Abstract
The majority of unsymmetrical dimethylhydrazine (UDMH) treatments produce lots of toxic by-products, among which N-Nitrosodimethylamine (NDMA) is a strong carcinogen. The compositions of the by-products are important for evaluating the treatment efficiency and understanding the UDMH degradation mechanism to achieve UDMH mineralization. The intermediate and end products of UDMH treatment with different oxidants were investigated by using a simple and fast method, solid-phase micro-extraction (SPME) in combination with gas chromatography–mass spectrometry (GC-MS). The effects of several parameters (coating fibre, salt addition, pH, sampling time and desorption time) were studied to optimize analyte recovery. The best response can be attained by the 65 µm PDMS/DVB fibre at pH 7 during 10 min after desorption of 1 min in the GC inlet. The intermediate and final oxidative products of UDMH wastewater treatment with different oxidants (O3, Mn2+/O3, Fe2+/H2O2) were investigated. The results showed that the UDMH treatment with O3 could lead to high yields of NDMA. Metal catalytic ozonation could largely minimize the formation of NDMA. No NDMA was observed in the final decontaminated samples after treatment with Fe2+/H2O2. The NDMA formation and degradation mechanism were discussed based on the intermediates. This study is expected to provide useful information for controlling NDMA formation during UDMH wastewater treatment.
Keywords: unsymmetrical dimethylhydrazine, degradation oxidation products, solid-phase micro-extraction, gas chromatography–mass spectrometry
1. Introduction
Unsymmetrical dimethylhydrazine (UDMH) has been widely used as a high-energy propellant for military applications. UDMH is known as a primary eco-toxicant with the maximum permissible concentration in water as low as 0.02 mg l−1 [1]. The higher health risks are associated with the rapid formation of a number of dangerous transformation products [2]. An important aspect of this problem is the wide use of different oxidants (hydrogen peroxide/copper sulfate (Cu2+/H2O2) [3–5], potassium permanganate [6], chlorine reagents [7], ozone [8–11] and Fenton's reactant [12–14]) for detoxification of the wastewater containing UDMH. However, these methods produce lots of toxic by-products. Hence, much attention has been paid to the degradation products of UDMH. The reported data on the compositions of UDMH after the treatment of polluted water with different reagents are summarized in table 1. The structural formula of the products is shown in figure 1. They contain N-Nitrosodimethylamine (NDMA), formaldehyde dimethylhydrazone (FDMH), tetramethyltetrazene (TMT), formaldehyde methylhydrazone (FMH), dimethylamine (DMA), formic acid (HCOOH), methanol (CH3OH), acetic acid (CH3COOH), nitromethane (NM), N,N-dimethylformamide (DMF), 1,1,5-trimethylformazane (TMFN), acetaldehyde dimethylhydrazone (ADMH), and formaldehyde (HCHO). It is notable that NDMA is an ineluctable by-product of most methods, which is a strong carcinogen with a cancer risk level for drinking water 0.7 ng l−1. An acceptable destruction scheme must eliminate UDMH without forming other toxic products. The efficiency of oxidants proposed for detoxification has also been estimated mainly based on the composition of UDMH degradation products. Moreover, the UDMH degradation mechanism and NDMA formation mechanism are important for NDMA controlling. The knowledge about the compositions of the intermediates and the end products of UDMH degradation is useful for NDMA formation and reduction mechanism. So, it is necessary to investigate the compounds of UDMH with different reagents.
Table 1.
Summary of UDMH degradation products via different oxidants in water.
| O3 | KMnO4 | Cu2+/H2O2 | Fe2+/H2O2 | chlorine reagents | cavitation | |
|---|---|---|---|---|---|---|
| NDMA | + | + | + | + | + | |
| FDMH | + | + | ||||
| TMT | + | + | + | |||
| FMH | + | + | ||||
| DMA | + | |||||
| HCOOH | + | + | ||||
| CH3OH | + | + | + | + | ||
| CH3COOH | + | + | ||||
| NM | + | + | ||||
| DMF | + | + | + | + | ||
| TMFN | + | |||||
| ADMH | + | |||||
| HCHO | + | + |
Figure 1.
The structural formula of the products in table 1.
Numerous investigations have been carried out regarding the environmental transformation effects of UDMH in water and in soil. Various analytical methods have been carried out, such as the high-performance liquid chromatography (HPLC)-MS method [15–17], gas chromatography–mass spectrometric (GC-MS) method [18–21], and solid-phase micro-extraction (SPME)-GC-MS method [22,23]. The GC-MS method provides sufficient lower detection limits for most UDMH transformation products compared to HPLC-MS methods. SPME combines sampling and sample preparation into one step. SPME is demonstrated to be rapid, simple and reproducible, with no solvent use. Moreover, SPME coupled with GC-MS provides high sensitivity. It has proven very useful for screening of UDMH transformation products in water. In addition, investigations on the intermediate and final products of UDMH are favourable to the understanding of the toxic by-product formation and degradation mechanism.
