Abstract
Gene fusions play an important role in the carcinogenesis of lung adenocarcinoma. The recent association of four oncogenic driver genes, ALK, ROS1, RET, and NTRK1, as lung tumor predictive biomarkers has increased the need for precision medicine. We used formalin‐fixed, paraffin‐embedded tissue samples of non‐small cell lung cancer from 150 EGFR mutation‐negative cases and 10 fusion status‐known cases and compared the performance of the Oncomine Dx Fusion Transcript Test (ODxFT) with FISH break‐apart for the detection of ALK, RET, and ROS1 fusion genes. RNA was extracted from the paraffin‐embedded tissue samples with or without macrodissection under hematoxylin and eosin staining, and the ALK fusion gene was independently determined using these assays. Fusion detection analyses were successfully carried out using ODxFT in 150 cases, with only one invalid case. ALK fusion genes were detected at a frequency of 7.3% (11/150) in the lung cancer specimens. Concordance rate between the ODxFT and ALK‐FISH analyses was 99.3% (148/149). Sensitivity and specificity were 91.7% and 99.3%, respectively. All the samples with a known fusion status were accurately matched between the two assays. Our results show a high concordance rate between the ODxFT and ALK‐FISH analyses. ODxFT was thus validated as an effective method for detecting clinically significant ALK fusion genes in paraffin‐embedded tissue samples.
Keywords: ALK fusion gene, companion diagnosis, FISH, next‐generation sequencer, non‐small cell lung cancer
1. INTRODUCTION
Non‐small cell lung cancer (NSCLC) is characterized by gene alterations. Activation of driver oncogenes such as EGFR, BRAF, and ERBB2 1, 2, 3 increases the sensitivity of tumors to tyrosine kinase inhibitors. Gene fusions involving tyrosine kinase receptor genes such as ALK,4 ROS1,5 RET,6, 7 and NTRK1 8 have also been described. These fusion events, which can involve a variety of partner genes, result in the formation of chimeric fusion kinases capable of oncogenic transformation and the induction of oncogene dependency within neoplastic cells. The individual prevalence of each of these chromosomal rearrangements ranges from 1% to 7% in NSCLC,4, 6, 9, 10 and, together, these rearrangements can be identified in approximately 5%‐9% of NSCLC.7, 11, 12 Lung cancers harboring these fusion genes show oncogene addiction to the respective fusion genes and are therefore sensitive to treatment with tyrosine kinase inhibitors (TKI), such as crizotinib, alectinib (CH5424802), and ceritinib (LDK378).
Fluorescence in situ hybridization, immunohistochemistry (IHC), and real‐time PCR can be used to detect fusion genes in clinical tissue samples. The FISH break‐apart assay is the gold standard for companion diagnosis. Development of the above‐mentioned agents8, 9, 13 has driven a rapid need for systematic and sensitive assays to detect fusion genes. The massively parallel nature of next‐generation sequencing (NGS) allows rapid characterization of point mutations, small insertions, and deletions as well as the detection of chromosome rearrangements in a large set of genes through the targeted sequencing of fusion junctions. In the present study, we validated the use of a new library kit, Oncomine Dx Fusion Transcript Test (ODxFT), for the characterization of the most frequent fusion genes in lung adenocarcinoma using NGS. This library kit is based on the high‐multiplexing capabilities of PCR and focuses on the identification of 72 different fusion genes. Herein, we report the clinical performance of this assay for the detection of fusion genes implicated in the occurrence of NSCLC.
2. MATERIALS AND METHODS
2.1. Samples
A total of 160 lung cancer samples were collected from Tokyo Medical University Hospital and Kindai University Hospital. All the samples were from resections that had been formalin‐fixed and paraffin‐embedded (FFPE) and had the wild‐type EGFR genotype. Ten of these samples had been previously tested for ALK, RET, or ROS1 fusion genes using an Ion AmpliSeq RNA Lung Cancer Research Fusion Panel for research purposes. All the patients enrolled in the study provided written informed consent for the use of their resected tissue. This study was approved by the ethics committee of Kindai University Faculty of Medicine (Authorization Number: 27‐228) and Tokyo Medical University (Authorization Number: 3380).
2.2. RNA extraction
RNA was extracted from each of the clinical research samples using the AllPrep DNA/RNA FFPE Kit (Qiagen, Valencia, CA, USA) based on the respective standard extraction procedures. RNA was quantified using the Nanodrop 2000 instrument (Thermo Fisher Scientific, Wilmington, DE, USA) and the RiboGreen RNA Assay Kit (Thermo Fisher Scientific).
