Abstract
Prediction of bathing water quality is recommended by the World Health Organization (WHO), the European Union (EU) and the United States Environmental Protection Agency (USEPA) and is an established element in bathing water management designed to protect public health. Most commonly, historical regulatory compliance data are used for model calibration and provide the dependent variable for modelling. Independent (or predictor) variables (e.g. rainfall, river flow and received irradiance) measured over some antecedent period are used to deliver prediction of the faecal indicator concentration measured on the day of the regulatory sample collection. The implied linked assumptions of this approach are, therefore, that; (i) the independent variables accurately predict the bathing-day water quality; which is (ii) accurately characterized by the single regulatory sample. Assumption (ii) will not be the case where significant within-day variability in water quality is evident. This study built a detailed record of water quality change through 60 days at a UK coastal bathing water in 2011 using half-hourly samples each subjected to triplicate filtration designed to enhance enumeration precision. On average, the mean daily variation in FIO concentrations exceeded 1 log10 order, with the largest daily variations exceeding 2 log10 orders. Significant diurnality was observed at this bathing water, which would determine its EU Directive compliance category if the regulatory samples were collected at the same time each day. A sampling programme of this intensity has not been reported elsewhere to date and, if this pattern is proven to be characteristic of other bathing waters world-wide, it has significance for: (a) the design of regulatory sampling programmes; (b) the use of historical data to assess compliance, which often comprises a single sample taken at the compliance point on a regular, often weekly, basis; and (c) the use of regulatory compliance data to build predictive models of water quality.
Keywords: Bathing water variability faecal indicators
Graphical abstract
Highlights
-
•
Half hourly samples were taken for 60 days in the UK bathing season.
-
•
Every day produced highly variable bacterial results covering 1–2.5 log10.
-
•
The daily variance was independent of antecedent conditions.
-
•
Samples taken at different times had different compliance outcomes.
1. Background
Prediction of bathing water quality, initially to characterise the bathing day, was first suggested as a bathing water management tool by a WHO expert group which developed the Annapolis Protocol (WHO, 1999). This was later incorporated into the first WHO Guidelines for Recreational Water Environments (WHO, 2003) and received its first regulatory application in the European Union (EU) Bathing Water Directive (EU, 2006) which sets legal compliance limits for over 20,000 European bathing waters. These criteria, using faecal indicator organism (FIO) measurements, are the basis of international bathing beach award and accreditation systems such as the Blue Flag awarded by the Foundation for Environmental Education (FEE, 2018). Parallel development of this broad approach is evident world-wide (USEPA, 2010a; b).
The purpose of predicting water quality on the bathing day is to protect public health by giving beachgoers 'informed choice' on days when adverse water quality is predicted and communicated through beach signage and internet resources (WHO, 1999). Also, in the EU, if this warning was in force on a day when the regulatory sample was taken then the adverse result on that day can be excluded from the 95th or 90th percentile calculations which determine legal compliance for an EU bathing water. This can also result in significant improvements in bathing water compliance, valued at several billion UK£ in benefits to UK communities dependent on tourism (EFTEC, 2002). To date, historical compliance data has provided the principal data resource for predictive model development and calibration in the UK.
Rapid and significant faecal indicator organism (FIO) variability in recreational waters has been reported in seminal studies by Boehm et al. (2002); Boehm (2007) and USEPA (2010a,b). The first centred on Huntington Beach in California. It was suggested that the observed variability invalidated the utility of single ‘spot’ determinations of faecal indicators as regulatory tools given the observed changes over minutes and hours: i.e. within the bathing day.
More recently, Lusic et al. (2017) reported significant diurnality in FIO concentrations at five Croatian bathing waters which were sampled at four hourly intervals between 02:00 and 20:00 local time, suggesting that the highest FIO concentrations occurred in the 06:00 samples, possibly driven by lower bactericidal solar irradiance in the preceding night-time period.
In two reports, the USEPA have summarised the drivers of variability in FIO concentrations across riverine, lacustrine and marine bathing waters and the implication for regulatory sampling (USEPA, 2010a; b). They note the impacts of tidal status and hydrograph events and comment on apparent diurnality, where early morning samples are expected to exhibit the highest FIO concentrations (USEPA, 2010a Page 3 Exhibit 2 and Page 11 Section 2.2). Interestingly, they cite the work of Boehm et al. (2002: (page 3891, right column, paragraph 3, lines 7–15)) to suggest regulatory monitoring of bathing waters exhibiting this pattern should, at a minimum, focus on early morning sampling to produce a 'precautionary' approach to health risk management (USEPA, 2010b Page 13 Section 2.2.3). This is an understandable regulatory response but, perhaps, it indicates a science need to facilitate more detailed modelling that can adequately predict the observed within-day patterns to furnish health risk predictions to beach management organisations and the public.
These international observations are consistent and of policy relevance because they reduce confidence in two foundations of current regulatory monitoring practice, namely:
-
i.
that a regulatory sample taken on the bathing day can characterise the day's water quality, and hence health risk, which is related to the faecal indicator organism (FIO) concentration that is used to characterise the pollution exposure level experienced by bathing beach users (WHO, 2003), or indeed, the confidence that a single check sample can be used to indicate the end of a short term pollution episode as is the case in current EU practice; and
-
ii.
that prediction models, advocated by WHO (WHO, 1999, 2003; EU, 2006; USEPA, 2012), and incorporated into legally enforced standard systems (e.g. EU, 2006) and award schemes (FEE, 2018), which use the ‘bathing day’ as the unit of prediction, and are often calibrated using historical compliance data or dedicated survey data; both being based on one, or possibly two, spot determinations assumed to characterise the bathing day; may not characterise relatively infrequent but important conditions producing non-compliance (Crowther et al., 2001; Francy et al., 2013; McPhail and Stidson, 2009; Nevers and Whitman, 2005; Olyphant, 2005; Seis et al., 2018; Stidson et al., 2012; Thoe et al., 2014; USEPA, 2012, 2018). Such an approach would be expected to produce relatively low explained variance (e.g. for US marine bathing waters, an average adjusted r2 of 0.39 has been reported by USEPA (2010a: Vol II Page xiii). However, overall model evaluation should be based on a broader set of tests than r2 alone, such as the percentage misclassification of 'acceptable' water quality when it was in fact 'poor' presenting an unpredicted health risk to bathers.
Many other workers have reported on the FIO response to hydrological events at regulated bathing waters (Stidson et al., 2012) but there have been few detailed reports describing modelling of sub-diurnal patterns in FIO concentration, which may present significant regulatory and prediction challenges. One exception is Bedri et al. (2016) who applied a hydrodynamic modelling approach to deliver within-day prediction.
