Abstract
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that present immunosuppressive effects in experimental and clinical trials targeting various rare diseases including inflammatory bowel disease (IBD). In addition, recent studies have reported tryptophanyl-tRNA synthetase (WRS) possesses uncanonical roles such as angiostatic and anti-inflammatory effects. However, little is known about the function of WRS in MSC-based therapy. In this study, we investigated if a novel factor, WRS, secreted from MSCs has a role in amelioration of IBD symptoms and determined a specific mechanism underlying MSC therapy. Experimental colitis was induced by administration of 3% DSS solution to 8-week-old mice and human umbilical cord blood-derived MSCs (hUCB-MSCs) were injected intraperitoneally. Secretion of WRS from hUCB-MSCs and direct effect of WRS on isolated CD4+ T cells was determined via in vitro experiments and hUCB-MSCs showed significant therapeutic rescue against experimental colitis. Importantly, WRS level in serum of colitis induced mice decreased and recovered by administration of MSCs. Through in vitro examination, WRS expression of hUCB-MSCs increased when cells were treated with interferon-γ (IFN-γ). WRS was evaluated and revealed to have a role in inhibiting activated T cells by inducing apoptosis. In summary, IFN-γ-mediated secretion of WRS from MSCs has a role in suppressive effect on excessive inflammation and disease progression of IBD and brings new highlights in the immunomodulatory potency of hUCB-MSCs.
Keywords: Aminoacyl-tRNA synthetase, Inflammation, Inflammatory bowel disease, Interferon-γ, Stem cell therapy
INTRODUCTION
Inflammatory bowel disease (IBD), a rare autoimmune disease, including Crohn’s disease and ulcerative colitis, presents excessive colonic inflammation and T cell infiltration to the intestine. Crohn’s disease (CD), a major form of IBD, is characterized by transmural inflammation, lymphocyte aggregation (1). Majority of patients present abdominal pain, diarrhea and intestinal obstruction, leading to a surgical option to remove the diseased bowel (2). However, stem cell therapy has emerged as a promising treatment in recent years. Injection of MSCs attenuates immune malfunction in patients with CD (3). Anti-inflammatory effects of MSCs have been explained by inhibition of dendritic maturation and inducing them into a regulatory-like profile (4). T cell population is shift to a more tolerogenic pattern by MSCs (5), leading macrophage phenotype to an anti-inflammatory M2 stage rather than inflammatory M1 type (6). Despite the auspicious role of MSCs in CD, multiple factors that may influence the positive result is not well addressed. Discovery of therapeutic factors novel to CD recovery and revealing the mechanism of therapeutic properties will bring efficient strategy for future clinical trials.
Aminoacyl-tRNA synthetases (ARSs) are enzymes that catalyze reaction of the attachment of amino acids to cognate tRNAs, the first reaction of protein synthesis. Because of their involvement in protein synthesis, ARSs have long been considered as ‘housekeepers’. However, mammalian ARSs acquired additional domains during evolution not involving protein synthesis (7). Recently, these domains are involved in diverse physiological activities including angiogenesis, RNA splicing, translational regulation and immune system regulation (8). New functions have been introduced in cytoplasmic forms as well as nuclear forms and even secreted extracellular forms. The N-terminal truncated form of tryptophanyl-tRNA synthetase (mini-WRS) is secreted as an angiostatic ligand (9), and secreted full-length WRS (FL-WRS) plays a primary defense system against infection (10).
This study examined the function of secreted WRS from MSCs and its role in experimental CD recovery. This study is designed to reveal the regulatory mechanism of WRS production in MSCs by proinflammatory cues in CD and validate immune-modulatory capability of WRS, indicating a possible mechanism of action of delivered MSCs.
