at 12 months, roughly equivalent to 20/50 vision; P=0.855). On average, eyes required one anti-VEGF injection every 1.9 months before and one every 6.6 months after the implant, while 60% of eyes did not require additional anti-VEGF injections. Small but significant increases in IOP at months 3, 6, and 9 were not sustained to month 12, and mean IOP was normal throughout follow-up.
Conclusion: In patients with DME previously treated with a steroid, and treated according to licensed indications in the US, an FAc implant not only reduces the burden of disease in the real-world setting, but also the burden of injections and office visits for patients.
Keywords: corticosteroid, retinal thickness, persistent DME, FAc, Iluvien®, visual acuity
Introduction
Diabetic retinopathy is a microvascular complication of diabetes mellitus. It is the leading cause of blindness in working-age adults and is increasing worldwide.1,2 The majority of eyes with diabetic retinopathy lose vision as a result of diabetic macular edema (DME), which results in retinal thickening involving the central macula.3 DME is characterized by capillary leakage and fluid accumulation following loss of pericytes and endothelial barrier decompensation.4–6 With a growing population that is becoming older with longer life expectancy, we can expect an increasing number of patients with vision impairment due to diabetes.7 Studies have also shown increased mortality secondary to visual impairment,8–12 so it is imperative to address vision impairment secondary to DME.
Although treating the underlying diabetes remains the best course for prevention of diabetic vision impairment,1,2,4 as demonstrated by multiple clinical studies such as the Diabetes Control and Complications Trial and the UK Prospective Diabetes Study,13,14 we continue to develop therapies to treat the symptoms and ocular manifestations of this disease, as a significant proportion of eyes go on to develop DME or proliferative changes that require DME-specific treatments.1,4 The Early Treatment of Diabetic Retinopathy Study (EDTRS) demonstrated the effectiveness of laser therapy in treating DME;15 however, although laser treatment reduces the risk of further vision loss, vision is improved in only about 12% of eyes after 3 years,1,4,6 and the treatment is not without complications either, including scotoma, altered color perception, night blindness, hemorrhage, transient vision loss, ME, and visual field defects.6,16
Medical therapies, such as anti-VEGF agents and corticosteroids, have shown significant benefit without the complications associated with laser treatment.4–6,17,18 As VEGF is believed to be a key mediator early in the development of edema, anti-VEGF therapy has superseded laser treatment as the first-line standard of care for clinically significant DME, unless clearly defined microaneurysms outside the fovea would benefit from targeted laser treatment.4,6 Although many eyes gain significant benefit from a single anti-VEGF injection, most need repeat treatments, as is the case in persistent or recurrent edema.4,6 Corticosteroids act on inflammatory cytokines and pathogenic mechanisms in addition to those associated with VEGF,5,17 leading to reductions in ME and improvements in vision.5,18 However, corticosteroids have tended to be used cautiously due to concerns about increased intraocular pressure (IOP) and worsening of cataracts.4
The corticosteroid fluocinolone acetonide (FAc) is available as a sterile, non-biodegradable, intravitreal implant (Iluvien®; Alimera Sciences, Inc., Alpharetta, GA, USA) containing 0.19 mg FAc, which is delivered over 36 months at a rate of 0.2 μg/day,18 reducing the need for repeat injections.4 The FAc implant is indicated in the US for the treatment of DME in eyes previously treated with a course of corticosteroids without a clinically significant rise in IOP.18 In trials in eyes with DME that had prior laser photocoagulation, the 0.19 mg implant significantly improved best-corrected visual acuity from as early as 3 weeks after injection, which was sustained for up to 2 years, and significantly reduced foveal thickness (FT).19,20 To date, however, there are very limited reports in the literature documenting the use of the FAc implant in general clinical practices in the US. In this retrospective observational study at a single center in the US, we show that the FAc implant, administered according to indicated use in the US, was effective in reducing central FT (CFT) and treatment burden for up to 1 year in eyes with DME.
Methods
Study design
This was a retrospective study of patients at one private practice in the US (Retina Associates of Cleveland, Cleveland, OH). The review was performed using information from electronic medical records collected between January 2011 and January 2018 following the practice’s own chart-review protocol using a chart review form developed by Barry Kapik (Senior Director Biostatistics, Alimera Sciences). The study included eyes with any stage of diabetic retinopathy, with persistent DME determined by optical coherence tomography, that had received at least one FAc intravitreal implant and had at least 1 year of follow-up. Eyes were included if they had been treated at least 1 year prior. Follow-up visits took place at 3, 6, 9, and 12 months. Multiple surgeons delivered the FAc implants. The practice received an institutional review board waiver for this study.