2. Materials and methods
2.1. Study site
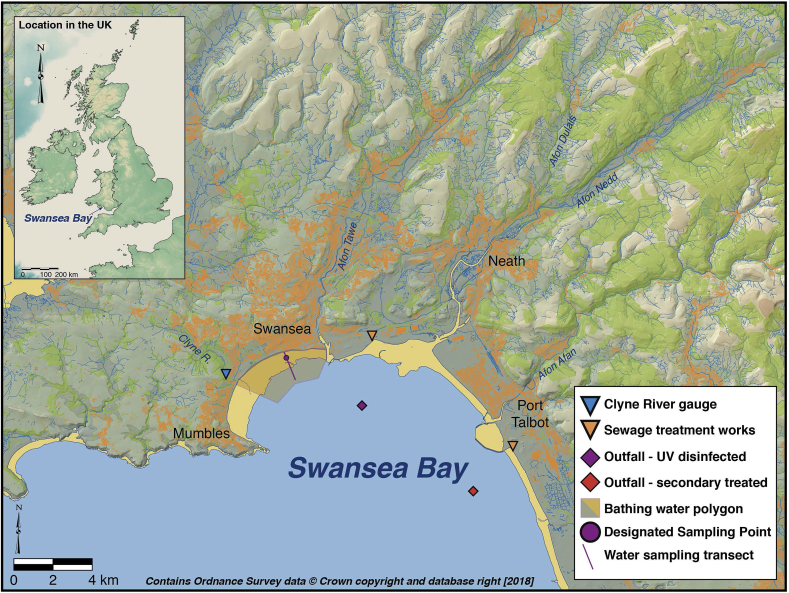
This paper reports on observed within-day patterns in FIO concentrations at Swansea Bay, in South Wales, UK (Fig. 1). This study site was selected because its bathing water had been considered an 'at risk' site by the UK authorities: i.e. at risk of non-compliance with European Union (EU) standards, leading to a 'Poor' classification against the EU Bathing Water Directive (EU, 2006) and, hence, potential permanent closure. The bathing water compliance point is in the approximate centre of the urban frontage of the bay which has significant freshwater inputs from three major rivers with diverse land use ranging from industrial and domestic urban areas (total population exceeding 250,000) through intensive livestock farming to upland mountain areas used for water gathering grounds, forestry and sheep grazing. These rivers drain a combined catchment area of approximately 600 km2. There are also two treated effluent discharges, entering the sea by long sea outfalls, from treatment works serving Swansea (ultra-violet (UV) disinfected) and Neath-Port Talbot (secondary treated). In addition, there are multiple small urban streams, some of which receive intermittent discharges of untreated, but dilute, sewage effluent when rainfall on the urban catchment results in the operation of combined sewer overflows. Streams also drain the urban infrastructure and may be impacted by what, in the UK, are termed 'cross-connections': i.e. the improper and unlicensed connection of domestic ‘foul’ drainage (i.e. toilets and ‘grey water’ from bathrooms, kitchens and washing machines etc.) into the 'non-foul' surface water urban drainage system. This complex pattern of land use and drainage is the principal reason why this bathing water has remained in the 'at-risk' category despite expenditures on sewage treatment and disposal exceeding 100 million UK£ over the past 20 years (DCWW, 2018), including UV disinfection of the sewage effluent from the city of Swansea itself.
Fig. 1.
Map of the Swansea Bay study area showing the locations of the Clyne River discharge gauge, the main sewage treatment works and corresponding outfalls and the designated water quality sampling point and transect.
2.2. Study design
The overall driver for this investigation was to generate the best possible model calibration data to effect bathing water prediction. The full study spanned 2010 to 2015 and investigated both black-box statistical and hydrodynamic water quality modelling approaches and a back-to back comparison of these two methods. The prediction modelling aspects are the basis of a current operational model deployed by Swansea Council, and a scientific paper reporting this is in preparation. This paper centres on the within-day variability in faecal indicator concentrations at this UK ′at-risk' bathing water.
Aseptic water quality sampling was undertaken at the Swansea Bay regulatory 'designated sampling point' (DSP) within the defined bathing water polygon (Fig. 1) at approximately half-hourly intervals between 07:00 GMT and 16:00 GMT during three days of each week (typically Monday to Wednesday) throughout the 20 week bathing season in 2011 (16/05/2011 to 28/09/2011).
Samplers followed the tidal transect along a line perpendicular to the foreshore as would be the case for regulatory sampling (Fig. 1) in tidal environments, which characterise UK coastal bathing waters. Samples were collected in sterile 1 L plastic containers (Aurora Scientific) by wading to a safe depth (between thigh and waist depth) and using a sampling pole to hold the container, thus avoiding any local disturbance from the sampler entering the water, and at approximately 30 cm below the surface. This was the safest and most consistent sampling approach achievable at low water, accounting for the characteristics of the site. The maximum tidal range here is large (>10 m) and the beach gradient extremely shallow towards low water, leading to maximum offshore sample collection distances in excess of 1.4 km during the largest spring tide. Samples were collected during 60 days in the UK bathing season (May to September), each day generating at least 19 water samples. This represents 43% of the 140-day bathing season and nearly as many samples were collected on individual sampling days as were collected during once weekly routine compliance monitoring at Swansea DSP in the entire 2011 bathing season (20 weekly samples). The within-day sampling period was extended to 19:00 GMT for 24 days between 18/07/2011 and 07/09/2011, yielding 25 samples per day over this part of the bathing season. This sampling regime allowed coverage of a wide range of tidal, meteorological and hydrometric conditions. For example, discharge data from a local river monitoring site operated by Swansea Council were available for the Clyne River (Fig. 1) to assess changes in the local freshwater input adjacent to the DSP.
2.3. Laboratory analysis
Samples were immediately refrigerated and analyzed in a dedicated microbiology laboratory within 24 h of collection as required by UK regulations (Mean: 10.77 h, Standard Deviation (SD): 8.12 h). The FIOs analyzed were Escherichia coli (E. coli) and confirmed intestinal enterococci (cIE) using standard membrane filtration techniques. E. coli were enumerated using membrane lactose glucoronide agar (MLGA, Oxoid/Glycosynth (SCA, 2009, 2011). Membranes were incubated for 4 h at 30 °C, followed by 14 h at 44 °C (±0.5 °C). All green colonies were counted as E. coli.
Enterococci were isolated using membrane enterococcus agar (MEA, Oxoid) by incubation for 4 h at 37 °C, followed by 44 h at 44 °C (±0.5 °C) (SCA, 2012). All maroon colonies were counted as presumptive intestinal enterococci (IE). Membranes were then transferred to kanamycin aesculin azide agar (KAAA, Oxoid) and incubated for 6 h at 44 °C (±0.5 °C). All colonies that developed black halos were counted as confirmed (cIE).
All microbiological analyses were undertaken in triplicate to reduce measurement imprecision (Fleisher and McFadden, 1980), which is significant for enumerations based on single filtrations employing an initial 10-fold dilution, which are generally used for UK bathing water regulatory compliance samples (SCA, 2009, 2011, 2012). Resulting concentrations were expressed as colony forming units per 100 ml (cfu/100 ml). Serial dilutions (generally two or three per sample) were made using sterile Ringer's solution in order to capture the appropriate range of FIO concentrations and limit censored data values (i.e. < and >values). The lower limit of detection (LLD) for E. coli was 3 cfu/100 ml. The theoretical LLD for cIE was 1 cfu/100 ml due to the two-stage incubation. All samples were analyzed within 24 h of collection. Samples were also analyzed for turbidity and salinity.
2.4. Statistical analysis and data preparation
Statistical analyses were performed using the SPSS statistical software package (Version 19, SPSS, 2010). The parametricity of distributions was assessed using the Shapiro-Wilk (S-W) normality test and Skewness statistic. General descriptive statistics included the mean, SD, range and the 95% confidence interval for the mean. Student's t-test was used to compare means between two groups. The outcome of the corresponding Levene test, for homogeneity of variances, was used to determine the appropriate type of t-test; based on either (i) separate or (ii) pooled variance estimates.
