Abstract
The immune checkpoint inhibitor nivolumab inhibits the programmed death 1 receptor and suppresses the immune resistance of cancer cells. This is a long‐term follow up of a single‐arm, open‐label, multicenter, phase II study of nivolumab in untreated Japanese patients with stage III/IV or recurrent melanoma. In addition, a post–hoc subgroup analysis stratified by melanoma types was performed. Nivolumab was administered intravenously at a dose of 3 mg/kg every 2 weeks. The primary endpoint was the overall response rate (ORR), and secondary endpoints included overall survival (OS), progression‐free survival (PFS), best overall response, the disease control rate and change in tumor diameter. Safety was assessed by recording treatment‐related adverse events (TRAE), including select immune‐related adverse events. Of the 24 patients initially included in the primary phase II study, 10 survived for over 3 years (41.7%). The ORR was 34.8% (90% confidence interval [CI]: 20.8, 51.9) for all patients. When analyzing by melanoma type, the ORR was 66.7% (90% CI: 34.7, 88.3) for superficial spreading, 33.3% (90% CI: 11.7, 65.3) for mucosal, and 28.6% (90% CI: 10.0, 59.1) for acral lentiginous tumors. The median OS was 32.9 months, the 3‐year OS rate was 43.5%, and the 3‐year PFS rate was 17.2%. A long‐term response was observed in all the tumor types. The most common TRAE included skin toxicity (45.8%) and endocrine disorders (29.2%). This study demonstrated the long‐term efficacy and tolerability of nivolumab in patients with advanced or recurrent melanoma, irrespective of melanoma type.
Keywords: clinical efficacy, Japan, malignant melanoma, nivolumab, survival rate
1. INTRODUCTION
In the past 10 years, the practice of medical oncology has been transformed by the development of immune checkpoint inhibitors for anticancer treatment.1 Immune checkpoint inhibitors are now at the forefront of cancer immunotherapy research as they enhance the body's own anticancer activities by modulating lymphocytes to recognize tumor cells.
The immune checkpoint inhibitor, nivolumab, is a human monoclonal antibody that inhibits the checkpoint receptor, programmed death 1 (PD‐1). In a previous phase II clinical trial (hereinafter referred to as the primary phase II study) that investigated nivolumab therapy in stage III/IV or recurrent melanoma, it was reported that nivolumab was clinically beneficial.2 The overall response rate (ORR) was 34.8%, and the overall survival (OS) rate at 18 months was 56.5%. A subgroup analysis also revealed that nivolumab was effective and safe regardless of BRAF genotype. This is clinically important because melanomas with the BRAF mutation are reported to be more aggressive and resistant to chemotherapy. Therefore, nivolumab is a clinically beneficial treatment option in Japanese patients with advanced or recurrent melanoma.3, 4, 5
The present study evaluated the long‐term follow‐up results (3‐year OS) in Japanese patients with advanced malignant melanoma from the primary phase II study.2 In addition, the OS of patients with acral lentiginous or mucosal melanoma types were also compared against the OS of patients with superficial spreading. This is because acral lentiginous and mucosal melanoma types are more prevalent in Japanese patients (40% and 10%, respectively) when compared with Caucasian populations, and, therefore, it would be of value to evaluate the efficacy of treatment in melanoma types that are specific to Japanese patients.6, 7
2. MATERIALS AND METHODS
2.1. Study design
The primary study was a single‐arm, open‐label, multicenter phase II study.2 Here, we report the long‐term (3‐year OS) follow‐up results of patients from the primary phase II study and the analysis of OS by melanoma types that are prevalent in the Japanese population. The primary study consisted of 3 stages: screening, intervention and post–treatment follow‐up. Patients were originally enrolled into a screening stage after which eligible patients were enrolled into the intervention stage. Nivolumab was administered intravenously at a dose of 3 mg/kg every 2 weeks in a 6‐week cycle until progressive disease (PD) or unacceptable adverse events (AE) were observed. The criteria for study drug discontinuation included the following: complete response (CR) based on Response Evaluation Criteria in Solid Tumors (RECIST) guidelines unless the patient was expected to experience recurrence, PD based on RECIST guidelines with no further clinical benefit expected, clinical symptoms that indicated cancer progression, grade ≥2 interstitial lung disease, grade ≥3 AE that were not ruled out to be related to nivolumab, or grade ≥2 AE (eye pain and visual acuity reduced) that could not be ruled out to be related to nivolumab. Tumors were evaluated at the end of each 6‐week cycle to determine whether treatment should be continued. The follow‐up stage started when treatment was discontinued or no new cycle was started.