Data were collected on demographics, CFT, visual acuity (VA), IOP, use of IOP-lowering drugs, and DME treatments before and after the implant. The primary outcome measure was change in CFT from baseline, determined by optical coherence tomography. VA was assessed using a Snellen chart and results were converted to EDTRS letters for data analysis.
Statistical analysis
Data are presented as mean ± SD unless otherwise stated, with ranges. Data were not available at all time points for all eyes. A statistical difference was taken as P<0.05 and was assessed using Student’s one-sample t-test. and values were compared against baseline values. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient population
The average age was 66.9 years (SD 10.5, range 35–82 years), and slightly more men than women were included (n=20 [62.5%] versus n=13 [40.6%]; Table 1). For patients whose medical history information was complete, the average duration of diabetes since diagnosis (n=16) was 19.1 years (SD 8.72, range 3–38 years) and average glycated hemoglobin (HbA1c; n=19) was 7.21% (SD 1.07%, range 5.8%–11.0%).
Table 1.
Patient demographics at baseline (n=33 patients, unless otherwise specified)
| Demographics | |
|---|---|
| Patients, n | 33 |
| Eyes, n | 40 |
| Mean age, years (range) | 66.85±10.57 (35–82) |
| Sex, n (%) | |
| Male | 20 (62.5) |
| Female | 13 (40.6) |
| Ethnicity, n (%) | |
| Caucasian | 28 (84.8) |
| African–American | 5 (15.2) |
| Other | 0 |
| Type of diabetes, n (%) | |
| Type 1 | 1 (3.0) |
| Type 2 | 32 (97.0) |
| Mean duration of diabetes, years (n=16) | 19.1±8.72 |
| Mean HbA1c, % (n=19) | 7.21±1.07 |
Note: Values reported as means ± SD unless otherwise stated.
Abbreviation: HbA1c, glycated hemoglobin.
Severity of diabetic retinopathy before treatment with the FAc implant had a slightly bimodal distribution, with 35% of eyes having moderate nonproliferative diabetic retinopathy and 40% proliferative diabetic retinopathy (Table 2). The average duration of DME (n=21) was 5.30 years (SD 2.86, range 1.1–10.5 years.) Seven eyes had been vitrectomized. After DME diagnosis, the first injection of anti-VEGF or corticosteroid had been required after a mean of 2.98 (SD 1.85) years, and eyes had received a mean of 12.25 (SD 8.94) anti-VEGF injections and 6.7 (SD 6.87) corticosteroid injections before the study. The mean number of focal laser treatments had been 2.6 (SD 3.75, range 0–20), with 18 eyes (45%) requiring one to three focal laser treatments and eleven (27.5%) requiring no such treatment. The mean number of panretinal photocoagulation treatments had been 0.35 (SD 0.8, range 0–3), with eight (20%) eyes requiring and 32 (80%) not requiring such treatment.
Table 2.
Ocular history at baseline (n=40 eyes, unless otherwise specified)
| n (%)/mean (range) | |
|---|---|
| Lens status, n (%) | |
| Pseudophakic | 31 (77.5) |
| Phakic | 9 (22.5) |
| Mean central foveal thickness, µm | 430.9±129.47 (160–842) |
| Mean intraocular pressure, mmHg | 15.9±4.03 (5–24) |
| Mean visual acuity, EDTRS letters | 66.2±10.46 (49–86) |
| Severity of NPDR, n (%) | |
| Mild | 6 (15) |
| Moderate | 14 (35) |
| Severe | 4 (10) |
| PDR | 16 (40) |
| Mean duration of DME, years (n=21 eyes) | 5.3±2.86 (1.1–10.5) |
| Mean duration since first treatment for DME, years | 2.98±1.85 (0–5.7) |
| Mean number of anti-VEGF injections, n (range) | 12.3±8.94 (0–32) |
| 0 injections, n (%) | 4 (10.0) |
| 1–3 injections, n (%) | 2 (5.0) |
| 4–6 injections, n (%) | 4 (10.0) |
| 7–9 injections, n (%) | 8 (20.0) |
| >9 injections, n (%) | 22 (55.0) |
| Mean number of steroid injections, n (range) | 6.7±6.87 (1–33) |
| 0 injections, n (%) | 0 |
| 1–3 injections, n (%) | 18 (45.0) |
| 4–6 injections, n (%) | 5 (12.5) |
| 7–9 injections, n (%) | 6 (15.0) |
| >9 injections, n (%) | 11 (27.5) |
| Mean number of focal laser treatments, n (range) | 2.6±3.75 (0–20) |
| 0 treatments, n (%) | 11 (27.5) |
| 1–3 treatments, n (%) | 18 (45.0) |
| 4–6 treatments, n (%) | 8 (20.0) |
| 7–9 treatments, n (%) | 1 (2.5) |
| >9 treatments, n (%) | 2 (5.0) |
| Mean number of panretinal photocoagulation treatments, n (range) | 0.35±0.80 (0–3) |
| 0 treatments, n (%) | 32 (80) |
| 1–3 treatments, n (%) | 8 (20) |
| 4–6 treatments, n (%) | 0 |
| 7–9 treatments, n (%) | 0 |
| >9 treatments, n (%) | 0 |
Notes: Values reported as means ± SD, unless otherwise stated, with ranges shown in parentheses.