RESULTS
Administration of hUCB-MSCs alleviates DSS-induced colitis
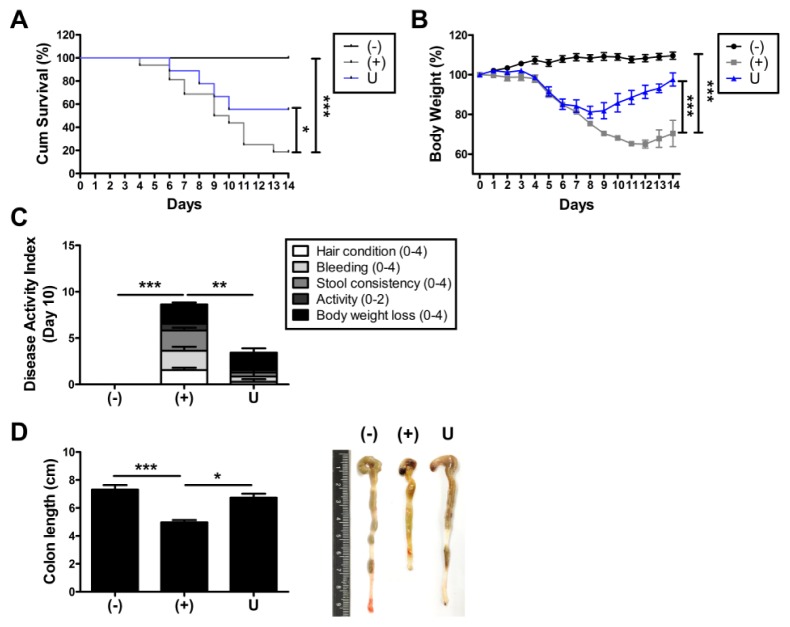
Adult stem cells including hUCB-MSCs have been considered as an alternative remedy for IBD (11, 12). To determine if administration of hUCB-MSCs can cure IBD, cells were intraperitoneally injected to DSS-induced colitis mice and therapeutic efficiency was assessed. Survival rate of DSS-treated mice significantly decreased and the rate was improved by injection of hUCB-MSCs (Fig. 1A). In addition, colitis-induced loss of body weight was markedly recovered by administration of hUCB-MSCs (Fig. 1B). To confirm the therapeutic efficiency of hUCB-MSCs against experimental colitis, disease activity index (DAI) was evaluated by scoring hair condition, bleeding, stool consistency, activity and the loss of body weight on day 10. Consistent with previous results, symptoms of colitis were attenuated by the hUCB-MSCs treatment (Fig. 1C). We next measured the length of colon, one of the disease indicators. DSS-mediated shortened colons were noticeably restored by administration of hUCB-MSCs (Fig. 1D). Together, administration of hUCB-MSCs as an alternative remedy for IBD ameliorates DSS-induced experimental colitis.
Fig. 1.
Administration of hUCB-MSCs attenuates symptoms of DSS-induced experimental colitis. Mice were administered with 3% DSS solution for seven days and infused intraperitoneally hUCB-MScs at day one. (A) Survival rate and (B) Body weight loss were monitored until day 14. (C) Disease activity index (DAI) was evaluated at day 10. (D) After mice were sacrificed, the length of colon was measured. n = 9–16 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001. Results are shown as the mean ± SEM.
Intraperitoneal injection of hUCB-MSCs attenuates intestinal inflammation and degeneration, and recovers the expression of WRS
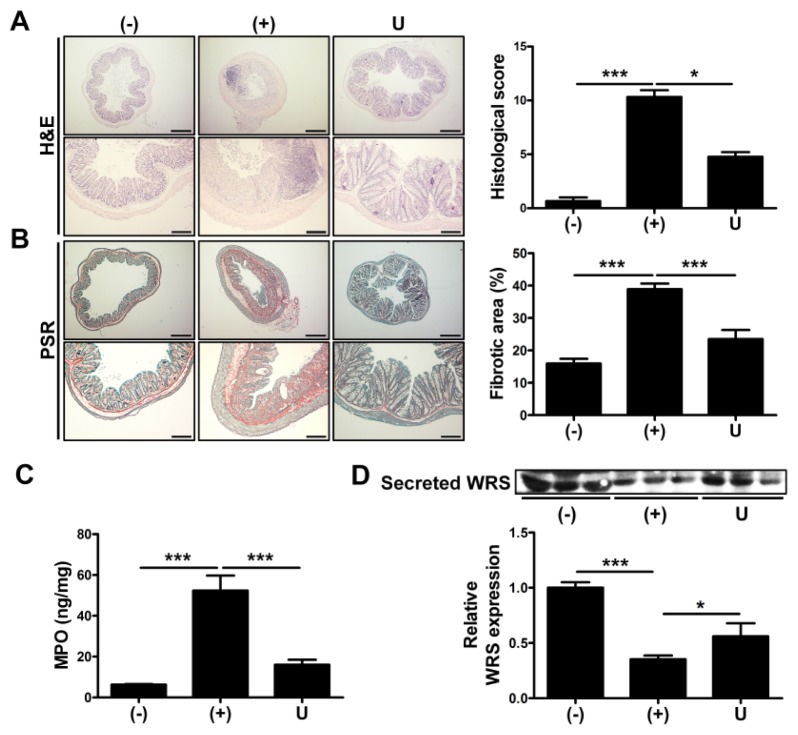
We further investigated therapeutic potential of hUCB-MSCs by analyzing a histopathology of inflamed colons of hUCB-MSCs-injected mice. We conducted a histopathologic evaluation by scoring the loss of goblet cells, hyperemia/edema, infiltration of immune cells, presence of crypt abscesses and loss of epithelium in H&E stained slides. As a result, the colon of the mice injected with hUCB-MSCs revealed attenuated disease phenotype compared to DSS-administered group (Fig. 2A). In addition to this, fibrotic degeneration, induced by excessive intestinal inflammation, was determined by analyzing picrosirius red (PSR) stained images (13). Colitis-induced increase of fibrogenesis was partially recovered by application of hUCB-MSCs (Fig. 2B). To confirm the infiltration of immune cells, particularly neutrophils, the activity of myeloperoxidase (MPO) was assessed in the serum of mice. Level of MPO activity was increased in the serum of DSS-treated mice and the activation was suppressed by treatment with hUCB-MSCs (Fig. 2C).