2.2. Patients
This study included Japanese patients with unresectable stage III/IV or recurrent malignant melanoma according to the Union for International Cancer Control‐TNM classification (version 7). Patients were included if the following criteria were met: age ≥20 years, patients with unresectable stage III/IV or recurrent malignant melanoma confirmed by biopsy or cytology, previously untreated with antineoplastic drugs (chemotherapy, molecular‐targeted therapy or immunotherapy), at least 1 measurable lesion as defined by the RECIST guideline version 1.1, Eastern Cooperative Oncology Group Performance Status (ECOG‐PS) of 0‐1, and patients that were expected to survive ≥90 days. In the case of preoperative or postoperative adjuvant therapy for malignant melanoma, patients whose treatment ended ≥6 weeks prior to enrollment and in whom all adverse drug reactions returned to baseline or stabilized at the time of enrollment were also included. Recurrence was defined as unresectable recurrence. The stage at diagnosis and adherence to inclusion/exclusion criteria regarding local recurrence were not regulated, and patients were included/excluded at the discretion of the attending physician; thus, it is possible that patients exhibiting local recurrence were included in the study.
Patients were excluded if they had severe hypersensitivity to other antibody preparations, residual effects of prior treatment with radiation therapy or surgical treatment, an autoimmune disease or a history of recurrent autoimmune disease, a primary tumor in the esophagus or rectum, multiple primary cancers, or an active primary lesion or metastatic lesion in the brain or meninges. Patients also had to provide a tumor section for BRAF V600 gene mutation analysis prior to enrollment (Cobas 4800 BRAF V600 Mutation Test; Roche Diagnostics).
2.3. Ethics
The institutional review board at each site approved the study protocol, and this study was carried out following the ethical principles described in the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Written informed consent was provided by all patients. The present study was registered at JapicCTI‐142533.
2.4. Efficacy endpoints
The primary endpoint was the ORR, which was centrally assessed. The secondary endpoints, assessed in all study patients and in the subpopulation of 3‐year survivors, included OS, progression‐free survival (PFS), best overall response (BOR), disease control rate (DCR), and the percent change in the sum of the diameter of the target lesion.
A subgroup analysis was conducted to evaluate the ORR, DCR, OS and PFS when stratified by the presence of the BRAF V600 mutation. Considering that the maximum time to response was 5.7 months in the previous analysis of this study, we used a post–hoc landmark analysis of OS after 6 months of treatment to evaluate the relationship between an early tumor response and subsequent survival. Post–hoc subgroup analyses included the investigation of ORR, OS, and change in tumor diameter for each melanoma type (superficial spreading, acral lentiginous, mucosal or unknown). In addition, post–hoc subgroup analyses were performed to investigate ORR, DCR, OS and PFS stratified by programmed death‐ligand 1 (PD‐L1) status (<1% or ≥1%), lactate dehydrogenase (LDH) levels and baseline tumor diameter (based on the first quartile [≤21.950 mm] and third quartile [>64.615 mm] of all tumor diameters at baseline).
2.5. Safety endpoints
Safety variables that were assessed included treatment‐related AE, which were graded using the Common Terminology Criteria for Adverse Events (version 4.0). The frequency of treatment‐related select AE, defined as AE with potential immunological causes, was also recorded.
2.6. Statistical analysis
The target sample size was ≥20 patients with at least 14 patients characterized as having a BRAF wild‐type malignant melanoma and at least 6 patients with a BRAF mutant malignant melanoma.2, 8 Sample size calculations were described in full in the primary phase II study.2
Efficacy endpoints were analyzed using the full analysis set, and the proportion of patients and the 2‐sided 90% confidence interval (CI) were calculated for ORR and DCR. The OS and PFS were reported as medians and 2‐sided 90% CI, which were estimated using the Kaplan‐Meier method. The proportion of patients with CR, partial response (PR), stable disease (SD) and PD, as well as that of patients who were not evaluable, were calculated. The 2‐sided 90% CI were calculated for BOR including CR, PR, SD and PD. We conducted a post–hoc landmark analysis to evaluate the difference in Kaplan‐Meier estimates of OS according to a BOR within 6 months. Safety endpoints were analyzed using the safety analysis set, which comprised patients who had received the study drug at least once.
For the subgroup analysis, the relationships between the BRAF V600 mutation, PD‐L1 status (<1% and ≥1%), LDH levels, baseline tumor diameter and efficacy were analyzed using the exploratory data analysis method. Analysis of PD‐L1 status (<1% and ≥1%), LDH levels and baseline tumor diameter were post–hoc subgroup analyses. The proportion of patients and 2‐sided 90% CI were calculated for ORR and DCR. Statistical analyses were performed using SAS software (version 9.4, SAS Institute).
3. RESULTS
3.1. Patient baseline characteristics and disposition
The study period was from March 2014 to September 2017, and the cut‐off date was 15 September 2017. Patient baseline characteristics are described in Table 1. Of the 24 patients included in the primary phase II study, 1 patient was excluded from the efficacy analysis because the patient was found to have multiple cancers after initiation of the study.2 In total, there were ten 3‐year survivors included, among whom tumor characteristics included superficial spreading (n = 5), acral lentiginous (n = 2), mucosal (n = 2), and unknown (n = 1). Patients had a median follow up of 32.9 months (min, max: 2.0, 39.6).