Abbreviations: DME, diabetic macular edema; EDTRS, Early Treatment Diabetic Retinopathy Study; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Outcome measures
Effectiveness
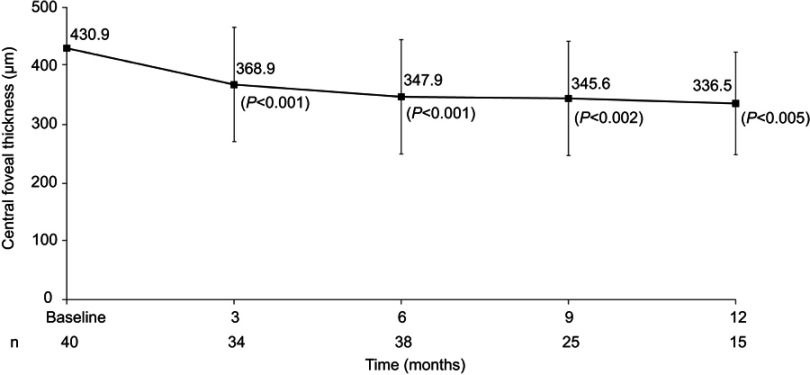
Mean CFT reduced from 430.9±129.47 µm just before the FAc implant was administered to 368.9±97.80 µm at 3 months and 336.5±87.68 µm at 12 months (Figure 1). Mean changes from baseline were statistically significant at all visits: –74.2 µm (SD 118.34, range –354 to 168 µm; P<0.001), –88.2 µm (SD 119.58, range –447 to 146 µm; P<0.001), –81.8 µm (SD 115.62, range –339 to 119 µm; P=0.002), and –55.3 µm (SD 64.66, range –161 to 67 µm; P=0.005) at 3, 6, 9, and 12 months, respectively.
Figure 1.
Mean change in central foveal thickness after FAc implant. Values reported as means ± SD.
Abbreviation: FAc, fluocinolone acetonide.
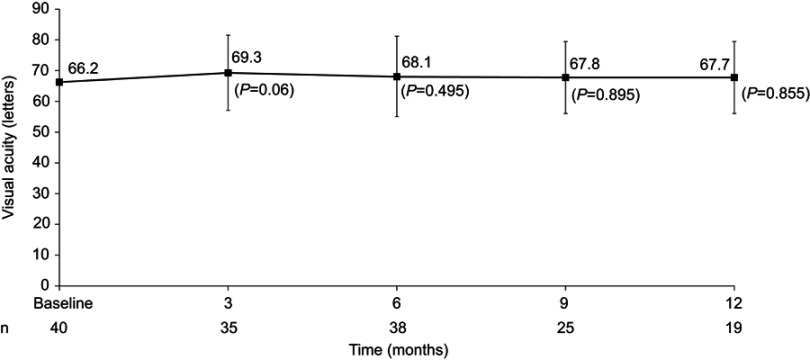
Mean VA before implant was 66.2±10.46 (49–86) letters and remained stable throughout the follow-up period, with a final value of 67.7±11.60 (50–85) letters at 12 months (P=0.855), roughly equivalent to 20/50 vision (Figure 2). Mean changes were stable at all visits: 3.8 letters (SD 11.46, range –36 to 25 letters; P<0.06), 1.7 letters (SD 15.08, range –35 to 35 letters; P=0.495), 0.3 letters (SD 12.03, range –15 to 28 letters; P=0.895), and –0.5 letters (SD 11.17, range –23 to 28 letters; P=0.855) at 3, 6, 9, and 12 months, respectively.
