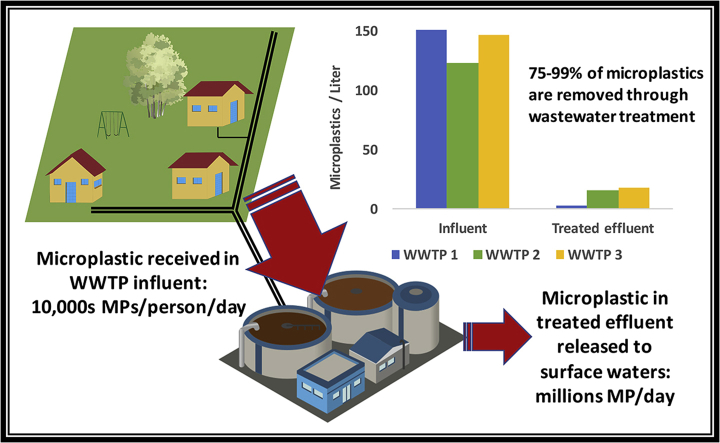
Abstract
Wastewater treatment plants serve to collect and treat wastes that are known to include microplastic (MP; synthetic polymer materials <5 mm in size) and other small anthropogenic litter as particles, fibers and microbeads. Here, we determined the microplastic loads and removal efficiencies of three wastewater treatment plants (WWTPs) with different treatment sizes, operations and service compositions discharging to Charleston Harbor, South Carolina, USA over the course of a year. Overall, we found that MP concentrations (counts per L) varied within a factor of 2.5 in influent and 4.8 in effluent at each WWTP, and that neither concentrations nor removal efficiencies demonstrated a seasonal trend. The largest wastewater treatment plant in the study, which also employed primary clarification, had the highest MP removal efficiency of 97.6 ± 1.2%. The other two smaller facilities had average removal efficiencies of 85.2 ± 6.0% and 85.5 ± 9.1%. We demonstrate through source modeling that microplastic fiber loads in influent were consistent with service area populations laundering textiles given previously published rates of microplastic generation in washing machines. Using measured WWTP flow rates and MP counts, we find a combined load of MPs leaving all three WWTPs with discharged effluent totaling 500–1000 million MPs per day. We estimate from this the emission of 0.34–0.68 g MP per capita per year in treated wastewater, which may only account for <0.1% of plastic debris input to this metropolitan area's surface waters on an annual mass basis when land-based (mis)managed plastic waste sources are also considered. However, the potential for sorption of chemicals present in wastewater to microplastics and their small size, which confers immediate bioaccessibility, may present unique toxicological risks for microplastics discharged from WWTPs.
Keywords: Microplastic, Microlitter, Wastewater treatment plants, Source modeling, Removal efficiency
Graphical abstract
Highlights
-
•
Microplastics were detected in influent and effluent at 3 WWTPs over 1 year.
-
•
Total microplastic counts were reduced by 75–99% through treatment.
-
•
Higher microplastic removal was found at the WWTP that uses primary clarification.
-
•
Fiber loading rate was consistent with the population's use of washing machines.
-
•
Treated wastewater likely emits less mass than non-point sources of plastic.
1. Introduction
Investigations into the abundance and risks associated with small plastic particles and fibers that are less than 5 mm in size, called microplastics, have accelerated worldwide in recent years. Microplastics originate from various primary and secondary sources. Primary microplastics are specifically engineered to be small, such as microbeads that are used for cosmetic, medicinal, and industrial purposes. Secondary microplastics derive from the fragmentation of larger plastics, such as by sunlight, wind, and water, or other chemical, biological, or mechanical forces (Andrady, 2011; Weinstein et al., 2016). Primary and secondary microplastics may enter the environment via direct release from shipping, fisheries, and shoreline activities and untreated sewage, treated effluents from industrial and municipal wastewater treatment plants (WWTPs), and stormwater runoff (Jambeck et al., 2015). While plastic materials that are inexpensive, strong, and lightweight offer many advantages for consumer goods, most plastics have a limited useful lifespan, and they may adversely affect the health of terrestrial and aquatic systems when introduced into the environment (Thompson et al., 2009). The risks posed by plastics will depend on their size, composition and life history (Wright et al., 2013; Koelmans et al., 2017; Jahnke et al., 2017).
Wastewater treatment plants serve to collect and treat wastes that are known to include microplastic sources, such as microplastic particles from personal care products and synthetic fibers from synthetic clothes washing (Prata, 2018). Field observations have found higher counts of microplastics in water and sediment near or downstream of wastewater treatment plants (e.g. Browne et al., 2011; Estahbanati and Fahrenfeld, 2016). Recently, several studies have reported microplastic in treated effluent from WWTPs around the world, with some studies also calculating removal efficiency of microplastics through treatment processes. Recent reviews of the existing literature on microplastics in wastewater treatment plants are available (Ziajahromi et al., 2016; Prata, 2018). A compilation of microplastic counts and removal efficiencies from the primary literature, provided in Table S1, finds that there is a 4-order of magnitude range in microplastic counts per liter of treated effluent. Within a single study of 12 WWTPs by Mason et al. (2016), microplastic concentrations in treated effluent were observed to vary among facilities by a factor of 50. While variation would be expected due to differences in the service areas and operations of wastewater treatment plants, comparing across studies is additionally complicated by variation in sample collection and processing methodologies. These observations demonstrate the difficulty in estimating the overall contribution of microplastics by WWTPs, and underscore a need to continue research towards understanding the methodological, socio-economic and technological factors influencing microplastic loading and removal through wastewater treatment.