The first goal of this research was to build a multianalyte method for a simple and sensitive qualification of oxidation products in as many treated water simples as possible based on SPME in combination with GC-MS. The effects of several sampling and sample preparation parameters were studied. The method was applied in identification of the intermediate and end products of UDMH solutions before and after treatment with O3, Mn2+/O3 and Fenton reagents. The NDMA formation and destruction mechanisms have been discussed. The results of this study are expected to provide useful data for controlling NDMA formation during UDMH wastewater treatment (figure 1).
2. Experimental
2.1. Materials
UDMH (98%, Henan Liming Chemical Reagent Co. Ltd. China), FDMH (99%, Tianjin Heowns Biochemical Co. Ltd. China), CH3NO2 (98%, Tianjin Heowns Biochemical Co. Ltd. China), TMT (99%, Tianjin Heowns Biochemical Co. Ltd. China), Dimethylamino-Acetonitrile (MSDS) (99%, Tianjin Heowns Biochemical Co. Ltd. China), 1-methyl-1,2,4-triazole (MT) (99%, Tianjin Heowns Biochemical Co. Ltd. China), DMF (99%, Tianjin Heowns Biochemical Co. Ltd. China), CH3OH (99%, Tianjin Heowns Biochemical Co. Ltd. China), CH3COOH (88%, Tianjin Heowns Biochemical Co. Ltd. China), HCHO (37.0%–40.0%, Tianjin Heowns Biochemical Co. Ltd. China), DMA (2.0 M in Methanol, Shanghai Mackin Chemical Co. Ltd. China), NDMA (1000 µg l−1 in Methanol, Accustandard Co. Ltd. USA), NaCl (Analytical grade, Sinopharm Chemical Co. Ltd. China), Fe2SO4 · 7H2O (analytical grade, Sinopharm Chemic al Co. Ltd. China) and H2O2 (30%, Sinopharm Chemical Co. Ltd. China) were commercially available. Ozone was generated by a laboratory ozonizer with an oxygen source (ModelCFK-3, Hangzhou Rongxin Electronic Equipment Co. Ltd. China).
In all of the experiments, deionized water was used to prepare the solutions. Measurement of the pH was done by a pH meter (PHSJ-4A, Shanghai Yidian Scientific Instrument Co. Ltd. China). The degradation samples were treated with an SPME device (85 µm SPME needle, USA) and were analysed by a GC/MS (Agilent 7890A-5795C, USA).
2.2. Reaction procedure
UDMH ozonation procedure: 200 ml of UDMH (1 g l−1) was added to the reaction chamber. Then, the reaction chamber was flushed with ozone (the production of ozonizer, 3 g h−1).
UDMH ozonation with catalyst Mn2+ procedure: 200 ml of UDMH (1 g l−1) and 0.755 g of MnSO4 were added to the reaction chamber in order. Then, the reaction chamber was flushed with ozone.
UDMH oxidation by Fenton reagents (Fe2+/H2O2) procedure: 100 ml of UDMH (1 gl−1), 4.08 ml of 30% H2O2, 7.8 ml of FeSO4 · 7H2O (0.5 mol l−1) were added to the reaction chamber in that order. An appropriate amount of distilled water was added to make a volume of 200 ml, so that the concentration of dimethyl hydrazine in the flask was 1000 mg l−1. At 20 min, 50 ml of the reaction solution was filtered and analysed. And the reaction mixture was added with 30% H2O2 (3.06 ml) and FeSO4 · 7H2O (5.85 ml).
Each experiment was repeated three to five times.
2.3. Samples
Fresh samples were prepared by mixing 50.0 ml of water with 100 µl of a standard solution of analytes in a 100 ml vial followed by sealing of the vial and agitation for 30 min. The fresh samples contained 12 oxidative products of UDMH.
The reaction samples were filtered at different times. All SPME fibres were conditioned in the GC inlet at 200°C before use. SPME-based sample desorption was carried out at 200°C for 30 min in the splitless mode. After adjusting the pH of the solution following the reaction, 8 ml of the sample was placed into a 10 ml beaker, and sodium chloride (NaCl) was added. The small beaker was placed on a magnetic stirrer. After the sodium chloride was completely dissolved, the SPME fibre was immersed in the solution with different times for adsorption equilibrium. Finally, the SPME fibre was inserted into the GC inlet by the SPME syringe.
2.4. Parameters of GC-MS analyses
The optimal injector temperature was 200°C. The separation was performed on a DB-225S (30 m × 250 µm × 0.25 µm) capillary column. The column was maintained at a constant flow of UHP-grade helium at 1.5 ml min−1. The GC oven programme was as follows: 35°C for the first 5 min, followed by 10°C min−1 ramping to 15°C. The split ratio was 1 : 1.