Table 1.
Baseline characteristics
| Characteristics | All patients (n = 24) |
|---|---|
| n (%) | |
| Sexa | |
| Male | 14 (58.3) |
| Female | 10 (41.7) |
| Age (y)a | |
| Median | 63.0 |
| <65 | 13 (54.2) |
| ≥65 | 11 (45.8) |
| ECOG‐PSa | |
| 0 | 16 (66.7) |
| 1 | 8 (33.3) |
| Stagea | |
| IV | 3 (12.5) |
| Recurrence | 21 (87.5) |
| Typea | |
| Acral lentiginous | 7 (29.2) |
| Mucosal | 6 (25.0) |
| Superficial spreading | 6 (25.0) |
| Nodular | 1 (4.2) |
| Lentigo maligna | 0 (0) |
| Unknown | 4 (16.7) |
| History of surgerya | |
| Yes | 23 (95.8) |
| History of radiotherapya | |
| Yes | 3 (12.5) |
| History of adjuvant therapya | |
| 0 | 9 (37.5) |
| 1 | 7 (29.2) |
| ≥2 | 8 (33.3) |
| BRAF V600 statusa | |
| Mutant | 6 (25.0) |
| Wild‐type | 18 (75.0) |
| PD‐L1 expression status | |
| ≥1% | 4 (16.7) |
| <1% | 14 (58.3) |
| Could not be determined or reported | 6 (25.0) |
| LDH | |
| ≤ULN | 17 (70.8) |
| >ULN | 7 (29.2) |
| Tumor diameter | |
| ≤21.950 mm | 6 (25.0) |
| >21.950 mm and ≤64.615 mm | 12 (50.0) |
| >64.615 mm | 6 (25.0) |
ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase; PD‐L1, programmed death‐ligand 1; ULN, upper limit of normal.
Data from Yamazaki et al, Cancer Sci 2017; 108: 1223‐30.
3.2. Efficacy analysis
The ORR was 34.8% (8/23 patients [90% CI: 20.8, 51.9]) with CR and PR both 17.4% each. The DCR was 65.2% (15/23 patients [90% CI: 48.1, 79.2]). The BOR assessed in ten 3‐year survivors included CR in 4 patients, PR in 1 patient, SD in 4 patients, unknown in 1 patient, and no PD reported (Table 2). The ORR by melanoma type showed that superficial spreading tumor type (66.7% [4/6 patients]; 90% CI: 34.7, 88.3) was associated with a greater response rate compared with mucosal (33.3% [2/6 patients]; 90% CI: 11.7, 65.3) or acral lentiginous tumor type (28.6% [2/7 patients]; 90% CI: 10.0, 59.1). The ORR was 66.7% (2/3 patients; 90% CI: 25.4, 92.2) for stage IV and 30.0% (6/20 patients; 90% CI: 16.4, 48.4) for recurrence.
Table 2.
Response rate
| Response | All patients (n = 23b) | 3‐y survivors (n = 10) | ||
|---|---|---|---|---|
| n | % | 90% CI | n | |
| BOR | ||||
| CR | 4 | 17.4 | 8.1‐33.6 | 4 |
| PR | 4 | 17.4 | 8.1‐33.6 | 1 |
| SDa | 7 | 30.4 | 17.4‐47.6 | 4 |
| PDa | 7 | 30.4 | 17.4‐47.6 | 0 |
| Unknownc | 1 | 4.3 | 1 | |
| ORR (CR + PR) | 8 | 34.8 | 20.8‐51.9 | 5 |
| DCR (CR + PR + SD) | 15 | 65.2 | 48.1‐79.2 | 9 |
BOR, best overall response; CI, confidence interval; CR, complete response; DCR disease control rate; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Data from Yamazaki et al, Cancer Sci 2017; 108: 1223‐30.
One patient who was found after study completion to have multiple cancers from the start was excluded from the efficacy analysis set.
No target lesions were identified at baseline.
The median OS was 32.9 months, and the OS rate after 1, 2 and 3 years was 69.6% (90% CI: 50.8, 82.3), 56.5% (90% CI: 38.0, 71.4) and 43.5% (90% CI: 26.4, 59.4), respectively (Figure 1A). The median PFS was 5.9 months (90% CI: 2.8, 12.2), and the PFS rate after 1, 2 and 3 years was 38.3% (90% CI: 21.8, 54.6), 28.7% (90% CI: 14.3, 44.9) and 17.2% (90% CI: 6.1, 33.1), respectively (Figure 1B).
Figure 1.