The objective of the present study was to characterize the loading of microplastic to and from three wastewater treatment plants directly and locally discharging to the Charleston Harbor estuary in South Carolina, USA and to determine whether there is a difference in the treatment effectiveness between WWTPs or variability over time. Previous studies in Charleston Harbor have measured 3 to 11 MP/L in the sea surface microlayer, with the majority found classified as fibers (Gray et al., 2018). Using data collected from local clean-ups, it has been estimated that there are 7 tons of macroplastic littering the shorelines of Charleston Harbor (Wertz, 2015), and conditions in the tidal marshes are conducive for degradation to microplastic (Weinstein et al., 2016). The present study is a component of on-going efforts to characterize the sources and scale of microplastics in this vital estuarine watershed. Therefore, by extension, this work allows the potential loading of microplastic from WWTPs to be compared to other sources to the estuary, and enables evaluation of best practices for the management of microplastic to prevent entry and dispersal in the environment.
2. Materials and methods
2.1. Study location
Three WWTPs in the Charleston Harbor watershed were included in the present study: 2 serving the city of Mount Pleasant, SC and 1 serving the City of Charleston and nearby unincorporated communities (map in Fig. S1). Mount Pleasant Waterworks operates two conventional activated sludge wastewater treatment plants, Center Street and Rifle Range Road, which both treat predominantly residential waste with treated effluent discharged into the Rebellion Reach Channel leading into the Charleston Harbor. Charleston Water System operates one conventional activated sludge wastewater treatment plant, Plum Island, which receives residential, commercial, and industrial waste. Plum Island discharges treated effluent into the Charleston Harbor. The characteristics of the service areas for the three WWTPs as well as treatment steps are compared in Table 1.
Table 1.
Comparison of service areas, treatment volumes and steps at wastewater treatment plants.
| Features | Charleston Water System (CWS) |
Mount Pleasant |
|
|---|---|---|---|
| Plum Island | Rifle Range Road | Center Street | |
| Population served (people) in 2017 | 180,000 | 53,000 | 32,000 |
| Plant Capacity (x106 L d−1) | 136 | 22.7 | 14.0 |
| Avg. Volume Treated in 2017 (x106 L d−1) | 83.3 | 18.9 | 11.4 |
| Treatment Steps |
|
|
|
| Service Composition |
|
|
|
2.2. Microplastic sampling and analysis
Influent and effluent were collected from each WWTP over the course of a year. Influent was sampled directly downstream of the headworks (i.e. bar or perforated screens) but upstream of sludge waste return flows at each facility, and effluent was sampled post-disinfection immediately prior to treated effluent discharge points. Sampling was conducted in June and October of 2016 and January, April, and July of 2017. Additional samples were taken at Rifle Range Road in June and July of 2017 to assess intra-month variability. Sampling dates and time for each plant are listed in Table S2. The strategy to sample over the course of a year was chosen to observe potential seasonal trends. Samples at the influent and effluent ends of each Mount Pleasant Waterworks facility were collected as 3.6 L, 24-h flow-weighted composite samples in glass jars with Teflon-lined lids. Samples at the influent and effluent ends of Plum Island were also collected as 24-h flow-weighted composite samples but galvanized steel containers with lids were used to accommodate larger sampling volumes (7.5–11.5 L for influent and 30 L for effluent). Composite sampling of a larger sample volume, especially for treated effluent, has been emphasized in other studies to limit the intra-day influence of source loading and peak flows (Talvitie et al., 2017a). The different sample sizes between WWTPs were chosen according to preliminary data indicating expected differences in microplastic concentrations in effluent. Flow data for treated effluent discharge and sludge wasting rate were collected by plant operators to inform calculations of microplastic loading. Inflow flow rate was assumed to be the sum of outflow and sludge wasting rate (Table S2).
Quality assurance and control steps were implemented to prevent contamination of samples. Cotton laboratory coats and glass or metal equipment were used when possible during sample collection and laboratory procedures. Glassware and filtration equipment were thoroughly rinsed with deionized (DI) water prior to use and routinely inspected under the microscope to check for contamination. Samples were covered with glass fiber filters (TCLP Filter, Fisher Scientific), stainless steel mesh, or aluminum foil when not being handled. Procedural blanks consisting of DI water (3.6–3.8 L) were processed alongside each sample batch to quantify background contamination of microplastics.
Methodology for analyzing wastewater treatment plant samples for microplastics was adapted from previously published methods (Nor and Obbard, 2014; Masura et al., 2015). Steps to isolate and enumerate microplastics from the wastewater matrices involved 1) physical separation by filtration, 2) chemical digestion to remove interferences in the matrix, and 3) counting and characterization. Duplicate subsamples of each wastewater influent (∼0.5 L) and effluent (∼1.5–15.5 L) sample were filtered through a 43 μm stainless steel mesh filter (Argus Steel, Richmond VA) to separate solids. The mesh filters with retained solids were then transferred to Petri dishes, submerged in DI water, sonicated in an ultrasonic bath for 10 min and thoroughly rinsed with DI water to separate solids. The mesh filters were viewed under the microscope to ensure all solids were transferred. Sonication of this type has been recommended for separating microplastics from matrices (Collard et al., 2015). Samples in Petri dishes were dried for 24 + hours at 65 °C while lightly covered. Samples were then treated with five, 10-mL aliquots of 30% hydrogen peroxide (H2O2(aq)), reacting for 30 min at 65 °C on a hot plate for each addition to remove organic material from the sample matrix. Next, 6 mL of 1 M HCl was added to dried samples, and reacted over 30 min at 65 °C to dissolve inorganics and non-plastic materials (e.g. cellulosic and semi-synthetic textiles like cotton, rayon and viscose) found in preliminary trials to interfere with plastic enumeration. An evaluation of the hydrogen peroxide and HCl additions employed in this method to remove fibers (silk, rayon, nylon, polyester, viscose, cotton and a polyester-cotton blend) is presented in Appendix A: Supplementary data. After acid treatment, samples were reconstituted in DI water and filtered by vacuum filtration through three stainless steel mesh screens in succession to result in three size fractions: >418 μm, 178–418 μm, and 60–178 μm. The filter for each size fraction was transferred into separate Petri dishes for a final round of sonication and rinsing, then the solids separated from the mesh filters were either transferred to a clean glass Petri dish or a gridded, colored (green) nonsterile cellulose membrane filter (Sartorius Stedim, Germany; Millipore, France) and dried in an oven while covered with glass fiber filters to prevent contamination.