Fig. 2.
Intraperitoneal injection of hUCB-MSCs reduces the intestinal inflammation and increases the secretion of WRS. Mice were administered with 3% DSS solution for seven days and infused intraperitoneally hUCB-MScs at day one. The mice were sacrificed at day 14 for more ex vivo examination. (A) Left: H&E-stained images of colon, bar = 500 μm (upper), 200 μm (lower). Right: Histopathological severity was quantified based on the criteria. (B) Left: PSR-stained images of colon, bar = 500 μm (upper), 200 μm (lower). Right: Fibrotic areas were calculated. (C) Myeloperoxidase (MPO) activity was detected in the colon of mice. (D) The level of secreted WRS in the serum of mice was determined by Western blot analysis and quantified. n = 3–9 mice per group. *P < 0.05, ***P < 0.001. Results are shown as the mean ± SEM.
Although it was reported that secretion of ARS triggered proinflammatory response in several disease, subtype-dependent controversial and noncanonical roles of ARS, such as an anti-inflammation, have been consistently revealed (14). It was recently demonstrated that drug-mediated upregulation of WRS has a role in suppressing proliferation of activated immune cells (15). Interestingly, secretion of WRS decreased in the serum of DSS-administered mice. Application of hUCB-MSCs facilitated partially, but significantly recovered secretion of WRS (Fig. 2D). These data indicate that hUCB-MSCs have a role in resolution of intestinal inflammation and fibrogenesis and the healing process is associated with secretion of WRS.
Secreted WRS inhibits the proliferation hUCB-MNCs and isolated T cells by inducing apoptosis
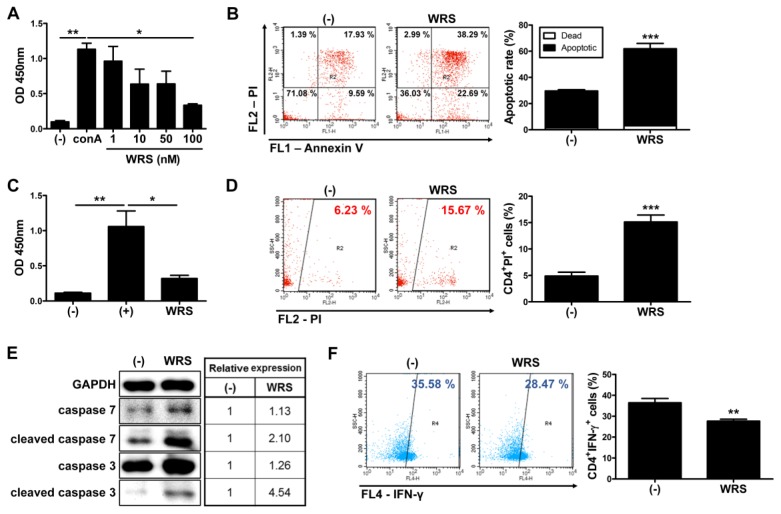
To investigate if secreted WRS facilitates immunomodulation with the previous study (15), we sought to determine proliferation level of isolated immune cells in presence or absence of WRS. Proliferation of human umbilical cord blood-derived mononuclear cells (hUCB-MNCs) was increased by lymphocyte mitogen, concanavalin A (conA) and treatment with WRS remarkably suppressed an increase of the proliferation (Fig. 3A). To address the mechanism underlying a suppression of immune cells, an apoptosis assay was further conducted. As a result, WRS inhibited excessive proliferation of hUCB-MNCs by inducing apoptosis (Fig. 3B). To