Robust analysis of variance (ANOVA) was employed to examine the significance of differences between more than two mean values. Here, the significance of the ANOVA is judged on: (i) the Levene test for homogeneity of group variances; and (ii) whether the numbers of observations (n) in groups can be considered equal. Where variances can be considered homogenous and n values are equal, the significance (p) of the F statistic is used. Where n values are equal but variances are not homogenous then the Brown-Forsyth statistic p value is used. Finally, when n values are unequal and variances not homogeneous the significance of the Welch statistic is employed. The Levene test also drives the selection of an appropriate post-hoc test to explore the significance of multiple paired comparisons between means. Where variances are homogenous the Tukey test is used, whilst the Tamhane test is employed when variances cannot be considered homogenous.
Bivariate linear regression was used to examine relationships between parameters where appropriate, the fitted function taking the form:
| (1) |
where:
Y is the dependent variable, X is the independent predictor variable, a is the intercept (Y at X = 0), b is the slope coefficient and U is the standard error of the estimate.
The strength of the relationship (proportion of variance in Y explained by X) was measured using the coefficient of determination, r2 (adjusted for degrees of freedom). The statistical significance of b provides an assessment of whether the slope is significantly different from zero.
The statistical significance of all tests was evaluated at the 95% confidence level.
3. Results
3.1. Designated sampling point monitoring
A total of 1303 samples were collected and analyzed from the 60 sampling days. Two results, one for each FIO parameter, were not reported due to analytical errors. No E. coli were recovered from 48 samples (3.7%) and no cIE from 116 samples (8.9%). Detection limit values were assigned to these samples for the purpose of statistical analysis. Descriptive statistics and normality tests showed that the raw FIO concentration distributions were positively skewed (skewness > 6) and demonstrated statistically significant departures from normality (S-W p < 0.05). Log10 transformation reduced skewness appreciably (<0.2), though the distributions still showed statistically significant departure from normality (S-W p < 0.05). Given the reduction in skewness, the FIO data were log10 transformed prior to further statistical analysis. Treating the log10 transformed FIO concentration data as parametric also allowed comparisons with classification systems such as the EU Directive (EU, 2006) and WHO guidelines (WHO, 2003).
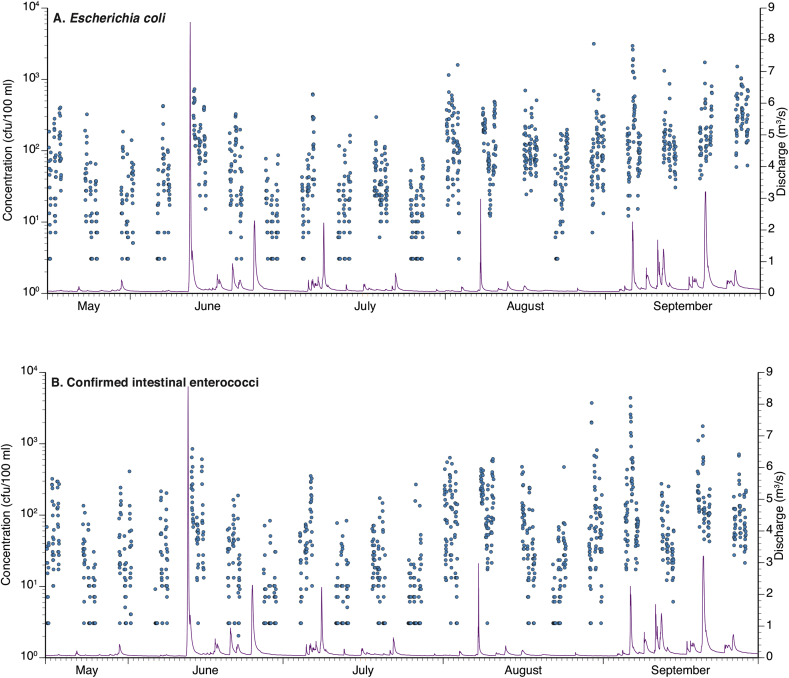
The FIO results for individual samples are shown in Fig. 2A and B. E. coli single sample concentrations ranged from <3 cfu/100 ml to 3100 cfu/100 ml (geometric mean (GM) 51 cfu/100 ml), whilst cIE single sample concentrations ranged from <2 cfu/100 ml to 4300 cfu/100 ml (GM 31 cfu/100 ml). The striking feature of this data set is the variation in FIO concentrations within individual days; a pattern that was continually repeated throughout the study period. For E. coli, the mean range of daily log10 concentrations was 1.34, ranging between 0.60 and 2.67. The corresponding mean range of daily log10 cIE concentrations was 1.43 and ranged between 0.48 and 3.08. Thus, on average, the mean daily variation in FIO concentrations exceeded 1 log10 order, with the largest daily variations exceeding 2 log10 orders.
Fig. 2.
Concentrations of: A. Escherichia coli and B. confirmed intestinal enterococci (colony forming units (cfu)/100 ml) (points) in marine water samples collected at Swansea Bay designated sampling point between 16/05/2011 and 28/09/2011 and discharge (m3/s) (line) at the Clyne River gauge (Fig. 1).
The plots also show the discharge record from the Clyne River, to the west of the DSP transect (Fig. 1), for comparison. There appears to be a general pattern of increased FIO concentrations and, thus, a decline in water quality, following hydrograph event conditions (Fig. 2A and B, Fig. 3A and B), although the size of the within-day variability, in log10 FIO concentrations, were consistently independent of antecedent rainfall and/or river flow (i.e. the principal predictor variables in short term pollution models deployed by UK and other regulatory agencies). Supplementary Material Fig. S1 contains detailed plots of FIO concentrations for the 60 days of sampling, whilst Fig. S2 presents results of corresponding turbidity and salinity analyses.
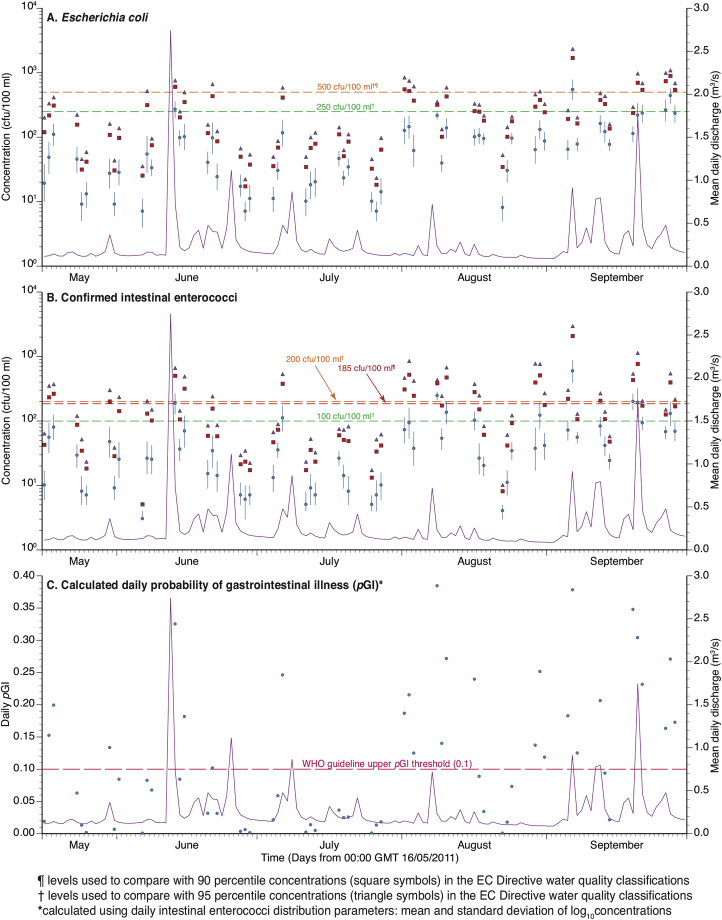
Fig. 3.