2.3. Oncomine Solid Tumor Fusion Transcript kit design
Primers spanning 72 fusions (37 ALK, 9 RET, 15 ROS1, and 11 NTRK1) were designed in order to to span all previously described fusions (as at time of development) of ALK, ROS1, RET, and NTRK1 by a research team at Thermo Fisher Scientific. Sources used for the curation of all known fusions included the COSMIC and NCBI databases and a review of current medical literature. Targeted fusion genes and numbers of subtypes are shown in Tables 1 and 2. The multiplex primer mixes also included primers for the amplification of five housekeeping genes: HMBS, ITGB7, LMNA, MYC, and TBP. Additionally, primers designed to amplify the 5′ and 3′ regions of ALK, RET, ROS1, and NTRK1 were included in the primer mix. Amplification of these regions for each gene of interest allowed for the comparison of expression levels between the 3′ end of the gene, which was part of the resulting fusion, and the non‐involved 5′ end of the gene, but was not used for the determination of fusion gene detection.
Table 1.
Partners for ALK, RET, ROS1, and NTRK1
| Target gene | ALK | RET | ROS1 | NTRK1 |
| Partner gene | EML4 | KIF5B | CD74 | CEL |
| KIF5B | CCDC6 | SDC4 | NFASC | |
| KLC1 | CUX1 | SLC34A2 | IRF2BP2 | |
| HIP1 | EZR | TFG | ||
| TPR | TPM3 | SQSTM1 | ||
| LRIG3 | SSBP2 | |||
| GOPC | CD74 | |||
| DYNC2H1 | ||||
| MPRIP |
Table 2.
Constitution of subtypes
| Fusion genes | No. of subtypes |
|---|---|
| EML4‐ALK | 18 |
| KIF5B‐RET | 7 |
| KIF5B‐ALK | 4 |
| SDC4‐ROS1 | 4 |
| SLC34A2‐ROS1 | 4 |
| CD74‐ROS1 | 2 |
| GOPC‐ROS1 | 2 |
| HIP1‐ALK | 2 |
| MPRIP‐NTRK1 | 3 |
| Other | 1 |
2.4. Detection of fusions
Total RNA was reverse transcribed using the SuperScript VILO cDNA Synthesis Kit (Life Technologies Japan, Tokyo, Japan) followed by library preparation using ODxFT (Life Technologies Japan). Reverse‐transcribed cDNA was amplified using the supplied reagent. The PCR product was digested and ligated with a barcode adaptor followed by purification (all from supplied reagent). The purified libraries were quantified using the Ion Library TaqMan Quantitation Kit (Life Technologies Japan), followed by a pooled and prepared sequencing template using the Ion OneTouch Dx Template Kit and an Ion OneTouch Dx Instrument (Life Technologies Japan). The sequencing template was purified using an Ion OneTouch ES Dx Instrument followed by sequencing on an Ion PGM Dx Sequencer using the Ion PGM Dx Sequencing Kit and the Ion 318 Dx Chip Kit (all from Life Technologies Japan).
Data analysis was carried out using an Ion Torrent T430 Server. This assay determined the fusion gene to be positive if the detection of the fused sequence was greater than the cutoff value.
2.5. Fluorescence in situ hybridization
Formalin‐fixed and paraffin‐embedded tissues sectioned at a thickness of 4 μm and placed on glass slides were subjected to FISH using a break‐apart probe for the ALK gene (Vysis ALK break‐apart FISH probe; Abbott Molecular, Des Plaines, IL, USA), RET gene (RET Split Dual Color FISH probe; GSP Research Inc.), or ROS1 gene (ROS1 Split Dual Color FISH probe; GSP Research, Inc., Kobe, Japan). FISH positivity was defined as the presence of >15% split signals in the tumor cells.
2.6. Statistical analysis
The kappa statistic and associated 95% confidence intervals were used to measure agreement among the assays.
3. RESULTS
3.1. Method correlation agreement analysis for the detection of ALK fusion transcripts
Of the 150 clinical research samples tested, 149 (99.3%) passed the quality control requirement. One failure case could not be analyzed using ODxFT because of the low yield of library.