define if WRS has suppressive effect on proliferation of T helper cells particularly, CD4+ T cells were isolated from hUCB-MNCs and cultured in the presence of WRS. Similar to hUCB-MNCs, isolated CD4+ T cells treated with WRS revealed decreased proliferation (Fig. 3C). Decrease in proliferation was caused by CD4+ T cell specific apoptosis (Fig. 3D). To investigate molecular mechanism involved in the WRS-induced apoptosis, we examined effects on expression of apoptotic markers. Among the caspase family, caspase-3 and caspase-7 are major cascade activation proteins. As rendered in Fig. 3E, WRS promoted expression of caspase-3 and caspase-7. Relative expression of cleaved form of enzymes was more remarkably up-regulated in WRS treated group (Fig. 3E). T helper cells, including CD4+IFN-γ+ presenting Th1 cells and CD4+IL-17+ presenting Th17 cells, have a major role in onset and progression of IBD (16). In the same line with apoptosis analysis, population of CD4+IFN-γ+ cells markedly decreased by treatment with WRS (Fig. 3F). Conversely, CD4+IL-17+ cells didn’t reveal significant change (Fig. S1). To investigate if immunosuppression is a unique property of WRS, hUCBMNCs were cultured with GRS, a member of ARS that is a novel regulatory cytokine in various diseases (17). As rendered in Fig. 3A, WRS effectively inhibited conA-mediated activation of cells. By contrast, GRS could not significantly reduce proliferation of cells compared to the WRS treated group (Fig. S2A). We next explored if cleaved form of WRS has similar effect on immune cell suppression. Similar to FL-WRS, same concentration of mini-WRS exerted significant changes in proliferation of hUCB-MNCs (Fig. S2B). Together, mini- or FL-WRS have a role in inhibiting activation of T cells by inducing apoptosis.
Fig. 3.
Secreted WRS inhibits the proliferation of CD4+ T cells by inducing apoptosis. Mononuclear cells and CD4+ T cells were isolated from human cord blood samples and cultured in the presence of WRS for three days. (A) Proliferation of hUCB-MNCs was measured by BrdU ELISA assay. (B) Apoptosis of hUCB-MNCs was determined by using flow cytometer. (C) Proliferation and (D) apoptosis of isolated CD4+ T cells were assessed. CD4+ T cells were isolated from hUCB-MNCs and cultured in the presence of WRS for three days. (E) Expression of signaling pathway molecules on apoptosis was determined by Western blot analysis. (F) Proportion of Th1 cells was measured by flow cytometric analysis after staining with cell surface CD4 and intracellular IFN-γ. *P < 0.05, **P < 0.01, ***P < 0.001. Results are shown as the mean ± SEM.