Geometric means (points), 95% confidence intervals (bars), 90th percentiles (square symbols) and 95th percentiles (triangle symbols) of A. Escherichia coli and B. confirmed intestinal enterococci concentrations (colony forming units (cfu)/100 ml) and C. calculated probability of gastrointestinal illness (pGI) (points) on 60 sampling days at the Swansea Bay designated sampling point during summer 2011. The plots also show: (i) the EC Directive levels used for comparison with 90th and 95th percentile values (Table 1) and (ii) mean daily discharge (m3/s) (line) at the Clyne River gauge (Fig. 1).
3.2. Variations in daily water quality and probability of gastrointestinal illness
The mean and SD for log10 FIO concentrations were calculated for each sampling day. The results are shown as GM values in Fig. 3A and B for E. coli and cIE, which also shows the corresponding 95% confidence intervals for each geometric mean. The plots show that the daily GM FIO concentrations varied considerably between days, but that the 95% confidence intervals (expressed as log10 values) remained similar and, perhaps surprisingly, appear independent of antecedent rainfall or river flow (This is further explored statistically and in Figs. 4 and 5).
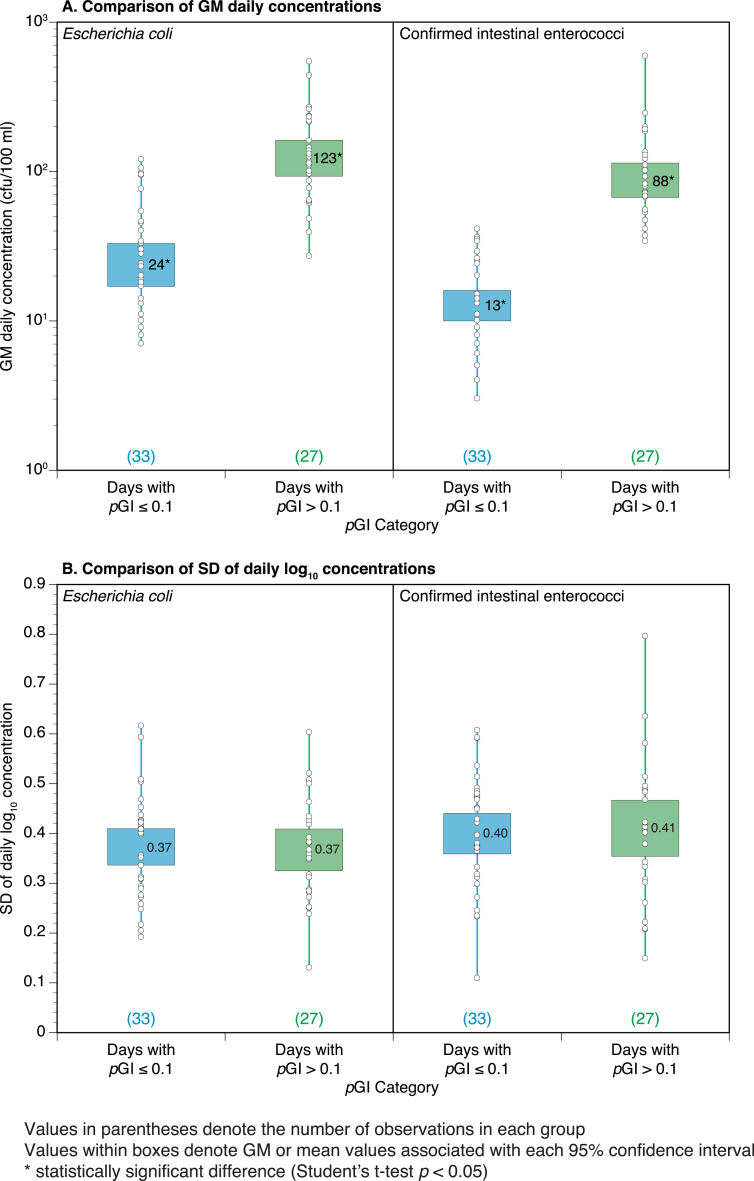
Fig. 4.
Box (95% confidence interval) and whisker (range) plots for comparisons of geometric mean (GM) daily faecal indicator organism (FIO) concentrations (colony forming units (cfu)/100 ml) and standard deviation (SD) of daily log10 FIO concentrations in groups of days (total = 60) categorized by daily probability of gastrointestinal illness (pGI, Fig. 3C) at the Swansea Bay designated sampling point during summer 2011.
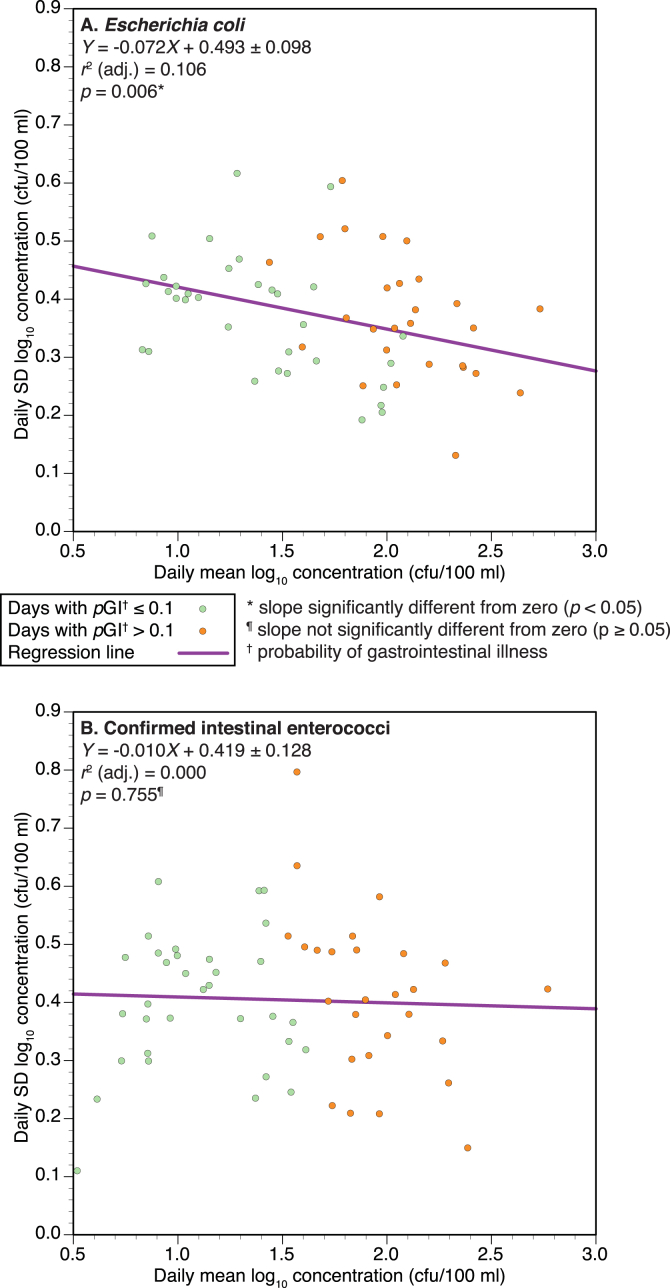
Fig. 5.