Microplastics were visually counted on glass Petri dishes underlain by a grid or on the gridded cellulose filter using a dissecting stereomicroscope (Leica, Stereozoom S9D) fitted with both UV (Stereo Microscope Fluorescence Adapter, Electron Microscopy Sciences, Hatfield, PA) and white light, and a digital camera (Jenoptik Progres Gryphax®). To count microplastics, characteristics derived from Nor and Obbard (2014) were adapted to fit the structure of this project. Fibers and particles were identified as microplastics if 1) there were no cellular or organic structures visible; 2) colors appeared homogenous throughout; 3) fibers were not segmented nor appeared as flat, twisted ribbon; and 4) fibers or particles did not fragment when pressed. There is potential for fiber damage and degradation during digestion, so shine and tapering, additional characteristics used by Nor and Obbard (2014), were not appropriate for this study. In addition to morphology (fiber or particle), color was noted. Examples of identified microplastics are shown in Fig. S2. During method development, a melting point analyzer was used to confirm thermoplastics with melting behavior, which was useful for evaluating the digestion method and training for optical identification. Fourier transform infrared microscopy (micro-FTIR; Bruker Hyperion with germanium attenuated total reflectance (ATR) crystal) was also attempted on select fibers and particles retained on filters following digestion.
In summary, over the course of this study there were 5 sampling dates for each of three WWTPs, and 3 additional sampling dates at the Rifle Range Road facility; 2 positions were sampled within each plant (influent and effluent) and duplicate subsamples processed through 3 sieve fractions. More than 200 microplastic sample counts and characterizations were performed over the 1-year study period.
2.3. Microplastic load and source estimates
The total number of microplastics counted in influent and effluent samples was blank-corrected and then expressed as microplastic concentrations (#MP/L). Removal efficiencies (RE%) for each WWTP were calculated using the concentrations of MPs counted for influent (CMP,infl) and effluent (CMP,effl) (Eq (1)):
| (1) |
Flow rates (106 L d−1) for influent (Qinfl) and effluent (Qeffl) (Table S2) were applied to calculate microplastic material loading, where influent loading is equal to and loading to Charleston Harbor in treated effluent is equal to . Microplastic loads entering WWTPs in sewage influent and discharged from WWTPs in treated effluents to surface waters (millions of MPs per day) were evaluated in the context of domestic sources and environmental sources, respectively, based on data and models available in the published literature as detailed in Appendix A Supplementary data.
2.4. Statistics
Standard statistics were performed to determine averages and variance of microplastic concentrations, and one-way ANOVA followed by Tukey's test used to analyze for differences between WWTPs. Statistics were performed using JMP Pro (Version 12.1.0, SAS Institute, Inc.).
3. Results and discussion
3.1. Quality control blanks
Average fiber and particle counts in DI water blanks (N = 2, each date) over the study period are presented in the supplementary data. In June 2016, there was an average of 26 MPs in duplicate blank samples, with 71% comprising fibers and most found in the smallest size fraction (Tables S3, S4). For the remainder of the study, only 3.5 to 8 MP total were detected in blanks and the improvement was seen across each size fraction. The decrease in MP counts in blanks over time can be attributed to the more stringent anti-contamination methods that were introduced after the June 2016 sampling, specifically, covering samples with glass fiber filters during digestion and drying, and using glass Pasteur pipettes instead of plastic wash bottles for rinsing glassware and mesh filters with DI water. As also evidenced in the literature, microplastic contamination of samples needs to be carefully monitored and mitigated. For example, in a study of microplastic in fish, Foekema et al. (2013) found small textile fibers in every sample that were largely attributed to contamination from atmospheric fallout; the authors subtracted fibers from all samples and took additional precautions to prevent fiber contamination.
3.2. Microplastics in WWTP influent
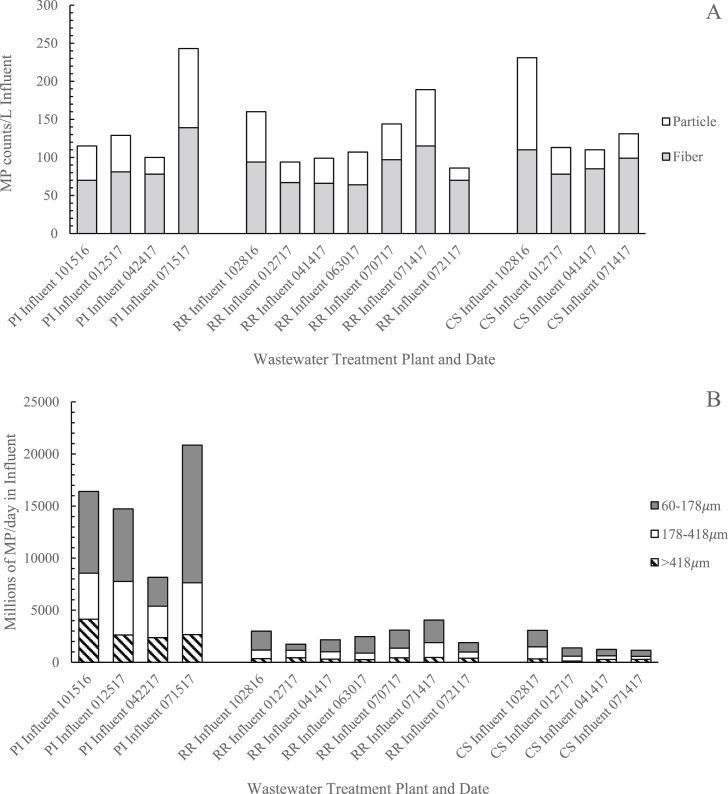
Average microplastic counts per liter influent by fiber and particle type and the total microplastic daily load in each size fraction for the three WWTPs are shown in Fig. 1a and b (all flow rates and microplastic count data in MP/L by date, sieve fraction, and type is provided in Appendix A. Supplementary material). Average influent microplastic concentrations were similar between treatment plants (Fig. 1a, statistics in Table S5). However, accounting for flow rates, Plum Island received the highest load of microplastics per day in influent: 8000 million MP/d to 20,000 million MP/d. Rifle Range and Center St. each received 1000 million to 4000 million MP/d (Fig. 1b and Table S6). Fibers were more prevalent than particles in influent across WWTPs and sampling dates (Fig. 1a and Table S6) and over 75% of microplastics were observed in the two smaller size fractions (Fig. 1b). MP color profiles were similar across the study and are represented in Fig. S3 as a combined average profile. The most common color was white/translucent (60%), followed by black (22%), blue/green (13%), and red (5%).