Secretion of WRS from hUCB-MSCs is upregulated in response to IFN-γ
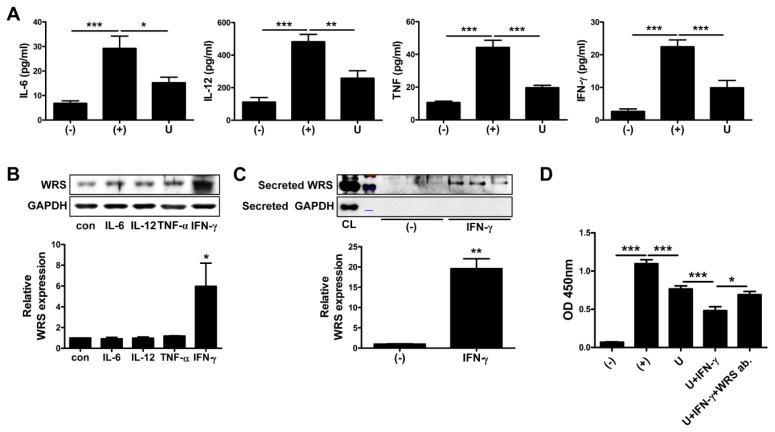
The level of proinflammatory cytokines such as IL-6, IL-12, TNF and IFN-γ increased in the serum of DSS-administered mice. In addition, increased level of proinflammatory cytokines was reduced by the application of hUCB-MSCs (Fig. 4A). IFN-γ upregulates production and secretion of WRS (14, 18). To determine if IFN-γ upregulates secretion of WRS from hUCB-MSCs and the other cytokines have influence on secretion, hUCB-MSCs were cultured with proinflammatory cytokines and expression level was detected. To determine production and secretion of WRS from hUCB-MSCs, WRS expression was assessed from cell lysates and cultured media, respectively. Production of WRS was not significantly changed by designated treatment with proinflammatory cytokines, but rather IFN-γ remarkably increased the level of WRS production (Fig. 4B). Consistently, significant increase in secretion of WRS followed only when hUCB-MSCs were treated with IFN-γ (Fig. S3 and Fig. 4C). We next determined that IFN-γ-mediated upregulation of WRS secretion influenced immunosuppressive capacity of hUCB-MSCs. As expected, IFN-γ primed hUCB-MSCs more efficiently suppressed proliferation of isolated T cells compared to naive cells. In addition, improved immunosuppressive effect was retrieved by neutralization using WRS antibody (Fig. 4D). It suggests that hUCB-MSCs secret WRS in response to proinflammatory cytokine, IFN-γ as a defense mechanism against robust inflammation.
Fig. 4.
IFN-γ plays a crucial role in WRS secretion of hUCB-MSCs for protection against excessive inflammation. (A) The mice were sacrificed at day 14 and serum samples were collected for more ex vivo examination. Level of IL-6, IL-12, TNF and IFN-γ in the serum of mice was measured by CBA analysis. (B–D) hUCB-MSCs were treated with indicated cytokines including IFN-γ for 24 hours. Expression level of WRS was determined in (B) the cell lysates and (C) cell-cultured media. (D) Inhibitory effect of IFN-γ-primed hUCB-MSCs on proliferation of hUCB-MNCs was detected by BrdU ELISA assay. n = 4–6 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001. Results are shown as the mean ± SEM.
DISCUSSION
Although clinical applications of human adult stem cells against several immune disorders including IBD have been consistently performed, results from clinical trials are variable and conflicting. In addition, mode of action of adult stem cells for treating those diseases was not thoroughly elucidated and it may correlate with variations in therapeutic outcomes. To broaden our knowledge and understanding, in this study, we investigated the role of WRS secreted by hUCB-MSCs in protecting against DSS-induced colitis.
The ARSs are the most primitive molecules that translate mRNA to protein. Consuming one ATP molecule, amino acid is activated to aminoacyladenylate and this activated amino acid is delivered to the acceptor end of tRNAs. However, ARSs evolved to maintain distinct catalytic domains other than mRNA to protein translation and these domains have recently gained functional versatility through research. In zebrafish, the Seryl-tRNA synthetase is an essential factor for development of the closed circularity system (19). This action was not associated with aminoacylation activity but a unique 45-residue domain, UNE-S (unique to SerRS), was essential for vascular development (20). Mini-WRS is well known for anti-angiogenesis function resulting from extracellular N-terminal fragments. N-terminal fragment interacts with VE-cadherin, an adhesion protein supporting blood vessel tube, and interferes with the assembly of network during neovascularization (21). However, FL-WRS is unable to interact with VE-cadherin because the binding domain of the synthetase is partially covered and this inhibits the interaction (8). We reported an immunomodulatory function of WRS and this function was visible in mini and FL form of WRS. Unlike the anti-angiogenesis function, the immunomodulation domain of the WRS is not masked by FL-WRS. Additionally, when compared with GRS, immunomodulation activity was only active in WRS, meaning the active domain may be a unique property of WRS.