The MS was operated in the full scan mode from 19 to 550 m/z. The ion source was held at 230°C and the electron multiplier was set at 70 eV.
2.5. Statistical analysis
The peak area was calculated by an RET integrator. The minimum integral is 0.1 of the maximum area, which is used as the integral parameter to ensure that the peak with a smaller area can be effectively integrated. Repeatability of the applied method was confirmed by the analysis of selected samples in triplicate. RSDs for replicates did not exceed 15%.
3. Results and discussion
3.1. Optimization of the sampling condition
The objective of this chapter is to screen UDMH in as many degradation oxidative products as possible and to obtain the best responses based on SPME-GC/MS. The effects of several sampling and sample parameters were studied. These involved SPME fibre coating type, effects of added salt, SPME sampling pH and time and optimization of GC injection/desorption.
3.1.1. Selection of SPME fibre coating
Polymeric coatings used in SPME vary in their layer materials and thicknesses, which affect SPME sensitivity towards specific chemical groups of compounds. The main coating materials, which are polydimethylsilane (PDMS), divinylbenzene (DVB), polyethylene glycol (CW), polyacrylate (PA) and activated carbon (Carboxen, CAR), are currently widely used. The most suitable SPME fibre coating for sampling of UDMH and its degradation products from water were selected from 65 µm PDMS/DVB, 85 µm CAR/PDMS, 85 µm PA and 100 µm PDMS tested for identifying the main UDMH degradation oxidative products. Extractions with SPME were carried out from the samples at 30 min. As shown in figure 2, the best response and range of extracted chemicals were obtained by applying the 65 µm PDMS/DVB.
Figure 2.
The response to the fresh sample with four different SPME fibres.
There were 12 kinds of UDMH and their oxidative products (DMA, FDMH, CH3OH, UDMH, NM, TMT, CH3COOH, MSDS, NDMA, DMF, MT, HCHO) in the fresh sample. Except for HCHO, they could all be detected. The identified transformation products are listed in table 2. Therefore, a 65 µm PDMS/DVB fibre was selected for further studies. Because the peak shapes of DMA, CH3OH and CH3COOH were unstable and poor, others were selected for further studies.
Table 2.
The compositions detected by SPME-GC/MS.
| product | retention time | CAS no | Pro. (%) |
|---|---|---|---|
| DMA | 1.053 | 124-40-3 | 78.5 |
| FDMH | 2.304 | 2035-89-4 | 80 |
| CH3OH | 2.731 | 67-56-1 | 87.5 |
| UDMH | 3.643 | 57-14-7 | 96.7 |
| NM | 4.429 | 75-52-5 | 97.8 |
| TMT | 6.610 | 6130-87-6 | 70.5 |
| CH3COOH | 6.813 | 2035-89-4 | 91.4 |
| MSDS | 7.830 | 924-64-7 | 97.4 |
| NDMA | 9.430 | 62-75-9 | 90.8 |
| DMF | 9.945 | 68-12-2 | 80.8 |
| MT | 11.503 | 6086-21-1 | 88 |
3.1.2. Effects of salt addition
The reports [2,23] showed that the water content in soil plays a significant role in the recovery of all UDMH transformation products and makes the quantification process difficult or even impossible. Salt was used to improve sample recovery, because the addition of salt could enhance the amount extracted by the fibre [24]. To investigate the effects of salt on the extraction efficiency of UDMH and its oxidation products, the different salt (NaCl) concentrations (0%, 10%, 20%, 30%, 40%) were selected to add to the samples. The results are shown in figure 3.
Figure 3.

Effect of salt concentration on extraction.
The amount of salt added resulted in increased recovery of almost all analytes. The increased recovery was observed when salt concentration increased from 10% to 30% while at a salt concentration of 40% (an oversaturation solution of NaCl) the recovery decreased. It was obvious that the peak recovery occurred with the salt concentration of NaCl (30%). The salt concentration of NaCl (30%) was therefore chosen to prepare the samples.
3.1.3. Effects of pH
pH plays a significant role in compound distribution between cation and neutral molecule, which affects the extraction effect. So the effect of pH on the peak area of degradation products was studied at 5.0, 7.0 and 10.0. The results are presented in figure 4.
Figure 4.
Effect of pH on extraction efficiency.
As we can see from figure 4, TMT could not be observed and the response of UDMH was poor at pH 5.0. The recovery of UDMH and its oxidative products at pH 7.0 was similar to that at pH 10.0. Generally, the solution is under neutral condition at pH 7.0. Thus, SPME at pH 7.0 provides the best combination of convenience and sensitivity.