ALK fusion genes were detected in 11/149 (7.4%) and 12/150 (8.0%) samples using ODxFT and ALK‐FISH, respectively (Table 3). The ODxFT results were 99.3% concordant (148/149 samples) with the FISH analysis. The invalid test rate for ODxFT was 0.7% (1/150). Positive predictive value and negative predictive value for ODxFT were 91.7% and 99.3% when the FISH results were assumed to be true. No statistically significant difference was observed between the correlations of ODxFT and ALK‐FISH (κ = 0.95, 95% CI 0.79‐1.11). Detailed findings for all the clinical samples are shown in Table S1. Representative FISH data and Integrative Genomics Viewer (IGV) diagram from ALK‐positive samples are shown in Figure 1A.
Table 3.
Concordance between ODxFT and FISH analysis for ALK fusion gene
| ALK FISH | Total | |||
|---|---|---|---|---|
| Positive | Negative | Invalid | ||
| ODxFT | ||||
| Positive | 11 | 0 | 0 | 11 |
| Negative | 1 | 137 | 0 | 138 |
| Invalid | 0 | 1 | 0 | 1 |
| Total | 12 | 138 | 0 | 150 |
ODxFT, Oncomine Dx Fusion Transcript Test.
Figure 1.
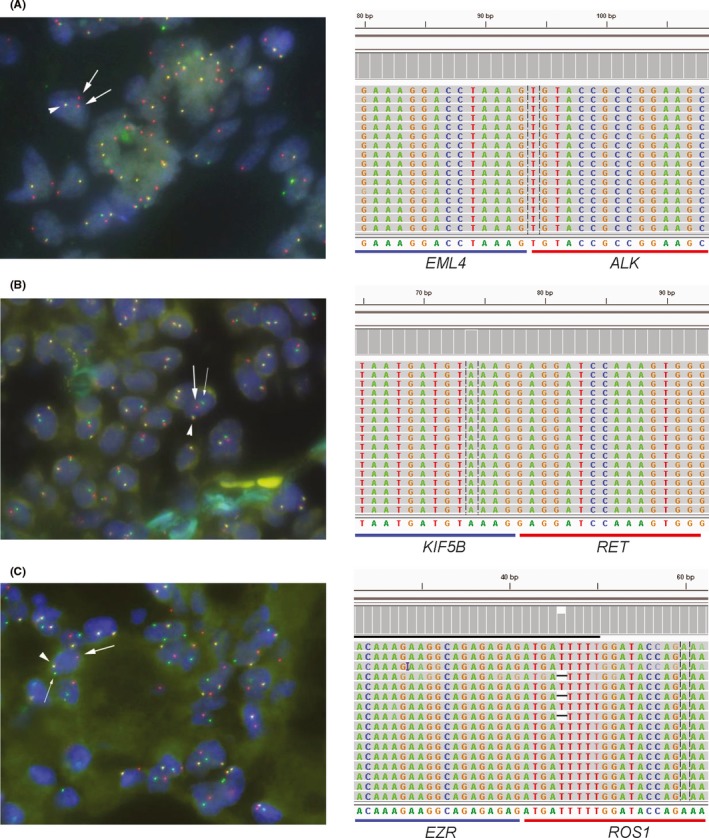
FISH analysis (left) and Integrative Genomics Viewer (IGV) diagram (right) of ALK, RET, and ROS1 fusion cases. A‐C, Representative FISH images for an ALK fusion‐positive specimen (A), a RET fusion‐positive specimen (B), and a ROS1 fusion‐positive specimen (C). Arrowhead, bold rightwards arrow, and the fine arrow show the pseudocolor signals of the 5′/3′ probe, the 5′ probe, and the 3′ probe, respectively
3.2. Detection of ROS1 and RET fusion transcripts
Oncomine Dx Fusion Transcript Test is a hotspot panel designed to detect ALK, ROS1, RET, and NTRK1 fusion transcripts. ODxFT detected five RET and three ROS1 fusions. Panel results for ROS1 and RET were concordant in 5/5 samples (100%) for ROS1 and in 3/3 samples (100%) for RET when compared with the respective FISH findings (Figure 1B,C). No NTRK1 fusion transcripts were detected using ODxFT.