Of note, therapeutic application of human adult stem cells including hUCB-MSCs revealed varied outcomes. To overcome this hurdle, several enhancement strategies such as an IFN-γ priming were used (22, 23). IFN-γ induces WRS expression via activation of transcription factor, STAT1 in several cells including innate immune cells (18) and upregulated WRS expression involves with suppression of activated immune cells (15). Although Wang et al. reported increased WRS expression from CD4+ T cells abrogated IDO-mediated immunosuppression from DCs (24), in this study, we demonstrated that production and secretion of WRS of hUCB-MSCs increased in response to IFN-γ resulting in suppression of excessive T cell proliferation. In addition to this, IFN-γ-induced rate-limiting enzyme IDO converts tryptophan into kynurenine and controls immune responses by inducing an anergy of effector T cells and a hyper-activation of regulatory T cells (25, 26). WRS has a role in tryptophan-kynurenine metabolism and it was elicited that increased production of WRS cooperates with IDO to regulate in vivo immune responses (27). Based on our results, we demonstrated upregulation of WRS may be a novel therapeutic mechanism underlying immunomodulation of hUCB-MSCs and one of the reasons why IFN-γ priming adult stem cells are more efficient for treating immune disorders.
In conclusion, this study revealed that secretion WRS of hUCB-MSCs in response to a proinflammatory cytokine, IFN-γ and consequent secreted WRS facilitated alleviation of experimental colitis by inducing apoptosis of immune cells including CD4+ T cells. With these findings, we demonstrated a new mechanism underlying stem cell therapy against autoimmune diseases including IBD and it may be a novel therapeutic target for improvement of therapeutic outcomes.
MATERIALS AND METHODS
Animals
All animal experiments were conducted in accordance with approved guidelines of Seoul National University Institutional Animal Care and Use Committee (IACUC No. SNU-130130-2). Six-week-old male C57BL/6 mice (Orientbio, Sungnam, Republic of Korea) were housed in animal facility of Seoul National University and grouped randomly. The mice were administered with 3% DSS in drinking water for seven days. On day one, the mice were injected intraperitoneally with hUCB-MSCs. Body weight of the mice were measured daily and the disease activity index (DAI), composed of body weight loss, activity, stool consistency, bleeding and hair condition, was evaluated on day 10. For more ex vivo examinations, colons and blood samples of mice were collected on day 14.
Isolation and culture of hUCB-MSCs
All experimental procedures using hUCB-MSCs were approved by the Boramae Hospital Institutional Review Board (IRB) and the Seoul National University IRB (IRB No. 1707/001-008). hUCB-MSCs were isolated and cultured as previously described (28). In brief, human cord blood samples were mixed with HetaSep solution (Stem Cell Technologies, Vancouver, Canada) at a ratio of 5:1 to remove red blood cells. Then, mononuclear cells were separated after centrifugation at 2,500 rpm for 20 minutes using Ficoll. Isolated cells were seeded in KSB-3 Complete media (Kangstem Biotech, Seoul, Republic of Korea) containing 10% fetal bovine serum (Gibco BRL, NY, USA) and antibiotics. After three days of stabilization, attached cells were washed out, and isolated stem cells were maintained. Isolated hUCB-MSCs were characterized by flow cytometric analysis (Fig. S4)
Statistical analysis
Mean values of all results were expressed as the mean ± SEM. Statistical analyses were performed using GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). Data were assessed for normality using the D’Agostino and Pearson normality test. For normally distributed data, significance was determined by using Student’s t tests for comparison of two groups or one-way ANOVA coupled to Bonferroni’s test for multiple groups. Wherein data were not normally distributed, Mann–Whitney U test or Kruskal-Wallis method coupled to Dunn’s multiple comparison test were used. For survival rate, Kaplan-Meier test and Mantel-Cox post-test were used. P-values less than 0.05 were statistically significant and statistical significance is indicated in the figure legend.
SUPPLEMENTARY INFORMATION
ACKNOWLEDGEMENTS
This paper was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1A2B3008483) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0421).