Figure 2.
Change in visual acuity after FAc implant. Values reported as means ± SD.
Abbreviation: FAc, fluocinolone acetonide.
Safety
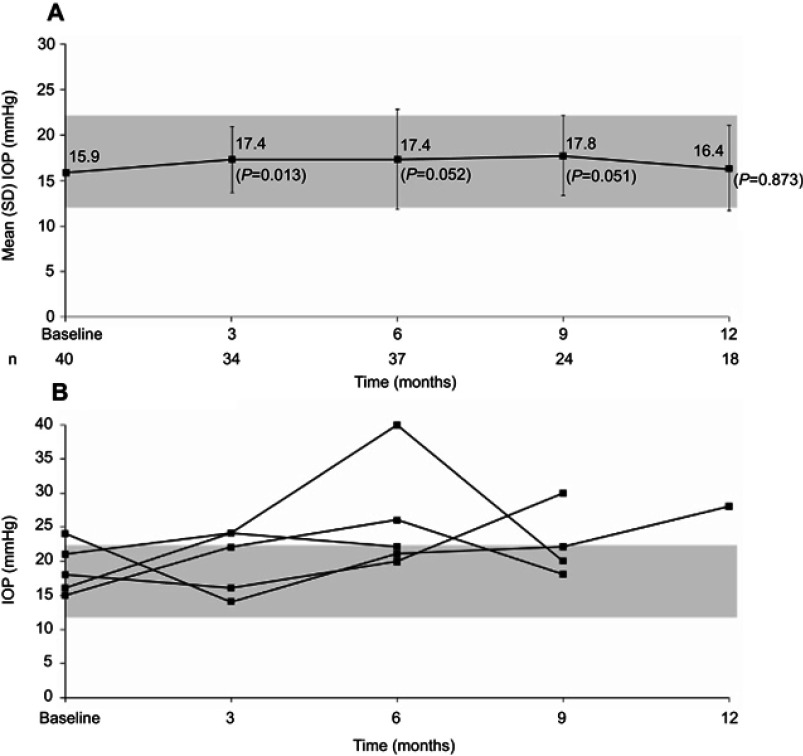
Small increases in IOP were seen at months 3 (1.8±3.93 mmHg, P=0.013), 6 (1.8±5.48 mmHg, P=0.052), and 9 (1.6±3.78 mmHg, P=0.051). The change at month 3 was not statistically significant at subsequent time points through to 12 months (0.2±4.37 mmHg, P=0.873). Mean IOP remained within the normal range (12–22 mmHg) throughout the follow-up period (Figure 3A). Five eyes developed pressure above the normal range (Figure 3B), and seven eyes with increases in IOP were managed easily with one treatment of IOP-lowering drops, including brimonidine tartrate/timolol maleate ophthalmic solution, brimonidine tartrate, and prostaglandin.
Figure 3.
Mean IOP after FAc implant (A) and IOP over time in five eyes with at least one IOP value above the normal range (B). Note: Gray box indicates normal range of 12–22 mmHg. Values reported as means ± SD in A.
Abbreviations: FAc, fluocinolone acetonide; IOP, intraocular pressure.
In the nine eyes that were phakic before treatment with the FAc implant, no effect on lens clarity was reported at 12 months. As the FAc implant is not biodegradable, there is concern about implant migration to the anterior chamber; however, implant migration was not observed in any of the eyes, including the one vitrectomized eye.