Fig. 1.
A) Average microplastic fiber and particle counts per liter influent and B) Millions of microplastics per day in influent for each size fraction for Plum Island (PI), Rifle Range Road (RR), and Center Street (CS) WWTPs in October 2016, and January, April, June and July 2017. (Date provided as 2-digit month, day, year.)
Microplastic concentrations in influent varied within a factor of 2.5 at each WWTP over the course of the year (Fig. 1a). Additional weekly sampling over 4 weeks at Rifle Range from late June to July 2017 found influent counts that varied by a similar factor. This series of samples indicates that within-season variability was similar to the degree of variability observed over the course of the year. Higher sampling frequency may be needed to detect seasonal dependence, if present. Seasonal trends in influent MP concentrations, therefore, could not be observed.
Plum Island showed the highest average MPs per day emitted per capita in sewage influent based on the service population, with 83,500 ± 29,200 MPs per capita per day, followed by Center St. and Rifle Range with 53,500 ± 28,400 and 49,600 ± 15,400 MPs per capita per day, respectively (Table S6). Microfibers constituted the majority of counts in samples (60–70%) (Table S6).
The validity of influent loading estimates can be cross-checked with published studies that have estimated the quantity of fibers released from synthetic clothing in washing machines. For instance, Napper and Thompson (2016) estimate that one, 6-kg load of laundry can release 138,000–728,000 fibers, depending on the type of synthetic textiles. We used these estimates to calculate how many loads of synthetic wash would produce the microplastic fiber loads per capita that were observed in the present study (Appendix A Supplementary Data; Table 2). Generation of microplastic is dependent on the composition of textiles being washed, the type of washing machine, and laundering preferences (e.g. water temperature), and therefore, is variable from wash to wash, and from household to household (Hartline et al., 2016; Salvador Cesa et al., 2017). Kruschwitz et al. (2014) estimate based on a survey in Germany that 4.5 kg–5.9 kg of clothes were washed per person per week, depending on the number of people in the household. In the United States, the average household of 2.6 people washes 289 loads per year, resulting in an average of 2.1 loads per person per week (Pakula and Stamminger, 2010). Therefore, we reasonably find that, depending on textile type, on average 0.3 to 2.5, 6 kg-loads of synthetic textiles per capita per week washed by the populations in each sewer service area would result in the influent fiber loads observed in the present study.
Table 2.
Estimates of number of laundry loads washed to produce microfiber counts observed in the present study, depending on laundered material type.
| Estimate by synthetic material type | Polyester:cotton blend | Polyester | Acrylic |
|---|---|---|---|
| # Microfibers per 6 kg load a | 138,000 | 496,000 | 728,000 |
| WWTP | # of 6 kg loads per capita per week, based on average MP/capita/week | ||
| Rifle Range Rd (RR) | 1.5 | 0.4 | 0.3 |
| Center Street (CS) | 1.6 | 0.5 | 0.3 |
| Plum Island (PI) | 2.5 | 0.7 | 0.5 |
| Range datab | # of 6 kg loads per capita per week, for lowest and highest count data | ||
| Low: RR 041417 | 1.4 | 0.4 | 0.3 |
| High: PI 071517 | 5.6 | 1.6 | 1.1 |
Lowest and highest count data from the data set: date is given as MMDDYY.
Influent is the raw wastewater that is collected from residences, commercial businesses, and industries for treatment. Since the waste-stream is characterized by the individuals and businesses that produce it and by alterations during the course of travel in the sewer pipe network, variability in MP influent counts should be explained by factors that are external to WWTPs. Service demographics, types of businesses or industry, and consumer behavior therefore must play an important role. Influent MP concentrations were statistically similar between plants, but at Plum Island WWTP the average per-person loading rate of microplastic was elevated compared to the other WWTPs and fiber loading was statistically significantly higher (fibers/person/day; Table S5). Plum Island treats a more diverse waste stream, which includes residential, commercial, and industrial waste, while Rifle Range and Center St. treat mostly residential and commercial waste. Since some industrial and commercial activities, such as media blasting or professional laundering, are known to include MPs, this may contribute to higher amounts of MP per day. A further look at the profiles of commercial or industrial customers in the service areas, and in the behaviors of residences, may improve understanding of the difference in microplastic loading to WWTPs.