3.3. Detection of fusion genes in 10 fusion status‐known cases
Because ALK, RET, or ROS1 fusion genes are not common oncogenic events in patients with NSCLC, we examined an additional 10 positive samples that had been previously tested using an Ion AmpliSeq RNA Lung Cancer Research Fusion Panel for research purposes (8 EML4‐ALK, 1 KIF5B‐RET, and 1 SLC34A2‐ROS1 cases). All the gene fusions were detected by ODxFT and FISH. The results of ODxFT and FISH matched exactly.
4. DISCUSSION
In the present study, we showed the feasibility of using the ODxFT assay to detect fusion genes in lung cancer tissues with a high rate of success. Discordance between ALK FISH and other methods has been previously reported.14, 15 A recent study using the Ion AmpliSeq RNA Lung Cancer Research Fusion Panel for fusion detection reported 100% concordance between this method and other methodologies.16 This study and ours confirm the clinical performance of inhouse NGS tests using RNA samples obtained from small tissue samples.
The simultaneous detection of fusions has important clinical implications for lung cancer patients in terms of turnaround time and cost. This method requires a very small amount of input RNA (10 ng). This advantage is particularly attractive for assays targeting lung cancers, as these samples are often obtained by biopsy and contained a limited amount of tissue. In clinical practice, lung cancer fusions have been detected using FISH, IHC, or RT‐PCR. Although FISH is considered to be the gold standard, especially for ALK testing because of the availability of an FDA‐approved ALK FISH assay, FISH analysis for multiple targets per sample can be costly and may potentially extend the time needed to rule out all relevant fusion genes. IHC staining offers a cheaper alternative; however, this methodology is subjective, and accurate interpretations are sometimes difficult.17 An RT‐PCR kit to detect ROS1 fusions is available as a companion diagnosis. The main limitation of traditional RT‐PCR is that this method typically focuses on only the most common fusion events and is thus limited when it comes to detecting rarer exon combinations and is unable to detect fusions involving novel 5′ partners.18 In contrast to FISH or IHC, detection of ALK, RET, ROS1, and NTRK1 fusions can be combined into a single assay using ODxFT. This allows the detection of fusions that might otherwise remain undetected.
In the present study, we had one case with sequencing failure (K013) and one case discordant with FISH analysis (T060). Sample K013 had a sufficient amount of RNA (10 ng RNA) for the library preparation, but the library concentration was low. Hence, we speculate that the NGS analysis of K013 failed as a result of poor‐quality RNA. One discordant case was shown to be ALK fusion negative in ODxFT but was positive in FISH. There are several fusion partners besides the partners contained in the ODxFT panel such as GCC2, DCTN1, and CLIP1.19 As the number of sequencing reads and alignment quality were sufficiently valid, it is possible that the partner gene was not included in the ODxFT panel.
Oncomine Dx Fusion Transcript Test includes a multiplexing of primers for 72 different fusion combinations and is thus not limited to only the most common fusions. For traditional RT‐PCR, one must have previous knowledge of all possible relevant fusions. The ODxFT assay addresses this problem in two ways. First, during the analysis of the sequenced reads, all reads that are initially unaligned to the reference sequence are split in half and allowed to realign. This step fosters the detection of novel fusions involving existing primers. Second, the assay includes a method for detecting fusions involving unknown partners using a 3′/5′ imbalance calculation. This step analyzes the expression levels of the 3′ and 5′ ends of each driver gene. For genes involved in a fusion event, the 3′ end of the gene is now under different regulatory control and typically shows overexpression relative to the 5′ end of the gene. Another recently described methodology using NanoString technology also exploits this phenomenon of 3′ overexpression.20 They found that looking at the imbalance between 3′ and 5′ expression works relatively well for ALK and RET, which are normally not expressed in lung tissue, but this calculation was more difficult for ROS1, as this gene is normally expressed at high levels. Therefore, in the process of developing this panel as an IVD (in vitro diagnostics), the use of 3′/5′ expression imbalance was resigned.
Oncomine Dx Fusion Transcript Test is focused on fusion genes using RNA materials obtained from FFPE tissue. In the present study, only samples that did not contain an epidermal growth factor receptor (EGFR) gene mutation were examined. In clinical practice, for Japanese lung cancer patients, EGFR gene mutation testing is the first gene screening test that is carried out because the prevalence of EGFR gene mutation positivity is relatively high (40%‐50%). Therefore, it is reasonable to screen for fusion genes in a single assay using ODxFT after the confirmation of a wild‐type EGFR genotype.