Bivariate relationships between daily mean log10 concentration (colony forming units (cfu)/100 ml) (X) and daily standard deviation (SD) of log10 concentrations (cfu/100 ml) (Y) of: A. Escherichia coli and B. confirmed intestinal enterococci on 60 sampling days at the Swansea Bay designated sampling point during summer 2011.
The plots also show the 90th and 95th percentile values used for comparison with EU Directive water quality standards, which were used to classify each day in terms of the percentile values employed in the EU (2006) standards (Table 1). At this monitoring site, the daily EU water quality classification is largely driven by cIE concentrations, with E. coli driving only three of the 23 ′Poor' overall outcomes. The results also demonstrate an apparent polarization of daily outcomes at Swansea Bay, with similar numbers of days in the EU ′Excellent' (42%) and 'Poor' (38%) categories. Of the remaining 12 days, six (i.e. 10% of 60 days) were classed as ‘Good’ and a further six were classed as ‘Sufficient’ (Table 1). The overall compliance outcome of this pattern is that Swansea Bay is considered 'at-risk' of failing to comply with the EU (2006) standards, with associated provisions for prohibition of bathing activities. It is, thus, critical to understand the factors affecting these observed daily EU Directive (2006) outcomes which have been uncovered by the intensive sampling undertaken in this study.
Table 1.
Classification of 60 sampling days at Swansea Bay designated sampling point during summer 2011 using the criteria of the European Bathing Water Directive (EU, 2006: Annex I and II, Pages 46 to 48).
| Category | Escherichia coli | Confirmed intestinal enterococci (cIE) | Overall |
|---|---|---|---|
| Excellenta | 33 | 25 | 25 |
| Goodb | 12 | 7 | 6 |
| Sufficientc | 6 | 8 | 6 |
| Poord | 9 | 20 | 23 |
95%ile E. coli ≤ 250 cfu/100 ml, 95%ile cIE ≤100 cfu/100 ml.
95%ile E. coli ≤ 500 cfu/100 ml, 95%ile cIE ≤200 cfu/100 ml.
90%ile E. coli ≤ 500 cfu/100 ml, 90%ile cIE ≤185 cfu/100 ml.
90%ile E. coli > 500 cfu/100 ml, 90%ile cIE >185 cfu/100 ml.
Where: cfu = colony forming units and the limit values are geometric, calculated using the mean and standard deviation (SD) of log10 concentrations:
The daily mean and SD of log10 cIE concentrations were also used to calculate the daily probability of gastroenteritis (pGI) values (Fig. 3C), as outlined in Wyer et al. (1999); Kay et al. (2004) and WHO (2003: Table 4.7 Page 70). The daily pGI values were then compared with the thresholds defined in the World Health Organization (WHO) guidelines for recreational waters, namely 1%, 5% and 10% pGI. Daily pGI values were variable, with 45% of days exceeding the upper pGI 0.1 (i.e. 10%) threshold, with a corresponding high risk of water associated GI (Table 2).
Table 2.
Classification of 60 sampling days at Swansea Bay designated sampling point during summer 2011 using probability of gastrointestinal illness (pGI) thresholds defined in the World Health Organization guidelines (WHO, 2003: Table 4.7 Page 70).
| Category | GI Risk | No. Days | % of Days |
|---|---|---|---|
| pGI < 1% | Negligible | 10 | 16.7 |
| pGI 1–5% | Low | 14 | 23.3 |
| pGI 5–10% | Moderate | 9 | 15.0 |
| pGI > 10% | High | 27 | 45.0 |
It should be noted that compliance with the EU standards and the associated WHO pGI thresholds do not correspond exactly. In the current study there were four more days with pGI > 0.1 than ‘Poor’ days classified using the EU (2006) standards. This is because: (i) the EU compliance assessment is based on both E. coli and cIE criteria and (ii) the original WHO percentile threshold values were derived assuming an SD of 0.8103 for log10 cIE concentrations (Kay et al. 2004). The data presented for Swansea Bay demonstrate that the daily SD can be much lower than this assumed value.
The daily results were split into two groups based on pGI, days with values > 0.1 (n = 27) and days with pGI ≤0.1 (n = 33). This split was chosen to facilitate exploration of any differences in variability or mean values for the FIO values on high and low health risk days. This pGI threshold is shown in Fig. 3C. The results are shown in Fig. 4 which presents box (in this case, 95% confidence intervals for the mean value) and whisker (presenting the range of values) plots. Unsurprisingly, the daily geometric mean FIO concentration associated with days with pGI >10% showed a clear elevation when compared to the days with pGI <10%. With such an obvious visual elevation (i.e. the lack of overlap in the 95% confidence intervals in Fig. 4A) and statistical significance testing may not furnish additional insight but, in this case, it was significant (Student's t tests p < 0.05). The average daily SDs for all 60 sampling days were: log10 E. coli = 0.37 and log10 cIE = 0.40. Thus, on average, the daily SD is approximately 0.4 log10 orders of magnitude, regardless of the grouping. However, it is also apparent that the absolute variability in concentrations will increase as the daily GM concentration increases. For example, taking the cIE results in Fig. 4A and applying a 0.4 log10 order of magnitude around the GM concentration would yield a range of 5–33 cfu/100 ml for the days with pGI ≤ 0.1 and 35 to 221 cfu/100 ml for the days with pGI > 0.1.
The relationships between daily mean log10 concentrations and corresponding daily SDs were further explored using regression analyses (Fig. 5), which models the change in within-day SD of log10 FIO concentrations (Y) against the mean daily log10 FIO concentrations (X). Both FIO plots exhibit a slight negative trend (i.e. Y decreases as X increases), although the slope is not significantly different from zero in the case of cIE (Fig. 5B).
These findings regarding the daily SD of log10 FIO concentrations, if they apply at other beaches, could be of potential interest with respect to water quality classification systems, which use the SD of log10 transformed FIO concentrations in their compliance calculations (e.g. EU (2006) Directive and WHO (2003) Guidelines criteria).