3.3. Microplastics in WWTP effluent
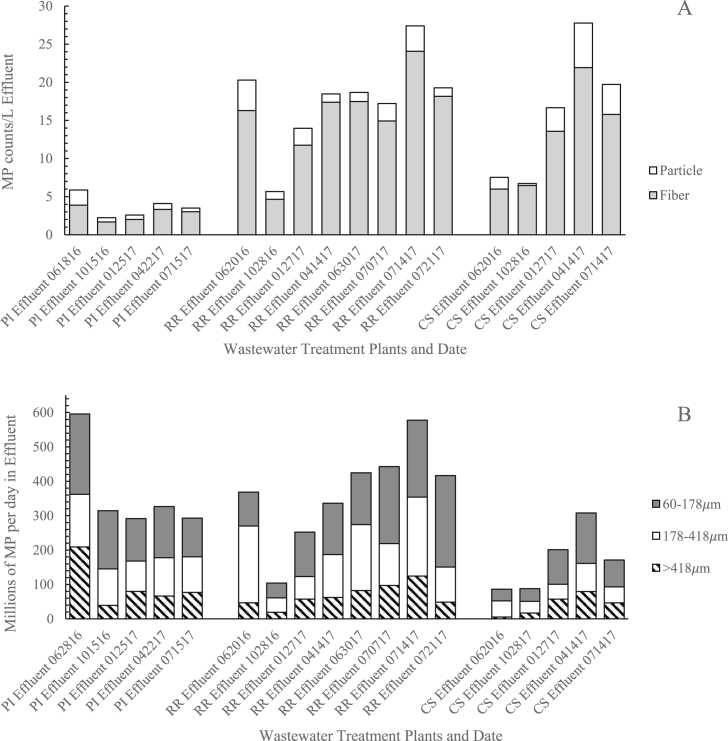
Average microplastics per liter effluent by fiber and particle and the total microplastic daily loads in each size fraction emitted in effluent for the three WWTPs are shown in Fig. 2a and b (all microplastic count data in MP/L by date, sieve fraction, and type is provided in Appendix A. Supplementary material). Average microplastic concentrations at Plum Island were statistically significantly lower than for Rifle Range and Center St. (Table S5). Fibers accounted for an average of 75% or more in all samples. Fibers have been seen to dominate the effluent microplastic or microlitter profile in many studies (e.g. 61–89% of all microplastic in treated effluents were fibers in Michielssen et al., 2016, and >99% were fibers in Dris et al., 2016), but others have found particles or fragments to dominate (e.g. ∼20–30% were fibers in Talvitie et al., 2017b and Murphy et al., 2016). For all WWTPs and sampling dates, an average of 75% or more of microplastics in effluent were observed in the two smaller size fractions. Talvitie et al. (2017a) found even higher prevalence of small size fractions, with 70% in effluent in the size range 20–100 μm, and >95% in the range of 20–300 μm. These results emphasize the importance of monitoring smaller size classes. MP color profiles in effluent were similar across WWTPs and were also similar to influent MP, except with a lower proportion of white/transparent (50%) (Fig. S3). Mintenig et al. (2017) also found a high proportion of transparent fibers in their analysis of treated wastewater (average 61%).
Fig. 2.
A) Average microplastic fiber and particle counts per liter effluent and B) Millions of microplastics per day in effluent discharging from WWTPs to Charleston Harbor for each size fraction for Plum Island (PI), Rifle Range Road (RR), and Center Street (CS) WWTPs in June and October 2016, and January, April, June and July 2017. (Date provided as 2-digit month, day, year.)
Microplastic concentrations in effluent shown in Fig. 2a, each collected as 24-h flow-weighted composites, varied within a factor of 4.8 and 4.1 at Rifle Range and Center St., respectively, and within a factor of 2.7 at Plum Island over the course of the year. Over the course of four weeks in July at Rifle Range, effluent microplastic concentrations varied by only a factor of 1.6. Therefore, there was more long-term variation in effluent at Rifle Range and Center St. While the lowest effluent MP concentrations were observed in October 2016, the variation over the year did not follow any recognizable seasonal pattern across WWTPs. Michielssen et al., (2016) also found no seasonal variation in treated effluent concentrations of small anthropogenic litter, and reported that effluent counts from an activated sludge WWTP with primary clarification, similar to Plum Island, varied by a factor of 2 over time. Lares et al. (2018) reported microplastic counts that varied within a factor of 10 in treated effluent collected over 7 sampling dates in a 3-month period; however, the authors note that their grab sampling technique likely contributed to the variation observed.
In the present study, effluent concentrations across all three WWTPs ranged 1–30 MP counts/L, which is within the range of several other studies reviewed in Table S1. Differences in MP concentrations in final effluent could be explained by a multitude of factors: i) differences between WWTPs (i.e. treatment process and technology, flow rate, service population, service compositions), ii) differences in sampling and processing methods (i.e. grab vs. composite sampling, microplastic isolation and analysis methods), and iii) sampling frequency.
After accounting for flow rates, releases of microplastics per day in treated effluents were found to be similar across WWTPs: Plum Island discharged 291–596 million MP/d, Rifle Range 104–578 million MP/d and Center St. 86–308 million MP/d (Fig. 2b). The combined loading of microplastic from all three WWTPs to Charleston Harbor results in an estimated 500 million to 1 billion microplastics released in treated effluent per day.
To compare the MP load per day from the combined WWTPs to plastics potentially entering Charleston Harbor from non-point sources, we employed a model for mismanaged plastic waste entering coastal oceans (see Appendix A Supplementary data for detailed calculations). Recent estimates show that 8 million metric tons of plastic wastes are mismanaged each year and enter coastal waterways globally (Jambeck et al., 2015). Jambeck et al. (2015) estimate that there are 0.1 million metric tons of plastic added to surface waters in the U.S. annually, which is a result of the activities of the 110 million people living within 50 km of U.S. coasts. The combined population served by the Plum Island, Rifle Range, and Center St. WWTPs was calculated as a fraction of the entire coastal population of the United States and then this fractional population multiplied by 0.1 million metric tons of plastic waste to determine this population's potential contribution, assuming no regional bias in plastic (mis)management. We estimate that the population living within the service areas of these three WWTPs in the Charleston area could be responsible for the release of 235 metric tons of plastic per year to coastal waterways, which in this area would flow predominately into the Charleston Harbor watershed. As reported above, a range of approximately 500 million to 1 billion microplastic particles were released per day in combined treated effluents from the three WWTPs. Making approximations about microplastic geometry and density that err towards overestimation, this count value is converted to a mass of microplastic per year of 0.1–0.2 metric tons, or about 0.34–0.68 g MP per capita per year. The magnitude of this microplastic mass loading in treated effluents is supported by a second derivation approach as detailed in Appendix A, and comparison to a recent study estimating Danish WWTPs emit 0.56 g MP per capita per year (Simon et al., 2018). While this analysis is subject to the assumptions outlined herein and in Jambeck et al. (2015), an order-of-magnitude level of comparison can be justified. Therefore, according to these estimates, plastic from WWTPs may account for <0.1% of the mismanaged plastic waste source to Charleston Harbor surface waters. The relative contribution of WWTPs to the microplastic load in Charleston Harbor, however, will depend on the fragmentation behavior of this litter resulting in microplastic generation. The comparison does not include emissions from sanitary sewer overflow events, or other potential sources of microplastic. Microplastic released by WWTPs will have different immediate bioaccessibility and chemical bioavailability that also needs to be factored into risk assessment of plastic sources.