In conclusion, the RT‐PCR NGS assay described here offers many advantages for laboratory testing in lung adenocarcinoma samples. The single‐assay format potentially allows for a faster turnaround time and a lower cost than carrying out the assays separately. Furthermore, the small amount of input RNA that is required is very advantageous for this type of sample. However, ODxFT primarily targets known fusion genes. Last, efforts to periodically update the primer pool as additional partner genes for ALK, ROS1, RET, and NTRK1 fusions are identified would aid in the continuing utility of this assay.
DISCLOSURE
This study was supported by a contract research fund from Life Technologies Japan Ltd. Emi Iwamatsu and Jin Katayama are employees of Life Technologies Japan Ltd and Thermo Fisher Scientific. Akihiko Ito, Norihiko Ikeda, and Kazuto Nishio received the research fund from Life Technologies Japan Ltd. Kazuko Sakai, Tatsuo Ohira, Jun Matsubayashi, Azusa Yoneshige, Tetsuya Mitsudomi, and Toshitaka Nagao have no conflicts of interest with any companies or organizations whose products or services were discussed in this article.
Supporting information
ACKNOWLEDGMENT
The authors wish to thank Ayaka Kurumatani of the Department of Genome Biology, Kindai University Faculty of Medicine and Kumiko Nagase of Department of Surgery, Tokyo Medical University for thier technical support.
Sakai K, Ohira T, Matsubayashi J, et al. Performance of Oncomine Fusion Transcript kit for formalin‐fixed, paraffin‐embedded lung cancer specimens. Cancer Sci. 2019;110:2044–2049. 10.1111/cas.14016
REFERENCES
- 1. Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sholl LM, Aisner DL, Varella‐Garcia M, et al. Multi‐institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10:768‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Network CGAR. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature. 2007;448:561‐566. [DOI] [PubMed] [Google Scholar]
- 5. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190‐1203. [DOI] [PubMed] [Google Scholar]
- 6. Kohno T, Ichikawa H, Totoki Y, et al. KIF5B‐RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378‐381. [DOI] [PubMed] [Google Scholar]
- 8. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug‐sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19:1469‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4‐ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275‐4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uguen A, De Braekeleer M. ROS1 fusions in cancer: a review. Future Oncol. 2016;12:1911‐1928. [DOI] [PubMed] [Google Scholar]
- 11. Scarpino S, Rampioni Vinciguerra GL, Di Napoli A, et al. High prevalence of ALK+/ROS1+ cases in pulmonary adenocarcinoma of adolescents and young adults. Lung Cancer. 2016;97:95‐98. [DOI] [PubMed] [Google Scholar]
- 12. Pan Y, Zhang Y, Li Y, et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern‐specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121‐126. [DOI] [PubMed] [Google Scholar]
- 13. Gainor JF, Shaw AT. Novel targets in non‐small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18:865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. To KF, Tong JH, Yeung KS, et al. Detection of ALK rearrangement by immunohistochemistry in lung adenocarcinoma and the identification of a novel EML4‐ALK variant. J Thorac Oncol. 2013;8:883‐891. [DOI] [PubMed] [Google Scholar]
- 15. Ali SM, Hensing T, Schrock AB, et al. Comprehensive genomic profiling identifies a subset of crizotinib‐responsive ALK‐rearranged non‐small cell lung cancer not detected by fluorescence in situ hybridization. Oncologist. 2016;21:762‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfarr N, Stenzinger A, Penzel R, et al. High‐throughput diagnostic profiling of clinically actionable gene fusions in lung cancer. Genes Chromosom Cancer. 2016;55:30‐44. [DOI] [PubMed] [Google Scholar]
- 17. Tembuyser L, Tack V, Zwaenepoel K, et al. The relevance of external quality assessment for molecular testing for ALK positive non‐small cell lung cancer: results from two pilot rounds show room for optimization. PLoS ONE. 2014;9:e112159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallander ML, Geiersbach KB, Tripp SR, Layfield LJ. Comparison of reverse transcription‐polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule‐associated proteinlike 4‐anaplastic lymphoma kinase fusion‐positive non‐small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med. 2012;136:796‐803. [DOI] [PubMed] [Google Scholar]
- 19. Vendrell JA, Taviaux S, Béganton B, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next‐generation sequencing approaches. Sci Rep. 2017;7:12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lira ME, Choi YL, Lim SM, et al. A single‐tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J Mol Diagn. 2014;16:229‐243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