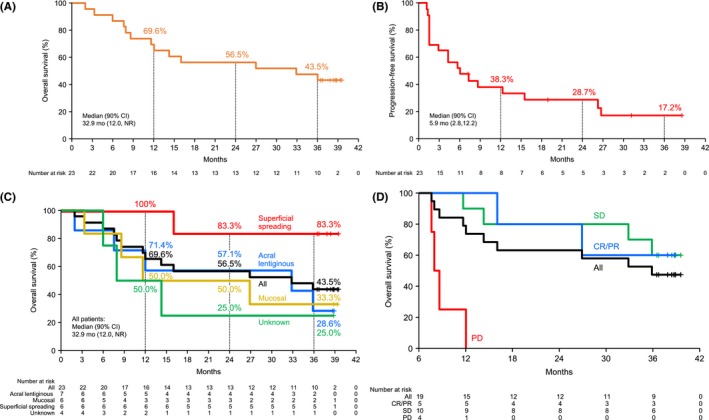
Kaplan–Meier curves for (A) OS, (B) PFS, (C) OS by melanoma type and (D) OS by response (landmark analysis). One patient found to have multiple cancers after initiation of the trial was excluded from the group to be analyzed for effectiveness. CI, confidence interval; CR, complete response; NR, not reached; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease
[Correction added on 05 June 2019, after first online publication: Figure 1C was replaced to include mucosal data.]
The 3‐year OS rates of acral lentiginous or mucosal melanomas (28.6% [90% CI: 6.4, 56.5] and 33.3% [90% CI: 7.4, 62.9], respectively) were lower compared with that of superficial spreading melanoma (83.3% [90% CI: 38.8, 96.5]) (Figure 1C). A landmark analysis of OS after 6 months of treatment showed that the OS rate at 36 months was similar between patients with CR/PR and SD at 6 months (Figure 1D), suggesting the possibility of long‐term survival even in patients with SD.
In Figure 2, the waterfall plot highlights the maximum change in target lesion size from baseline (%) by tumor type (superficial spreading, acral lentiginous and mucosal). All 6 patients with the superficial spreading type, all 3 PD‐L1 positive (≥1%) patients, and 5 out of 6 patients with a BRAF mutation showed a reduction in target lesion size from baseline. A spider plot of the percent change in target lesion diameter from baseline (%) by tumor type is shown in Figure 3A (mucosal and acral lentiginous) and Figure 3B (superficial spreading and unknown). A long‐term response was obtained in all the tumor types.
Figure 2.
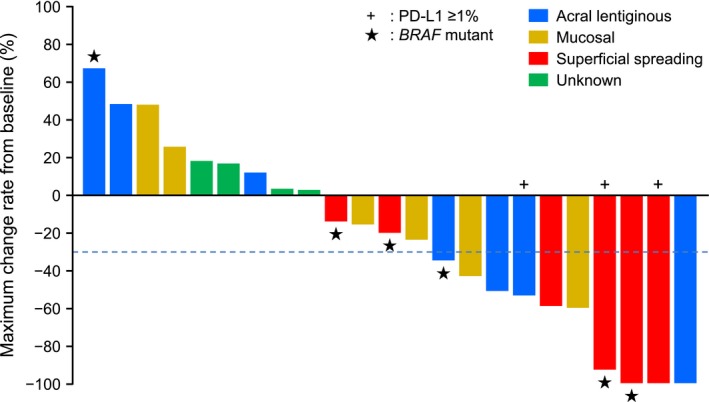
Maximum change in target lesion size from baseline (%) by tumor type (in the presence or absence of PD‐L1 expression or BRAF mutation). One patient found to have multiple cancers after initiation of the trial was excluded from the group to be analyzed for effectiveness. PD‐L1, programmed death 1 ligand
Figure 3.