3.4. Microplastic removal efficiency
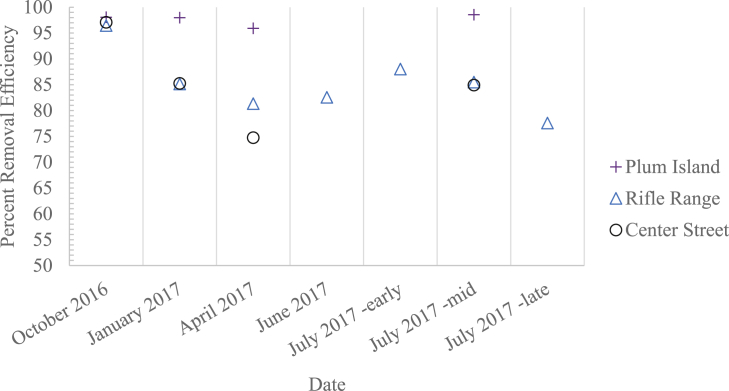
Removal efficiencies (RE) of microplastics were calculated for Plum Island, Rifle Range, and Center St. WWTPs in October of 2016, and January, April, and July of 2017. Plum Island had the highest RE, ranging from 95.9% to 98.1%, while Rifle Range and Center St. REs ranged from 74.8% to 97.1%. Weekly sampling at Rifle Range from June 30, 2016 to July 21, 2017 showed consistent removal (77.6–88.0% removal) (Fig. 3). Results at Rifle Range and Center St. were lower than most other published studies, which have found microplastic REs at WWTPs to be typically greater than 95% (minimum literature value of 71%; Table S1). Differences in study methodologies may complicate comparisons. However, an additional factor in our study is that the influent sampler pumps were located downstream of the headworks of the WWTPs (after influent screens/grit removal), which may have resulted in lower counts in influent samples and skewed towards lower calculated removal efficiencies. Though we did not find a clear trend in this study or a factor to explain why variation in treatment efficacy occurs, the longitudinal data inform our understanding of loading rates and removal effectiveness at individual facilities.
Fig. 3.
Microplastic percent removal efficiencies calculated at WWTPs over the study period.
Variations in REs across WWTPs are likely due to differences in treatment units. In addition to potential variation in the treatment efficiencies in the headworks across plants (that would affect influent MP concentrations), Plum Island has four large, rectangular primary clarifiers with hydraulic detention times of ∼2 h. The purpose of primary clarification is to promote solid settling before biological treatment. Each primary clarifier is also equipped with surface skimmers to skim floating solids off the surface of the supernatant water prior to secondary treatment. Depending on density, MPs have the potential to be removed by sedimentation or flotation during primary clarification. This suggests that additional MPs may be removed at Plum Island via primary clarification. Michielssen et al. (2016) found that primary screening and primary clarification removed 84–88% of microlitter. Similarly, Murphy et al. (2016) found that primary treatment removed up to 78% of microplastics at a WWTP in Glasgow, Scotland, UK.
Particles were more effectively removed than fibers in all WWTPs in this study (Table 3). While we hypothesized that fibers would be more effectively removed due to greater surface area for flocculation, that was not observed. Also finding higher removal of particles than fibers, Michielssen et al. (2016) reported removal efficiencies of 99% and 97% for particles and fibers during treatment, respectively. However, on the other hand, Lares et al. (2018) found higher removal efficiency for fibers (99.1%) than particles (89.9%). Unit operations at facilities, sampling and sample processing steps likely influence these comparisons. Fiber removal rates have been estimated between 66% and >99% in previous studies (Michielssen et al., 2016).
Table 3.
Average MP percent removal efficiency (±S.D.) through wastewater treatment for Plum Island, Rifle Range Road, and Center Street by fiber, particle and total microplastics.
| WWTPs | Total MP Removal, % | Fiber Removal, % | Particle Removal, % |
|---|---|---|---|
| Plum Island | 97.6 ± 1.2 | 97.2 ± 1.0 | 98.4 ± 1.3 |
| Rifle Range | 85.2 ± 6.0 | 80.2 ± 8.0 | 95.4 ± 2.4 |
| Center Street | 85.5 ± 9.1 | 83.7 ± 8.2 | 88.8 ± 9.6 |
3.5. Microplastic or small anthropogenic litter
Currently, methods for MP sampling, isolation, and enumeration are not standardized, and studies, such as those focused on wastewater treatment plants (Table S1), collect grab or composite samples, isolate microplastics via various digestion or separation techniques or omit altogether, and classify with varying size fractions. Grab sampling is a technique that is useful to capture waste during peak flows or to detect changes over short time periods, but it may not provide a representative sample of the waste stream. Digesting samples to remove organics and other interferences can improve visibility and selectivity during microplastic enumeration. Wet peroxide oxidation is often used to remove organics and cellular material before microplastic enumeration, but cellular material may still remain in the sample matrix (Dyachenko et al., 2017; Lares et al., 2018). Based on our method development (Appendix A), adding a small acid digestion step following hydrogen peroxide treatment reduces interferences from cotton and semi-synthetic textiles, although this may also reduce microplastic polymer types that are sensitive to acids. Studies using mesh screens >125 μm may underreport microplastics, since the present study, among others, finds a high contribution of smaller size fractions.