Footnotes
AUTHORS’ CONTRIBUTIONS
I.K. and B-C.L. designed the study, collected and analyzed the data and wrote the manuscript; J.Y.L. and J-J.K. collected and analyzed the data and contributed to the writing of the paper; S.E.L. and N.S. collected and analyzed the data; SW.C. contributed to the writing of the paper; K-S.K. designed and supervised the study, analyzed the data and wrote the manuscript.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB. Crohn’s disease: step up or top down therapy. Best Pract Res Clin Gastroenterol. 2003;17:131–137. doi: 10.1053/bega.2003.0361. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Liu Z, Chen J, et al. Resveratrol induces autophagic apoptosis via the lysosomal cathepsin D pathway in human drug-resistant K562/ADM leukemia cells. Exp Ther Med. 2018;15:3012–3019. doi: 10.3892/etm.2018.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccocioppo R, Cangemi GC, Kruzliak P, et al. Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn’s disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact. Stem Cell Res Ther. 2015;6:137. doi: 10.1186/s13287-015-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE 2-dependent mechanism. Sci Rep. 2016;6 doi: 10.1038/srep38308. 383 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, You S, Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: more than housekeeping. Nat Rev Cancer. 2011;11:708. doi: 10.1038/nrc3124. [DOI] [PubMed] [Google Scholar]
- 8.Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9:145. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakasugi K, Slike BM, Hood J, et al. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A. 2002;99:173–177. doi: 10.1073/pnas.012602099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn YH, Park S, Choi JJ, et al. Secreted tryptophanyl-tRNA synthetase as a primary defence system against infection. Nat Microbiol. 2017;2:16191. doi: 10.1038/nmicrobiol.2016.191. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Shin TH, Lee BC, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013;145:1392–1403. doi: 10.1053/j.gastro.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Fichtner-Feigl S, Strober W, Geissler EK, Schlitt HJ. Cytokines mediating the induction of chronic colitis and colitis-associated fibrosis. Mucosal Immunol. 2008;1(Suppl 1):S24–27. doi: 10.1038/mi.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005;30:569–574. doi: 10.1016/j.tibs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Noh KT, Cho J, Chun SH, et al. Resveratrol regulates naive CD 8+ T-cell proliferation by upregulating IFN-gamma-induced tryptophanyl-tRNA synthetase expression. BMB Rep. 2015;48:283–288. doi: 10.5483/BMBRep.2015.48.5.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-gamma+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321–2329. doi: 10.1097/MIB.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 17.Park SR, Kim HJ, Yang SR, Park CH, Lee HY, Hong IS. A novel endogenous damage signal, glycyl tRNA synthetase, activates multiple beneficial functions of mesenchymal stem cells. Cell Death Differ. 2018;25:2023–2036. doi: 10.1038/s41418-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav MC, Burudi EM, Alirezaei M, et al. IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55:1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui H, Hanaoka R, Kawahara A. Noncanonical activity of seryl-tRNA synthetase is involved in vascular development. Circ Res. 2009;104:1253–1259. doi: 10.1161/CIRCRESAHA.108.191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Shi Y, Zhang H-M, et al. Unique domain appended to vertebrate tRNA synthetase is essential for vascular development. Nat Commun. 2012;3:681. doi: 10.1038/ncomms1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q, Kapoor M, Guo M, et al. Orthogonal use of a human tRNA synthetase active site to achieve multifunctionality. Nat Struct Mol Biol. 2010;17:57. doi: 10.1038/nsmb.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang I, Lee BC, Choi SW, et al. Donor-dependent variation of human umbilical cord blood mesenchymal stem cells in response to hypoxic preconditioning and amelioration of limb ischemia. Exp Mol Med. 2018;50:35. doi: 10.1038/s12276-017-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DS, Jang IK, Lee MW, et al. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-gamma. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Mao C, Zhao Z, et al. Increased TTS abrogates IDO-mediated CD4(+) T cells suppression in patients with Graves’ disease. Endocrine. 2009;36:119–125. doi: 10.1007/s12020-009-9184-0. [DOI] [PubMed] [Google Scholar]
- 25.Chen W. IDO: more than an enzyme. Nat Immunol. 2011;12:809. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyanokoshi M, Yokosawa T, Wakasugi K. Tryptophanyl-tRNA synthetase mediates high-affinity tryptophan uptake into human cells. J Biol Chem. 2018;293:8428–8438. doi: 10.1074/jbc.RA117.001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BC, Kim HS, Shin TH, et al. PGE2 maintains self-renewal of human adult stem cells via EP2-mediated autocrine signaling and its production is regulated by cell-to-cell contact. Sci Rep. 2016;6:26298. doi: 10.1038/srep26298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.