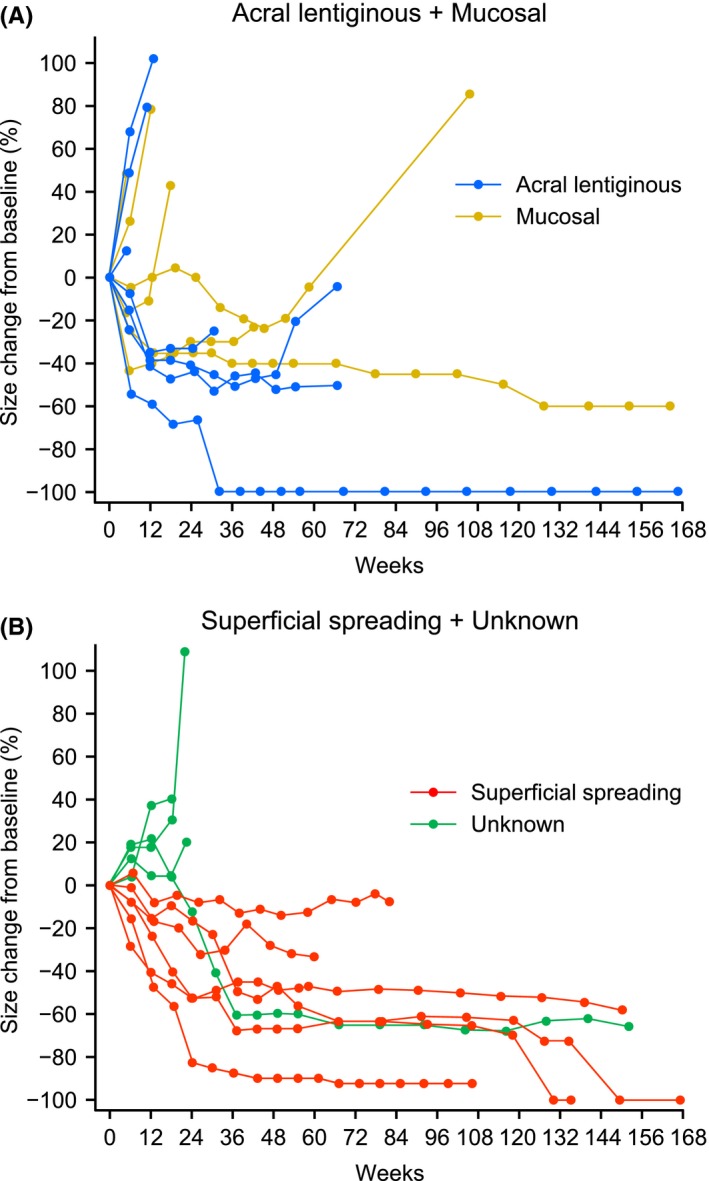
Percent change in target tumor diameter over time in (A) acral lentiginous and mucosal tumor types and (B) superficial spreading and unknown tumor types
Table 3 shows the clinical efficacy (ORR, DCR, OS and PFS) as stratified by the presence of the BRAF V600 mutation, the PD‐L1 status, normal or abnormal serum LDH levels, and baseline tumor diameter of another subgroup analysis. There was a clinical response regardless of the presence or absence of the BRAF V600 mutation; OS rates after 24 months in patients with BRAF wild‐type and BRAF mutation were 52.9% and 66.7%, respectively, and OS rates after 36 months were 41.2% and 50.0%, respectively (Figure S1). In addition, PFS rates after 24 months in patients with BRAF wild‐type and BRAF mutation were 17.6% and 62.5%, respectively (Figure S2). The PFS rate at 36 months could not be estimated because none of the patients were followed up for 36 months. As for PD‐L1, a notable effect on clinical efficacy was observed in 2 out of 3 patients who had a PD‐L1 positive (≥1%) status. The median OS was not reached during the study period, whereas a PFS of 26.2 months was associated with a PD‐L1 positive status (Table 3). In comparison, a PD‐L1 status of <1% was associated with a median OS of 14.0 months and a median PFS of 3.5 months. Similarly, serum LDH levels also greatly affected the median OS and PFS. A serum LDH ≤ the upper limit of normal (ULN) resulted in a median OS that was not reached and a median PFS of 7.9 months. In contrast, a serum LDH > the ULN resulted in a median OS of 11.7 months and a median PFS of 1.4 months (Table 3). In addition, OS and PFS were also found to be associated with tumor diameter at baseline. In patients with smaller tumors (≤21.950 mm), the median OS was not reached during the study period, while the median PFS was 26.6 months, compared with 11.8 months and 2.8 months, respectively, in patients with larger tumors (>64.615 mm).
Table 3.
Subgroup analysis of clinical efficacy
| All patients (n) | ORR | DCR | OS | PFS | |
|---|---|---|---|---|---|
| n (%) [90% CI] | n (%) [90% CI] | Median (mo) | Median (mo) | ||
| BRAF | |||||
| Wild‐type | 17 | 4 (23.5) [11.0, 43.3] | 10 (58.8) [39.3, 75.9] | 26.9 | 4.2 |
| Mutant | 6 | 4 (66.7) [34.7, 88.3] | 5 (83.3) [49.8, 96.2] | NR | 26.2 |
| PD‐L1a | |||||
| ≥1% | 3 | 2 (66.7) [25.4, 92.2] | 3 (100.0) [52.6, 100.0] | NR | 26.2 |
| <1% | 14 | 4 (28.6) [13.5, 50.6] | 8 (57.1) [36.0, 75.9] | 14.0 | 3.5 |
| LDH | |||||
| ≤ULN | 16 | 6 (37.5) [20.8, 57.8] | 12 (75.0) [54.5, 88.2] | NR | 7.9 |
| >ULN | 7 | 2 (28.6) [10.0, 59.1] | 3 (42.9) [18.6, 71.1] | 11.7 | 1.4 |
| Tumor diameter at baseline (mm) | |||||
| ≤21.950b | 5 | 3 (60.0) [27.2, 85.7] | 4 (80.0) [43.5, 95.4] | NR | 26.6 |
| >21.950b and ≤64.615c | 12 | 3 (25.0) [10.5, 48.7] | 8 (66.7) [43.1, 84.1] | 29.9 | 4.9 |
| >64.615c | 6 | 2 (33.3) [11.7, 65.3] | 3 (50.0) [22.1, 77.9] | 11.8 | 2.8 |
CI, confidence interval; DCR disease control rate; LDH, lactate dehydrogenase; NR, not reached; PD‐L1, programmed death‐ligand 1; PFS, progression‐free survival; ORR, overall response rate; OS, overall survival; ULN, upper limit of normal.