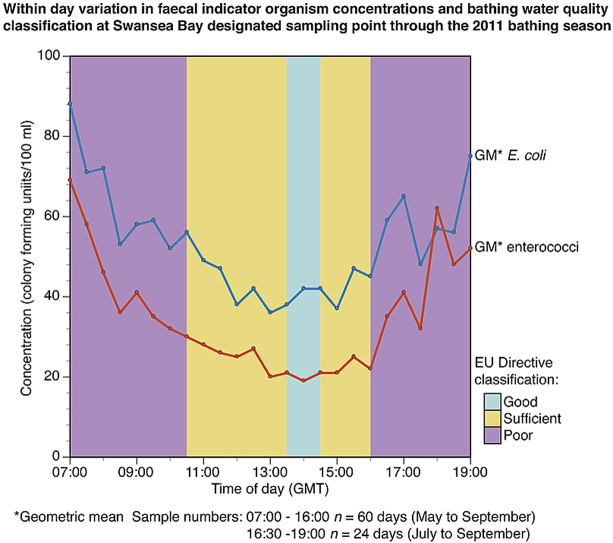
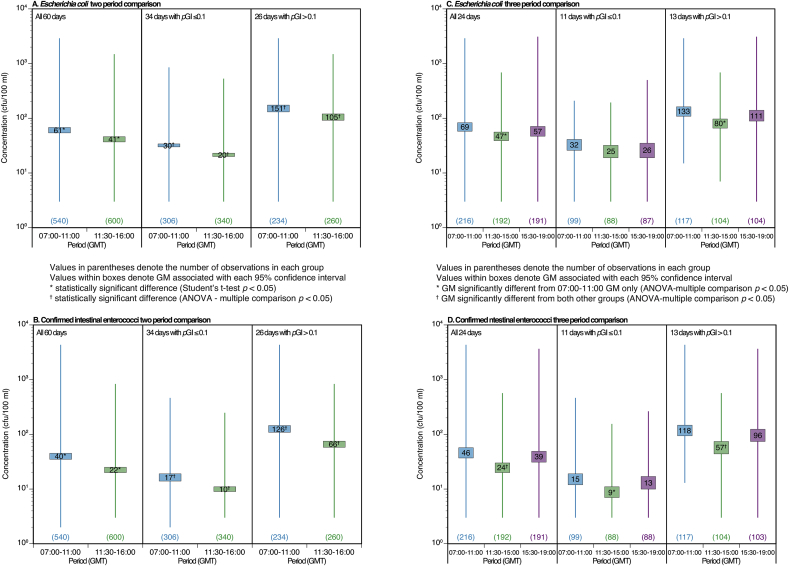
Fig. 6A and B presents GM FIO concentrations, their 95% confidence intervals and range for predominantly morning (07:00 to 11:00 GMT) and afternoon sample groups (11:30 to 16:00 GMT) for: (i) all 60 sampling days; (ii) the 34 days when pGI≤10%; and (iii) the 26 days when pGI>10%. In all cases, the predominantly afternoon samples have statistically significantly lower GMs than the predominantly morning samples. Fig. 6C and D provide a similar analysis using data from the 24 days during which sampling was extended to 19:00 comparing three time periods of 07:00 to 11:00: 11:30 to 15:00 and 15:30 to 19:00 again reported for the all 24 days and the days in the two pGI risk categories. In all cases, the early afternoon period exhibits the lowest GM concentration for both E. coli and cIE. Despite the relatively small number of sampling days available for the comparisons, the early afternoon GM is significantly different to the morning period GM for all comparisons except the comparison of GM E. coli concentrations for the days when pGI was ≤10% (Fig. 6C). In the case of cIE, the early afternoon GM is significantly lower than the GMs for both other periods when comparing data for all 24 days and the 13 days with pGI>0.1 (Fig. 6D).
Fig. 6.
Box (95% confidence interval) and whisker (range) plots for comparisons of geometric mean (GM) faecal indicator organism (FIO) concentrations (colony forming units (cfu)/100 ml) in samples collected from Swansea DSP during two periods within the day on 60 sampling days (07:00–11:00 GMT and 11:30–16:00 GMT (plots A and B)) and three periods within the day on 24 sampling days when sampling was extended to 19:00 GMT (07:00–11:00 GMT, 11:30–15:00 GMT and 15:30–19:00 GMT (plots C and D)). Results are shown for all sampling days and groups of days split by the daily probability of gastrointestinal illness (pGI - ≤ 0.1 and >0.1) calculated using the data for the relevant daily sampling period.
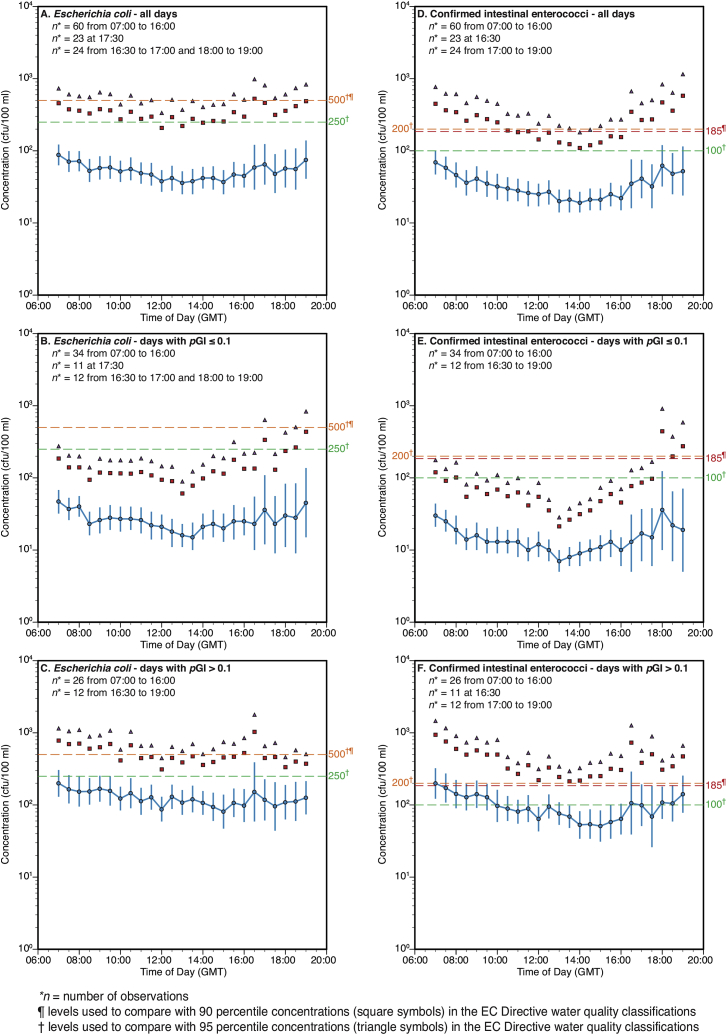
Fig. 7 presents the GM FIO concentrations in samples taken at each half-hour time point through the full study. The pattern for all days shows a steady decline through the morning and early afternoon, with the GM concentrations steadily increasing again in the late afternoon and evening, from 16:00 onwards. However, it must be noted that the post 16:00 values are based on smaller numbers of sample results as indicated by the wider confidence intervals shown in Fig. 7. On the days with pGI ≤10% this diurnal pattern is more subdued. For example, the daily maxima and minima for the cIE GM half hourly concentrations during the morning/early afternoon decline (Fig. 7 D, E and F) are:
-
(i)
all data - maximum 07:00 69 cfu/100 ml, minimum 14:00 19 cfu/100 ml;
-
(ii)
days with pGI≤0.1 - maximum 07:00 30 cfu/100 ml, minimum 13:00 7 cfu/100 ml;
-
(iii)
days with pGI>0.1, maximum 07:00 199 cfu/100 ml, minimum 15:00 51 cfu/100 ml.
Fig. 7.
Geometric means (points), 95% confidence intervals (bars), 90th percentiles (square symbols) and 95th percentiles (triangle symbols) of faecal indicator organism concentrations (colony forming units (cfu)/100 ml) by time of day at Swansea Bay designated sampling point during summer 2011 for all days (plots A and D) and groups of days with probability of gastrointestinal illness (pGI) ≤ 0.1 (Plots B and E) and >0.1 (Plots C and F).
Fig. 7 presents the full season's data and there is of course considerable variability in this diurnal pattern for individual days where, for example, rainfall events increase bacterial inputs and/or spring-neap tidal cycles vary the intertidal swept area of beach, which is a likely source of avian and possibly canine FIO loadings to the beach surface. Thus, a high between group (i.e. pGI ≤10% vs pGI >10%) variability is to be expected, as indicated in Fig. 3A, and this exceeds the within-day variability for the full data set.