The majority of studies, including the present, have relied on visual-sensory identification to confirm and count microplastics using a set of qualitative characteristics. Even studies employing FT-IR microscopy to confirm polymer composition typically analyze only sub-samples. While chemical oxidation with hydrogen peroxide and acid was employed to remove interferences, the presence of any remaining natural materials appearing visually like microplastic may confound results. Both positive and negative biases are possible when visual-sensory traits alone are used to classify microplastic. Without confirmation of polymer identity, results may represent “microlitter” or “small anthropogenic litter” more broadly, terms used in previous studies at wastewater treatment plants by Talvitie et al. (2017a) and Michielssen et al. (2016), respectively, although in analyses that did not involve digestion or separation steps in methods. Especially white/translucent fibers and smaller size fractions may be evaluated as potential microplastics. In the present study, we attempted to use FT-IR microscopy with attenuated total reflectance (ATR) to identify plastic composition for fibers and particles, however we encountered difficulties in adjusting the ATR crystal contact with MP to obtain useful spectra. Similar difficulties were observed by Mintenig et al. (2017) during the identification of plastic material < 500 μm. The authors cite fiber thinness, spherical shapes, and protrusion of fibers as complications to identification. Similarly, Leslie et al. (2017) confirmed by FT-IR analysis that all spheres and colored fibers in a subsample of those isolated from sediment and biota samples had been correctly identified as microplastic (>300 μm mesh) using optical microscopy; however, FT-IR spectra could not be obtained for 75% of very thin colorless fibers. In the present study, in the absence of advanced analytical confirmation, the findings are supported by rigorous quality control, method development that included melting point analysis, and laundry source modeling.
Methods to identify plastic composition and to discern synthetic from non- or semi-synthetic materials (especially for fibers and smaller size classes) by FT-IR, Raman spectroscopy, or other analytical instrumentation is a crucial research need (Lares et al., 2018). However, even cellulosic or semi-synthetic anthropogenic microlitter may present similar risks to human and ecological health as microplastic (Remy et al., 2015). Polymer typing may help in identifying and mitigating sources, and in evaluating the potential for toxicological harm posed by materials. Broad initiatives to survey microplastics through wastewater treatment using consistent, validated methodologies could lead to better understanding of the needs for source management in wastewater.
4. Conclusions
Municipal sewage contains high levels of microplastic and other anthropogenic microlitter, and therefore although wastewater treatment plants are effective at removing these through treatment operations, the small fraction of microplastic released factored with large treatment volumes equates to a significant environmental source of microplastic. Higher removal efficiency observed in the present study at the WWTP employing primary clarification suggests that retrofitting secondary plants with primary clarifiers could improve microplastic removal, while also likely improving treatment of other contaminants of concern. Upgrading plants to include primary clarification is dependent on site-specific factors, such as existing plant design, service composition, service population, cost, and co-benefits which have to be considered before investments in capital improvements occur. The high loading of microplastics into wastewater treatment plants presents a point of intervention at the level of the individual consumer. Given information and opportunity, households’ choice of clothing, washing machine models or wash temperatures and frequency could collectively aim to reduce this microplastic source to wastewater treatment plants and ultimately, the environment. However, we estimate that wastewater treatment plants may contribute <0.1% of total plastic mass into waterways such as Charleston Harbor, and therefore the introduction of mismanaged plastic waste into the environment and fragmentation over time is another concern. Plastics of different size and composition may present acute or chronic risks to different receptor organisms. Microplastics emitted from wastewater treatment plants may present unique toxicological risks posed by their immediate bioaccessibility and their potential to have relatively high concentrations of sorbed pharmaceuticals and other chemicals found in wastewater compared to natural organic matter or dietary items found in the environment, depending on factors such as sorption capacity and desorption kinetics (Beckingham and Ghosh, 2017; Li et al., 2018; Seidensticker et al., 2017). In order to reduce the dispersal and potential harmful effects of microplastic pollution, a broad view on managing point and non-point sources of plastics is needed.
Acknowledgements
We thank Stephanie Cretté at the Clemson University Warren Lasch Conservation Center for assistance with micro-FT-IR, and Logan Johnson for laboratory support. Images in the graphical abstract are attributed to the Integration and Application Network, University of Maryland Center for Environmental Science, authored by Tracey Saxby, Catherine Collier, Kim Kraeer and Lucy Van Essen-Fishman (ian.umces.edu/imagelibrary/). Mount Pleasant Waterworks and Charleston Water System leadership and staff are greatly appreciated for collaboration and help with sampling and data collection, especially Jillian Phillips, Tyler Waterhouse, Jackie Alston, Patricia Iler, and Meghan Dailey.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wroa.2019.100030.
Funding
We would like to thank the Masters of Environmental Studies Program at the College of Charleston for a Research Assistantship for K. Conley. The College of Charleston School of Science and Mathematics provided a stipend which supported H. Lane. Additional funding was kindly provided by Mount Pleasant Waterworks, Charleston Water System and the College of Charleston through the Department of Geology and Environmental Geosciences and a Faculty Research and Development grant awarded to B. Beckingham.