Could not be determined or reported (n = 6).
First quartile.
Third quartile.
3.3. Safety analysis
Treatment‐related AE were observed in 100.0% (10/10 patients) of 3‐year survivors and in 83.3% (20/24 patients) of the total patient group. In the total patient group, the most common treatment‐related AE that were observed in ≥10% of patients are shown in Table 4. Four grade 3‐4 treatment‐related AE (anemia, fever, colitis and renal impairment) were reported in 3 patients.
Table 4.
Incidence of treatment‐related adverse events observed in ≥10% of overall patients (median follow up: 32.9 mo)
| Preferred term | All patients (n = 24) | 3‐y survivors (n = 10) | ||
|---|---|---|---|---|
| All grades, n (%) |
Grade 3‐4 n (%) |
All grades n (%) |
Grade 3‐4 n (%) |
|
| Overall | 20 (83.3) | 3 (12.5) | 10 (100.0) | 0 |
| Vitiligo | 9 (37.5) | 0 | 7 (70.0) | 0 |
| Hypothyroidism | 6 (25.0) | 0 | 2 (20.0) | 0 |
| Malaise | 6 (25.0) | 0 | 4 (40.0) | 0 |
| Pruritus | 6 (25.0) | 0 | 3 (30.0) | 0 |
| Nausea | 3 (12.5) | 0 | 1 (10.0) | 0 |
| Weight decreased | 3 (12.5) | 0 | 1 (10.0) | 0 |
| Appetite decreased | 3 (12.5) | 0 | 1 (10.0) | 0 |
| Arthralgia | 3 (12.5) | 0 | 2 (20.0) | 0 |
| Rash maculo‐papular | 3 (12.5) | 0 | 2 (20.0) | 0 |
There were only 2 incidences of treatment‐related AE leading to discontinuation in all patients (n = 1 for colitis and pleural diffusion) (Table 5). The most common treatment‐related select AE were skin toxicity (n = 11, 45.8%), endocrine disorders (n = 7, 29.2%), gastrointestinal toxicity (n = 2, 8.3%), hepatotoxicity (n = 2, 8.3%) and pulmonary toxicity (n = 1, 4.2%) (Table 6). There were no treatment‐related deaths.
Table 5.
Incidence of serious treatment‐related AE and treatment‐related AE leading to discontinuation
| All patients (n = 24) | ||
|---|---|---|
|
All grades n (%) |
Grade 3‐4 n (%) |
|
| Treatment‐related AEs leading to discontinuation | ||
| Overall | 2 (8.3) | 1 (4.2) |
| Colitis | 1 (4.2) | 1 (4.2) |
| Pleural effusion | 1 (4.2) | 0 |
| Serious treatment‐related AE | ||
| Overall | 3 (12.5)a | 2 (8.3) |
| Colitis | 1 (4.2) | 1 (4.2) |
| Hepatic function abnormal | 1 (4.2) | 0 |
| Renal impairment | 1 (4.2) | 1 (4.2) |
| Pleural effusion | 1 (4.2) | 0 |
Abbreviation: AE, adverse event.
One patient had 2 events.
Table 6.
Incidence of treatment‐related select adverse events (defined as AE with potential immunological causes)
| All patients (n = 24) | ||
|---|---|---|
|
All grades n (%) |
Grade 3‐4 n (%) |
|
| Overall | 14 (58.3)a | 1 (4.2) |
| Skin toxicity | 11 (45.8) | 0 |
| Endocrine disorders | 7 (29.2) | 0 |
| Gastrointestinal toxicity | 2 (8.3) | 1 (4.2) |
| Hepatotoxicity | 2 (8.3) | 0 |
| Pulmonary toxicity | 1 (4.2) | 0 |
| Nephrotoxicity | 0 | 0 |
| Hypersensitivity/infusion reaction | 0 | 0 |
Abbreviation: AE, adverse event.
Some patients had multiple adverse events.