3.3. Compliance implications of the daily patterns in microbial water quality
This apparent diurnal pattern has a major impact on the compliance level achieved over the bathing season during each hour. Table 3A presents the compliance levels for the two periods in Fig. 6A and B. The three periods in Fig. 6C and D are presented in Table 3B; and for each hour of the day, from 07:00 to 19:00, in Table 3C. One implication of the results in Table 3C is that, on average, a ‘Poor’ classification results from sampling before 11:00 or after 17:00 with compliance at the minimum (EU ′Sufficient' or better) level between 11:00 and 17:00 and a 'Good' classification if regulatory samples were taken between 14:00 and 15:00. However, the conclusions regarding the post 16:00 time period in Table 3C should be regarded tentatively due to the reduced number of sample results available for the 90th and 95th percentile calculations. The principal parameter driving the classifications in Table 3 is cIE, which tends to be a common observation at UK coastal bathing waters. It should be noted that these projected classifications use average data over the full 60-day (and 20 week) study period and, within specific hourly time intervals, a range of compliance outcomes will be experienced due to changes in FIO influx following, for example, storm hydrograph events.
Table 3.
Water quality classifications based on European Bathing Water Directive (BWD) criteria for Escherichia coli and confirmed intestinal enterococci (cIE) results from Swansea Bay designated sampling point during summer 2011 for two time period groupings and hourly time of day (EU, 2006: Annex I and II, Pages 46 to 48).
| A. Two period classification (60 days): | |||
|---|---|---|---|
| Time of day (GMT) | BWD E. coli classification | BWD cIE classification | Number of observations |
| 07:00–11:00 | Sufficient | Poor | 540 |
| 11:30–16:00 | Good | Sufficient | 600 |
|
| |||
|
B. Three period classification (24 days): | |||
|
Time of day (GMT) |
BWD E. coli classification |
BWD cIE classification |
Number of observations |
| 07:00–11:00 | Sufficient | Poor | 216 |
| 11:30–15:00 | Good | Sufficient | 192 |
| 15:30–19:00 | Sufficient | Poor | 191 |
|
| |||
|
C. Hourly classification (07:00–16:00–60 days, 16:30–19:00–24 days) | |||
|
Time of day (GMT) |
BWD E. coli classification |
BWD cIE classification |
Number of observations |
| 07:00 | Sufficient | Poor | 60 |
| 08:00 | Sufficient | Poor | 60 |
| 09:00 | Sufficient | Poor | 60 |
| 10:00 | Good | Poor | 60 |
| 11:00 | Good | Sufficient | 60 |
| 12:00 | Good | Sufficient | 60 |
| 13:00 | Good | Sufficient | 60 |
| 14:00 | Good | Good | 60 |
| 15:00 | Good | Sufficient | 60 |
| 16:00 | Sufficient | Sufficient | 60 |
| 17:00 | Sufficient | Poor | 24 |
| 18:00 | Sufficient | Poor | 24 |
| 19:00 | Sufficient | Poor | 24 |
Excellent: 95%ile E. coli ≤ 250 cfu/100 ml, 95%ile cIE ≤100 cfu/100 ml.
Good: 95%ile E. coli ≤ 500 cfu/100 ml, 95%ile cIE ≤200 cfu/100 ml.
Sufficient: 90%ile E. coli ≤ 500 cfu/100 ml, 90%ile IE ≤ 185 cfu/100 ml.
Poor: 90%ile E. coli > 500 cfu/100 ml, 90%ile cIE >185 cfu/100 ml.
Where: cfu = colony forming units and the limit values are geometric, calculated using the mean and standard deviation (SD) of log10 concentrations:
4. Discussion
The empirical data collected in this study at the designated bathing water monitoring site in Swansea Bay UK (i.e. half hourly samples through 60 bathing days in 2011) fully supports the US assessments of Boehm et al. (2002), Wymer et al. (2007) and the USEPA 2010a,b), that there is considerable within-day variability in FIO concentrations at compliance monitoring sites both on days when antecedent weather produces increased FIO flux to the bathing water but also, surprisingly, when antecedent meteorological conditions are more quiescent. In the study reported above, this typically spanned 1–2 log10 orders within each day of sampling. It is clear, therefore, that a single compliance sample should not be treated as representative of the bathing day water quality even on the day of sample collection. The application of consistent compliance sampling times through the bathing season could drive the compliance outcome at any bathing water that exhibits this type of diurnal pattern of water quality (see Table 3C). However, at present, it is not clear whether this observed, and extreme, within-day variability is a generic observation for all bathing waters or is a characteristic of Swansea Bay and similar urbanized UK bathing waters. An alternative compliance sampling approach worth attention would be to vary the time of sampling (ideally randomized), through adjustment of sampling runs where they cover multiple bathing waters. This approach should provide data more representative of the bathing day. There is, therefore, an urgent need; to both assess: (i) within-day variability at other sites and, importantly; (ii) to explore within-day prediction modelling.
5. Conclusions
-
1.
Significant within-day variability has been observed in this urbanized UK bathing water which suggests that a single compliance sample should not be used to characterise bathing water quality on the bathing day. It remains to be seen whether similar conclusions can be made for other UK bathing waters (particularly more rural resorts) and indeed whether these observations have wider international application.
-
2.
Thus, the use of compliance data to build and calibrate prediction models, which forecast a single value for water quality on the bathing day and are used as a means of public health protection for bathers should be examined to ensure that the predicted water quality provides an adequate representation of health risk throughout the bathing day.
-
3.
Where bathing water FIO concentration exhibits the extreme variability reported in this paper, research and management attention should focus on delivery of accurate within-day prediction of bathing water microbial quality. This could allow warnings for potentially short time periods, as is commonly done for adverse tidal conditions, which are often marked, in the UK, by red flags erected by lifeguards at the water's edge.
-
4.
Such an approach would require intensive sampling to drive model building and testing. Whilst this would be relatively expensive, it could improve both regulatory compliance and public health protection. In addition, it could also offer a cost-effective solution for many 'at-risk' (in EU Directive terms 'Sufficient') bathing waters, like Swansea Bay in Wales, which has not improved over many years despite expenditures of sewerage and other improvements of over £100 m UK£.
-
5.
If the diurnal pattern observed at Swansea Bay is a generic observation, it would be wholly inappropriate for compliance sampling programme design to be directed to achieve higher compliance levels by choosing the most beneficial time of day for sample collection. We would hope that regulatory authorities would choose either the precautionary approach of Boehm et al. (2002) and USEPA (2010b) of early morning sampling, or seek to characterise the bathing day when most bathers are present in the water by more nearly random sampling during this period (allowing for tidal factors and sample transport logistics).