Conflicts of interest
None.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Andrady A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Beckingham B., Ghosh U. Differential bioavailability of polychlorinated biphenyls associated with environmental particles: microplastic in comparison to wood, coal and biochar. Environ. Pollut. 2017;220:150–158. doi: 10.1016/j.envpol.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Crump P., Niven S.J., Teuten E., Tonkin A., Galloway T., Thompson R. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Collard F., Gilbert B., Eppe G., Parmentier E., Das K. Detection of anthropogenic particles in fish stomachs: an isolation method adapted to identification by Raman Spectroscopy. Arch. Environ. Contam. Toxicol. 2015;69:331–339. doi: 10.1007/s00244-015-0221-0. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Saad M., Mirande C., Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Dyachenko A., Mitchell J., Arsem N. Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal. Methods. 2017;9:1412–1418. doi: 10.1039/C6AY02397E. [DOI] [Google Scholar]
- Estahbanati S., Fahrenfeld N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere. 2016;162:277–284. doi: 10.1016/j.chemosphere.2016.07.083. [DOI] [PubMed] [Google Scholar]
- Foekema E.M., De Gruijter C., Mergia M.T., van Franeker J.A., Murk A.J., Koelmans A.A. Plastic in north sea fish. Environ. Sci. Technol. 2013;47:8818–8824. doi: 10.1021/es400931b. [DOI] [PubMed] [Google Scholar]
- Gray A.D., Wertz H., Leads R.R., Weinstein J.E. Microplastic in two South Carolina Estuaries: occurrence, distribution, and composition. Mar. Pollut. Bull. 2018;128:223–233. doi: 10.1016/j.marpolbul.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Hartline N.L., Bruce N.J., Karba S.N., Ruff E.O., Sonar S.U., Holden P.A. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016;50:11532–11538. doi: 10.1021/acs.est.6b03045. [DOI] [PubMed] [Google Scholar]
- Jahnke A., Arp H.P.H., Escher B.I., Gewert B., Gorokhova E., Kühnel D., Ogonowski M., Potthoff A., Rummel C., Schmitt-Jansen M., Toorman E., MacLeod M. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 2017;4:85–90. doi: 10.1021/acs.estlett.7b00008. [DOI] [Google Scholar]
- Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Koelmans A.A., Besseling E., Foekema E., Kooi M., Mintenig S., Ossendorp B.C., Redondo-Hasselerharm P.E., Verschoor A., Van Wezel A.P., Scheffer M. Risks of plastic debris: unravelling fact, opinion, perception, and belief. Environ. Sci. Technol. 2017;51:11513–11519. doi: 10.1021/acs.est.7b02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz A., Karle A., Schmitz A., Stamminger R. Consumer laundry practices in Germany. Int. J. Consum. Stud. 2014;38:265–277. doi: 10.1111/ijcs.12091. [DOI] [Google Scholar]
- Lares M., Ncibi M.C., Sillanpää M., Sillanpää M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–246. doi: 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Leslie H., Brandsma S.H., van Velzen M.J.M., Vethaak A.D. Microplastics en route: field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017;101:133–142. doi: 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang K., Zhang H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018;237:460–467. doi: 10.1016/j.envpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Mason S.A., Garneau D., Sutton R., Chu Y., Ehmann K., Barnes J., Fink P., Papazissimos D., Rogers D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016;218:1045–1054. doi: 10.1016/j.envpol.2016.08.056. [DOI] [PubMed] [Google Scholar]
- Masura J., Baker J.E., Foster G.D., Arthur C., Herring C. NOAA; 2015. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments. Technical Memorandum. NOS-OR-R-48. [Google Scholar]
- Michielssen M.R., Michielssen E.R., Ni J., Duhaime M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016;2:1064–1073. doi: 10.1039/C6EW00207B. [DOI] [Google Scholar]
- Mintenig S.M., Int-Veen I., Löder M.G.J., Primpke S., Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017;108:365–372. doi: 10.1016/j.watres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Murphy F., Ewins C., Carbonnier F., Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;50:5800–5808. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- Napper I.E., Thompson R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Nor N.H., Obbard J.P. Microplastics in Singapore's coastal mangrove ecosystems. Mar. Pollut. Bull. 2014;79:278–283. doi: 10.1016/j.marpolbul.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Pakula C., Stamminger R. Electricity and water consumption for laundry washing by washing machine worldwide. Energy Efficiency. 2010;3:365–382. doi: 10.1007/s12053-009-9072-8. [DOI] [Google Scholar]
- Prata J.C. Microplastics in wastewater: state of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018;129:262–265. doi: 10.1016/j.marpolbul.2018.02.046. [DOI] [PubMed] [Google Scholar]
- Remy F., Collard F., Gilbert B., Compère P., Eppe G., Lepoint G. When microplastic is not plastic: the ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 2015;49:11158–11166. doi: 10.1021/acs.est.5b02005. [DOI] [PubMed] [Google Scholar]
- Salvador Cesa F., Turra A., Baruque-ramos J. Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017;598:1116–1129. doi: 10.1016/j.scitotenv.2017.04.172. [DOI] [PubMed] [Google Scholar]
- Seidensticker S., Zarfl C., Cirpka O.A., Fellenberg G., Grathwohl P. Shift in mass transfer of wastewater contaminants from microplastics in presence of dissolved substances. Environ. Sci. Technol. 2017;51:12254–12263. doi: 10.1021/acs.est.7b02664. [DOI] [PubMed] [Google Scholar]
- Simon M., van Alst N., Vollertsen J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018;142:1–9. doi: 10.1016/j.watres.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Talvitie J., Mikola A., Koistinen A., Setälä O. Solutions to microplastic pollution – removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017;123:401–407. doi: 10.1016/j.watres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Talvitie J., Mikola A., Setälä O., Heinonen M., Koistinen A. How well is microlitter purified from wastewater? – a detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017;109:164–172. doi: 10.1016/j.watres.2016.11.046. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Moore C.J., vom Saal F.S., Swan S.H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J.E., Crocker B.K., Gray A.D. From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ. Toxicol. Chem. 2016;35:1632–1640. doi: 10.1002/etc.3432. [DOI] [PubMed] [Google Scholar]
- Wertz H. College of Charleston; Charleston, SC, USA: 2015. Marine Debris in Charleston Harbor: Characterizing Plastic Particles in the Field and Assessing Their Effects on Juvenile Clams (Mercenaria mercenaria) [Google Scholar]
- Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Ziajahromi S., Neale P., Leusch F.D.L. Wastewater treatment plant effluent as a source of microplastics: review of the fate, chemical interactions and potential risks to aquatic organisms. Water Sci. Technol. 2016;74:2253–2269. doi: 10.2166/wst.2016.414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.