Treatment burden
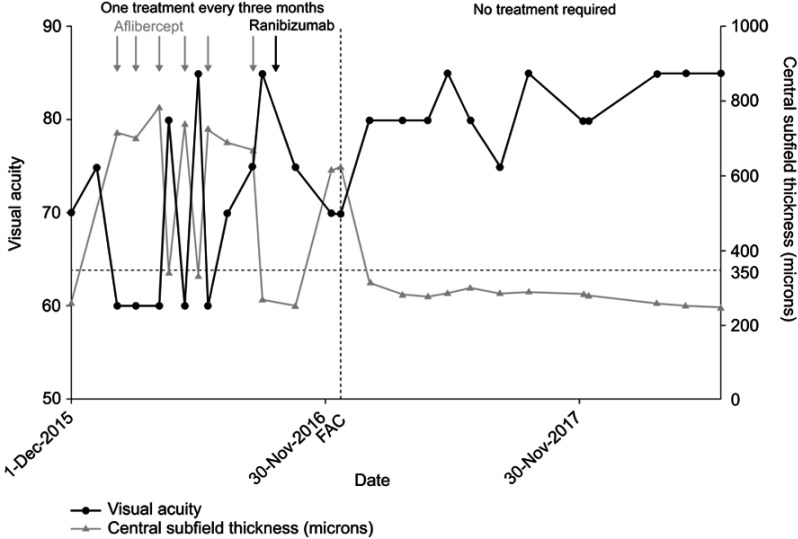
On average, fewer injections of anti-VEGF agents and steroids were required after the FAc implant was administered: 12.25 versus 1.83 injections for anti-VEGF agents (before versus after) and 6.7 versus 0.96 injections for steroids (Figure 4A). The frequency of anti-VEGF and steroid injections was also lower during the follow-up period, with the average number of anti-VEGF injections decreasing from one treatment every 1.9 months before the implant to one every 6.6 months after the FAc implant. Most eyes (60% and 67.5%, respectively) did not require additional anti-VEGF and steroid injections (Figure 4, B and 4C), but some requiring further FAc implants due to persistent edema (n =3, 7.5% of eyes) were treated with a second FAc implant, and no patients required a third implant (a mean of 1.08 implants). No patients required laser treatment after the FAc implant. Figure 5 shows the treatment outcomes for a single patient before and after the FAc implant, illustrating that no further anti-VEGF treatments were required after the implant compared with multiple injections during a comparable time frame before the implant.
Figure 4.

Number of anti-DME treatments (A) and frequency of anti-VEGF (B) and steroid (C) injections before and after FAc implant.
Abbreviations: DME, diabetic macular edema; FAc, fluocinolone acetonide.
Figure 5.
Single-patient case study to show treatment outcomes (visual acuity, central subfield thickness, and treatment burden) before and after FAc implant.
Abbreviation: FAc, fluocinolone acetonide.
Discussion
This study evaluated the use of the FAc intravitreal implant in the treatment of persistent DME in a real-world setting. The implant produced statistically significant improvements in CFT that were evident at 3 months and sustained through 12 months for most eyes. VA remained stable during the follow-up period. Treatment burden was reduced, with the average number of anti-VEGF and steroid injections decreasing after the FAc implant and most eyes not requiring additional anti-VEGF or steroid injections. Although some eyes experienced increases in IOP, these were not sustained at 12 months and were managed with just one treatment of IOP-lowering drops. No phakic eyes developed cataracts, and implant migration was not observed in any eye.
The results of this study are in line with the findings of clinical trials and previous case series with the FAc implant, showing improvements in FT and improvements or maintenance of VA, as well as few increases in IOP, which were small and easily manageable; significant reductions in treatment burden; and no evidence of cataracts or implant migration of cataracts or implant migration of cataracts or implant migration17,19–27. One strength of this study is the setting of a single private practice that covers the greater Cleveland area, which has a broad range of demographics and underlying disease severity. Multiple specialists were involved in the care of the patients, but practice across the clinic would have varied little between ophthalmologists, who follow standard procedures in terms of administration of the implant, follow-up, outcome measures, and equipment used. A limitation of the study is the small number of eyes, as well as some data on medical history being incomplete, not allowing more in-depth subgroup analysis. As is usual in retrospective observational studies, not all outcomes were available for all eyes at all time points, further reducing the population analyzed. Errors may have been made when data were extracted from patients’ records, and positive or negative placebo effects cannot be excluded without a control group.
Conclusion
This study adds to the current body of evidence of the safety and effectiveness of using the FAc intravitreal implant in a real-life setting. The findings suggest that in patients previously treated with a steroid and according to the licensed indications in the US, the FAc implant is an appropriate therapy that not only reduces disease burden but also reduces injection and office-visit burden for patients. A longer-term study with more complete medical histories for patients may help elucidate any influence from systemic factors.
Acknowledgments
Jemma Lough, independent medical writer, drafted this paper. I would like to acknowledge the support of my colleagues Dr David G Miller, Dr Jerome P Schartman, Dr Hernando Zegarra, and Dr Llewelyn J Rao for providing data on their patients treated with the FAc implant. I would also like to acknowledge Barry Kapik, an employee of Alimera Sciences Inc at the time of the analyses, for providing statistical support. This paper was presented in part at the Association for the Research of Vision and Ophthalmology Meeting, Hawaii, USA, 2018.