Declarations of Interest
None
Acknowledgements
This work, under the project titled 'Smart Costs Sustainable Communities', was funded by the European Union, Ireland-Wales Interreg programme with additional inputs from the Swansea Council, Natural Resources Wales, Dŵr-Cymru/Welsh Water and Aberystwyth University. Parallel research was conducted under the same grant by University College Dublin. We are grateful to all our partner organisations and particularly to the staff of the Welsh European Funding Office managing the Ireland-Wales Programme and their desk officer Mr Patrick Lilley, who gave generously of his time and advice throughout. Related work has been awarded further funding in the 2014–2020 EU Interreg programme entitled 'Acclimatize'. We are grateful to Professor Stephen Tooth who offered useful comment and suggestions on an early draft of this paper. We are grateful to the three reviewers who made perceptive and detailed points which have significantly enhanced the clarity of this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.wroa.2018.10.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bedri Z., Corkery A., O'Sullivan J.J., Deering L.A., Demeter K., Meijer W.G., O'Hare G., Masterson B. Evaluating a microbial water quality prediction model for beach management under the revised EU Bathing Water Directive. J. Environ. Manag. 2016;167:49–58. doi: 10.1016/j.jenvman.2015.10.046. https://doi/org/10.1016/j.marpolbul.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Boehm A.B., Grant S.B., Kim J.H., Mowbray S.L., McGee C.D., Clark C.D., Foley D.M., Wellman D.E. Decadal and shorter period variability of surf zone water quality at Huntington beach, California. Environ. Sci. Technol. 2002;36(18):3885–3892. doi: 10.1021/es020524u. [DOI] [PubMed] [Google Scholar]
- Boehm A.B. Enterococci concentrations in diverse coastal environments exhibit extreme variability. Environ. Sci. Technol. 2007;41(24):9227–9232. doi: 10.1021/es071807v. [DOI] [PubMed] [Google Scholar]
- Crowther J., Kay D., Wyer M.D. Relationships between microbial water quality and environmental conditions in coastal recreational water: the Fylde coast, UK. Water Res. 2001;35(17):4029–4038. doi: 10.1016/s0043-1354(01)00123-3. [DOI] [PubMed] [Google Scholar]
- DCWW (2018) Dŵr-Cymru/Welsh Water. Pers. Comm. From the Coastal Waters Lead Scientist Mr N. Barcock, Email 26th January 2018, Outlining Estimated Costs of Bathing Water Improvements in Swansea Bay.
- EFTEC (2002) reportValuation of Benefits to England and Wales of a Revised Bathing Water Quality Directive and Other Beach Characteristics Using the Choice Experiment Methodology. Final report submitted to UK Department for Environment, Food and Rural Affairs. Economics for the Environment Consultancy Ltd. 97 Pages. Available from::https://ukbeachmanagementforum.files.wordpress.com/2014/03/defra-beach-user-study-2002.pdf
- EU European union directive 2006/7/EC, of the European parliament and of the Council of 15 february 2006 concerning the management of bathing water quality and repealing directive 76/160/EEC. Official Journal of the European Union. 2006;4(3) 2006, L64/L37. [Google Scholar]
- FEE (2018) Foundation for Environmental Education. http://www.blueflag.global/accessed 12/07/2018.
- Fleisher J.M., McFadden R.Y. Obtaining precise estimates in coliform enumeration. Water Res. 1980;14:477–483. [Google Scholar]
- Francy D.S., Stelzer E.A., Duris J.W., Brady M.G., Harrison J.H., Johnson H.E., Ware M.W. Predictive models of Escherichia coli concentrations in inland lake beaches and relationship of model variables to pathogen detection. Appl. Environ. Microbiol. 2013;79(5):1676–1688. doi: 10.1128/AEM.02995-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D., Bartram J., Pruss A., Ashbolt N., Wyer M.D., Fleisher J.M., Fewtrell L., Rogers A., Rees G. Derivation of numerical values for the World Health Organization guidelines for recreational waters. Water Res. 2004;38:1296–1304. doi: 10.1016/j.watres.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Lusic D.V., Kranjcevik L., Macesic S., Lusic D., Jozic S., Linsak Z., Bilajac L., Grbcic, Neiro B. Temporal variations analyses and predictive modeling of microbiological seawater quality. Water Res. 2017;119:160–170. doi: 10.1016/j.watres.2017.04.046. [DOI] [PubMed] [Google Scholar]
- McPhail C., Stidson R. Bathing water signage and predictive water quality models in Scotland. Aquat. Ecosys. Health Manag. 2009;12(2):183–186. doi: 10.1080/14634980902907540. [DOI] [Google Scholar]
- Nevers M.B., Whitman R.L. Nowcast modelling of Escherichia coli concentrations at multiple urban beaches of Southern Lake Michigan. Water Res. 2005;39:5250–5260. doi: 10.1016/j.watres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Olyphant G.A. Statistical basis for predicting the need for bacterially induced beach closures: emergence of a paradigm? Water Res. 2005;39:4953–4960. doi: 10.1016/j.watres.2005.09.031. [DOI] [PubMed] [Google Scholar]
- SCA (2009). Standing Committee of Analysts. The Microbiology of Drinking Water - Part 4 -Methods for the Isolation and Enumeration of Coliform and Escherichia coli (Including E. coli O157:H7). Methods for the Examination of Waters and Associated Materials. Environment Agency, Bristol UK, 101pp.
- SCA (2011). Standing Committee of Analysts. The Microbiology of Recreational and Environmental Waters (2011) – Part 3 - Methods for the Isolation and Enumeration of Coliform and Escherichia coli (Including E. coli O157:H7). Methods for the Examination of Waters and Associated Materials. Environment Agency, Bristol UK, 111pp.
- SCA (2012). Standing Committee of Analysts. The Microbiology of Drinking Water - Part 5 - Methods for the Isolation and Enumeration of Enterococci. Methods for the Examination of Waters and Associated Materials. Environment Agency, Bristol UK 31pp.
- SPSS 19 (2010). IBM SPSS Statistics Brief Guide. 161pp SPSS inc.
- Stidson R.T.C.A., Gray C.A., McPhail C.D. Development and use of modelling techniques for real-time bathing water quality predictions. Water Environ. J. 2012;26:7–18. doi: 10.1111/j.1747-6593.2011.00258.x. [DOI] [Google Scholar]
- Thoe W., Gold M., Griesbach A., Grimmer M., Taggart M.L., Boehm A.B. Predicting water quality at Santa Monica beach: evaluation of five different models for public notification of unsafe swimming conditions. Water Res. 2014;67:105–117. doi: 10.1016/j.watres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- USEPA (2010a). United States Environmental Protection Agency. Predictive Tools for Beach Notification: Review and Technical Protocol. Predictive Modeling at Beaches volume I. EPA-823-R-10e003 and Predictive Tools for Beach Notification Volume II EPA-600-R-10-176 Available from: https://www.epa.gov/beach-tech/models-predicting-beach-water-quality
- USEPA (2010b). United States Environmental Protection Agency. Sampling and Consideration of Variability (Temporal and Spatial) for Monitoring of Recreational Waters. EPA- 823-R-10e005. Available from: https://www.epa.gov/sites/production/files/2015-11/documents/sampling-consideration-recreational-waters.pdf.
- USEPA (2012) Recreational Water Quality Criteria. U.S. Environmental Protection Agency, Washington, DC, 820-F-12-058. https://www.epa.gov/wqc/2012-recreational-water-quality-criteria See also: https://www.epa.gov/wqc/five-year-review-2012-recreational-water-quality-criteria see also
- USEPA (2018). United States Environmental Protection Agency. Virtual Beach. https://www.epa.gov/exposure-assessment-models/virtual-beach-vb.
- WHO (1999) Health Based Monitoring of Recreational Waters: the Feasibility of a New Approach (The Annapolis Protocol). WHO/SDE/WSH/99.1, Geneva. http://www.who.int/water_sanitation_health/bathing/Annapolis.pdf
- WHO (2003) Guidelines for Safe Recreational Water Environments Volume 1: Coastal and Freshwaters. World Health Organisation, Geneva. http://www.who.int/water_sanitation_health/publications/srwe1/en/
- Wyer M.D., Kay D., Fleisher J., Jackson G., Fewtrell L. An experimental health-based standard system for marine waters. Water Res. 1999;33:715–722. doi: 10.1016/j.watres.2006.02.009. [DOI] [Google Scholar]
- Wymer, L., Dufour, A. and McGee, C. (2007). Temporal variability of microbial indicators of faecal contamination of marine and freshwater beaches. Presented at American Society for Microbiology General 107th Meeting, Toronto, CANADA, May 21-25th.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.