4. DISCUSSION
The present study demonstrated the long‐term efficacy and safety of nivolumab in Japanese patients with advanced or recurrent melanoma. The median OS reported here was similar to that of patients who received nivolumab monotherapy in the CheckMate 067 study (32.9 and 37.6 months, respectively).9 There were long‐term survivors even among patients with SD. This phenomenon may occur due to an equilibrium being achieved between tumor growth and tumor shrinkage by therapy, or the tumor may have lost its proliferative activity despite being observed during computed tomography scan imaging.10
In this study, patient characteristics were generally consistent with those who received nivolumab monotherapy in the CheckMate 067 study.9 However, the percentage of tumor types reported in Japan11 for acral lentiginous (46%), superficial spreading (23%) and mucosal (10%) is different to what has been reported in other countries (5%, 54% and .4% respectively).12 It has been reported that acral lentiginous (50%‐58%)13 and mucosal (15%)14 melanoma are common subtypes in Asian countries. Thus, the percentage of tumors that are acral lentiginous or mucosal is higher in East Asian countries, including Japan, than in other countries. In the present study, the distribution of acral lentiginous (29.2%) and mucosal (25.0%) types accounted for over half of the study population and when investigating the 3‐year OS rate, nivolumab was not as effective in patients with mucosal or acral lentiginous types compared with patients with the superficial spreading type. Similar results have been reported previously.15 This difference in tumor response by melanoma type may be the reason why the ORR and 3‐year OS rate in this study was lower than what was reported in the CheckMate 067 study.9 To explain these differences in response rates, the tumor mutation burden (TMB) may be less in acral lentiginous and mucosal types compared with the superficial spreading type.16
In cancer cells with a high TMB level, the immune system is thought to more easily recognize the tumor due to increased neoantigen levels. Here, an interferon gamma messenger RNA signature has been previously reported to correlate with higher TMB levels in tumor biopsies, which results in greater T‐cell activation.17 In addition, it was also reported that the OS was better in patients with a high TMB when nivolumab was administered in the ipilimumab‐naïve group in the CheckMate 038 study.18 According to an interim analysis of a Japanese post–marketing surveillance study, there was no difference in OS between cutaneous and mucosal melanoma types, although the cutaneous type does include acral lentiginous, superficial spreading and nodular types.19 Therefore, further research is required to fully elucidate the efficacy of nivolumab treatment in different melanoma types.
In this study, only 3 patients (superficial spreading [n = 2] and acral lentiginous [n = 1]) were confirmed to be positive for PD‐L1. None of the patients with mucosal melanomas were confirmed to be PD‐L1 positive, which is consistent with previous reports that have shown there were fewer PD‐L1‐positive patients with the mucosal type than the cutaneous type.20, 21 The ORR was relatively higher in PD‐L1‐positive patients versus those with PD‐L1 <1%. However, this result is limited by the small number of PD‐L1 positive patients (n = 3).
The percentage of patients with a BRAF mutation in this study was 25%, which was similar to that reported in 2 previous Japanese studies.22, 23 The incidence of BRAF mutations is lower in Japanese patients than in Caucasian patients, although in the CheckMate 067 study, efficacy was confirmed regardless of BRAF status.9 We report that nivolumab was effective in both BRAF wild‐type and BRAF mutant melanomas based on our reported median OS and PFS.
The observation period in this report was 14.1 months longer than in our previous report (18.8 months vs 32.9 months), but there were no additional AE of clinical concern observed over time.2 This confirms the long‐term safety of nivolumab treatment.
This study had limitations, which included the open‐label design, the small sample size and the absence of a control group.
In conclusion, we report the long‐term survival data for nivolumab monotherapy in previously untreated Japanese patients with advanced or recurrent malignant melanoma. We also show that long‐term survival can be expected, even in patients with SD, and that efficacy was observed, irrespective of melanoma type, including acral lentiginous and mucosal types, which are more prevalent in Japanese patients. The long‐term safety of nivolumab therapy was demonstrated and these results were consistent with other safety data reported to date.
CONFLICT OF INTEREST
N.Y. has received grants and personal fees from Ono Pharmaceutical and Bristol‐Myers Squibb. Y.K. has received grants from MSD, Bristol‐Myers Squibb and Takara Bio and personal fees from Ono Pharmaceutical. Hisashi Uhara has received grants from Maruho and Fujita Health University; personal fees from Ono Pharmaceutical, MSD, Novartis and Mitsubishi Tanabe Pharma; and scholarship funds from Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Maruho and Kyowa Hakko Kirin. T.T. has received personal fees from MSD and Ono Pharmaceutical. M.H. is an employee of Ono Pharmaceutical. H.M. has received grants from Ono Pharmaceutical, Bristol‐Myers Squibb, Chugai Pharmaceutical and Taiho Pharmaceutical, and personal fees from Ono Pharmaceutical. J.U., Y.F., M.O., Hiroshi Uchi and H.I. have no conflict of interest related to this research. The study was designed under the responsibility of Ono Pharmaceutical Co., Ltd., in conjunction with the steering committee. The study was funded by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb. Nivolumab was provided by Ono Pharmaceutical Co., Ltd. Ono Pharmaceutical Co., Ltd. collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supporting information
ACKNOWLEDGMENTS
The authors are grateful to the patients and their families, and to the investigators, nurses, and staff members who participated in this study. The authors wish to thank James Graham, PhD, of Edanz Medical Writing for providing medical writing assistance, which was funded by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). We also acknowledge the statistical support of Eiichiro Morishima of Ono Pharmaceutical Co., Ltd., and Masatada Hirata and Yoshihiro Ogawa of CAC Croit Corporation.