Statement of ethics
This article does not contain any new studies with human or animal subjects performed by any of the authors. Patient consent to review medical records was not required by the institutional review board, as this was a not a clinical trial, but rather a retrospective audit of the usage of the implant in general clinical practice. All data are anonymized and confidential and in compliance with the Declaration of Helsinki.
Disclosure
This study was supported by Alimera Sciences, the manufacturer of the FAc implant, who funded the writing and project management and commented on the manuscript. JMC reports personal fees from Alimera and grants from Aerpio outside the submitted work, and no other conflicts of interest.
References
- 1.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Council of Ophthalmology. ICO Guidelines for Diabetic Eye Care. San Francisco, CA: ICO; 2017. [Google Scholar]
- 3.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XV: the long-term incidence of macular edema. Ophthalmology. 1994;102(1):7–16. doi: 10.1016/S0161-6420(95)31052-4 [DOI] [PubMed] [Google Scholar]
- 4.Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res. 2015;2015:8. doi: 10.1155/2015/815839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves RS, Teixeira C, Coelho P. Recurrent diabetic macular edema: what to do. Case Rep Ophthalmol. 2017;8(3):465–474. doi: 10.1159/000480119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dugel PU, Parrish R. Iluvien – a new approach to the treatment of diabetic macular edema. US Ophthalmic Rev. 2015;8:110–115. doi: 10.17925/USOR.2015.08.02.110 [DOI] [Google Scholar]
- 7.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002;120(11):1544–1550. [DOI] [PubMed] [Google Scholar]
- 9.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care. 2011;34(5):1238–1244. doi: 10.2337/dc11-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hecke MV, Dekker JM, Stehouwer CDA, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence. EURODIAB Prospective Complications Study. 2005;28(6):1383–1389. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X-R, Zhang Y-P, Bai L, Zhang X-L, Zhou J-B, Yang J-K. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: evidence from epidemiological observational studies. Medicine. 2017;96(3):e5894. doi: 10.1097/MD.0000000000005894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siantar RG, Cheng C-Y, Gemmy Cheung CM, et al. Impact of visual impairment and eye diseases on mortality: the Singapore Malay Eye Study (SiMES). Sci Rep. 2015;5:16304. doi: 10.1038/srep16304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 14.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594 [DOI] [PubMed] [Google Scholar]
- 15.Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033–1040. doi: 10.1001/jamaophthalmol.2013.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddrill M 2017. http://www.allaboutvision.com/conditions/diabetic-treatment.htm. Accessed April3, 2017.
- 17.Saedon H, Anand A, Yang YC. Clinical utility of intravitreal fluocinolone acetonide (Iluvien) implant in the management of patients with chronic diabetic macular edema: a review of the current literature. Clin Ophthalmol. 2017;11:583–590. doi: 10.2147/OPTH.S131165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alimera Sciences. Iluvien (fluocinolone acetonide intravitreal implant) 0.19 mg for intravitreal injection. Alpharetta, GA: Alimera Sciences, Inc; 2016. [Google Scholar]
- 19.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635.e622. doi: 10.1016/j.ophtha.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 20.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 21.Elaraoud I, Attawan A, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (Iluvien) in patients with diabetic macular edema. Ophthalmol Ther. 2016;5(1):95–104. doi: 10.1007/s40123-016-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meireles A, Goldsmith C, El-Ghrably I, et al. Efficacy of 0.2 mug/day fluocinolone acetonide implant (Iluvien) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond). 2017;31(5):684–690. doi: 10.1038/eye.2016.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currie CJ, Holden SE, Berni E, Owens DR. Evaluation of the clinical effectiveness of fluocinolone acetonide 190 microg intravitreal implant in diabetic macular edema: a comparison between study and fellow eyes. Curr Med Res Opin. 2017;33(sup2):19–31. doi: 10.1080/03007995.2017.1366659 [DOI] [PubMed] [Google Scholar]
- 24.Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 microg intravitreal implant for diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):33–43. doi: 10.1080/03007995.2017.1366662 [DOI] [PubMed] [Google Scholar]
- 25.Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 microg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):5–17. doi: 10.1080/03007995.2017.1366645 [DOI] [PubMed] [Google Scholar]
- 26.Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant (Iluvien) in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series. Clin Ophthalmol. 2015;9:801–811. doi: 10.2147/OPTH.S79785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton A, Koh SS, Jimenez J, Riemann CD. The USER study: a chart review of patients receiving a 0.2 µg/day fluocinolone acetonide implant for diabetic macular edema. Opthalmology Ther. 2019;8(1):51–62. doi: 10.1007/s40123-018-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]