Yamazaki N, Kiyohara Y, Uhara H, et al. Long‐term follow up of nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. Cancer Sci. 2019;110:1995–2003. 10.1111/cas.14015
Clinical trial register: JapicCTI‐142533 [http://www.clinicaltrials.jp/]
REFERENCES
- 1. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a phase II study. Cancer Sci. 2017;108:1223‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239‐1246. [DOI] [PubMed] [Google Scholar]
- 4. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arozarena I, Sanchez‐Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP‐specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45‐57. [DOI] [PubMed] [Google Scholar]
- 6. Ishihara K, Saida T, Otsuka F, Yamazaki N; Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society . Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33‐41. [DOI] [PubMed] [Google Scholar]
- 7. Tomizuka T, Namikawa K, Higashi T. Characteristics of melanoma in Japan: a nationwide registry analysis 2011–2013. Melanoma Res. 2017;27:492‐497. [DOI] [PubMed] [Google Scholar]
- 8. Yamazaki N, Tahara H, Uhara H, Moroi Y, Kiyohara Y. Phase 2 study of nivolumab (Anti‐PD‐1; ONO‐4538/BMS‐936558) in patients with advanced melanoma [abstract no. 3738]. Eur J Cancer. 2013;49(Suppl 2):S868. [Google Scholar]
- 9. Hodi F, Chiarion‐Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4‐year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480‐1492. [DOI] [PubMed] [Google Scholar]
- 10. Schliep S, Agaimy A, Cavallaro A, Kiesewetter F, Schuler G, Heinzerling L. Concealed complete response in melanoma patients under therapy with immune checkpoint inhibitors: two case reports. J Immunother Cancer. 2018;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujisawa Y. Japanese melanoma study: annual report 2017. http://www.skincancer.jp/report-skincancer_melanoma_2017.pdf. Accessed November 15, 2018.
- 12. Tsoutos D, Papadopoulos S, Kehagias G, et al. Epidemiological trends in the diagnosis of melanoma in a Southern European population: analysis of a large database from a tertiary referral center. Melanoma Res. 2018;28:348‐358. [DOI] [PubMed] [Google Scholar]
- 13. Chang JW. Acral melanoma: a unique disease in Asia. JAMA Dermatol. 2013;149:1272‐1273. [DOI] [PubMed] [Google Scholar]
- 14. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019. 10.1097/DSS.0000000000001759. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15. Nakamura Y, Fujisawa Y, Tanaka R, et al. Use of immune checkpoint inhibitors prolonged overall survival in a Japanese population of advanced malignant melanoma patients: retrospective single institutional study. J Dermatol. 2018;45:1337‐1339. [DOI] [PubMed] [Google Scholar]
- 16. Hayward NK, Wilmott JS, Waddell N, et al. Whole‐genome landscapes of major melanoma subtypes. Nature. 2017;545:175‐180. [DOI] [PubMed] [Google Scholar]
- 17. Higgs BW, Morehouse CA, Streicher K, et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non‐small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res. 2018;24:3857‐3866. [DOI] [PubMed] [Google Scholar]
- 18. Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171:934‐949.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiyohara Y, Uhara H, Ito Y, Matsumoto N, Tsuchida T, Yamazaki N. Safety and efficacy of nivolumab in Japanese patients with malignant melanoma: an interim analysis of a postmarketing surveillance. J Dermatol. 2018;45:408‐415. [DOI] [PubMed] [Google Scholar]
- 20. Thierauf J, Veit JA, Affolter A, et al. Identification and clinical relevance of PD‐L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015;25:503‐509. [DOI] [PubMed] [Google Scholar]
- 21. D'Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashida A, Uhara H, Kiniwa Y, et al. Assessment of BRAF and KIT mutations in Japanese melanoma patients. J Dermatol Sci. 2012;66:240‐242. [DOI] [PubMed] [Google Scholar]
- 23. Sakaizawa K, Ashida A, Uchiyama A, et al. Clinical characteristics associated with BRAF, NRAS and KIT mutations in Japanese melanoma patients. J Dermatol Sci. 2015;80:33‐37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