3.1.4. Effects of SPME sampling time
SPME is often used for equilibrium sampling, resulting in an equilibrium between the analytes in the aqueous phase and the fibre, and also including any other matrix capable of binding the analyte. The extraction time was determined based on sufficient response and reproducibility within a practical time frame. Several sampling times (1 min, 5 min, 10 min, 20 min and 30 min) were studied to determine the practical SPME sampling time. Mass detector responses versus SPME sampling time are presented in figure 5. The increase of peak area with the increase of sampling time was observed for all the analytes. No significant difference in the area counts was found ranging from 10 to 30 min. The extraction time was, therefore, chosen to be 10 min which resulted in sufficient signal and precision for all the detected UDMH oxidation products.
Figure 5.

Effect of SPME sampling time on extraction.
3.1.5. Optimization of SPME desorption time
Studies have shown that excessive desorption time will cause the fibre coating to degrade slowly in a high-temperature environment, affecting the service life of the extracted fibre. But, too short a desorption time cannot fully release the target component, resulting in reduced desorption efficiency and material residue.
In this experiment, different desorption times (10 s, 30 s, 1 min, 2 min and 5 min) were selected for the experiment. The results are shown in figure 6. The extension of desorption time has little effect on the mass detector responses. Moreover, after the secondary desorption of the extracted fibres, it was found that the six substances did not re-peak again, which indicated that when the GC inlet temperature was set to 250°C, the eight kinds of odours adsorbed on the fibres were obtained using the desorption time of 1 min. The substance is completely desorbed. Therefore, the optimal SPME desorption time is 1 min.
Figure 6.

Effect of SPME desorption time on extraction.
3.2. MDL of this method
Generally, the revised definition of the method detection limit (MDL) [25] is an estimate of the measured concentration on which there is 99% confidence that a given analyte is present in the given sample matrix. Here, the MDL is roughly determined by
| 3.1 |
where ki is the confidence factor, generally 3, si is the standard deviation of the sample measurement reading. c is a sample content value and is the average value of sample measurement readings.
As shown in table 3, SPME-GC/MS allowed the MDL of UDMH, NM, DMF and MT to be as low as 9.3 mg l−1, 3.3 mg l−1, 5.0 mg l−1 and 7.1 mg l−1, respectively. The MDL of FDMH and NDMA is as low as 20 µg l−1. The MDL of TMT as low as 6.9 µg l−1.
Table 3.
MDL of UDMH and its degradation products.
| compound | peak area | average value | Si | MDL (mg l−1) |
|---|---|---|---|---|
| UDMH | 11083274 10562878 11843866 10025687 11568974 10025698 12365897 11044562 |
11065104.5 | 842288.8 | 9.3 |
| FDMH | 14435654 15579654 16129543 15388590 15532927 13140917 14435654 16564560 |
15150937.4 | 1095812.3 | 0.021 |
| TMT | 106618338 139934000 122036490 130536890 104620609 100700160 121498760 115608923 |
117694271.3 | 13520422.8 | 0.0069 |
| NM | 7001567 7750996 7713547 6464465 7128949 7402588 7458925 7546321 |
7308419.75 | 401052.7 | 3.3 |
| NDMA | 6498546 7386548 6895268 7025864 6985474 6854956 7056982 7165832 |
6983683.75 | 257010.1 | 0.011 |
| DMF | 2711471 2904413 2714194 3270874 3349445 2746290 2918300 2895645 |
2938829 | 245401.7 | 5.0 |
| MT | 1872495 1974081 1927596 2342147 2435296 2557846 2114088 2041569 |
2158139.8 | 254836.0 | 7.1 |
3.3. Identification of the products of the treated water
Here, we selected three oxidative reagents (O3, Mn2+/O3, Fe2+/H2O2) to treat UDMH wastewater. The compositions of the fresh UDMH solutions and solutions after treatment with oxidative reagents were analysed using GC-MS based on SPME.
3.3.1. O3
Figure 7 shows the composition of extracts from fresh UDMH solutions with a concentration of 1 g l−1 after being treated with ozone.
Figure 7.

Gas chromatogram of an extraction of a fresh UDMH solution (1 g l−1) treated with O3 at different times.
It follows from figure 7 that there was no UDMH in the extract after purification within 20 min. The extract also contained DMA, FDMH, TMT, MSDS, NDMA and DMF. After treatment with 6 h, the main product was NDMA, which was higher than the samples at 20 min. Others are DMA, FDMH, DMF and NM. It was discovered that after treatment with ozone, the solution contained no UDMH but did contain high yields of NDMA, which poses a high risk for human beings. This implies that O3 plays an important role in NDMA formation from UDMH.
The NDMA formation mechanism during wastewater ozonation has attracted much attention [10,26–28]. Transformation of UDMH to NDMA is mainly induced by ozone or HO· rather than the dissolved oxygen proposed previously. Pisarenko et al. [28] found that wastewater with higher HO· scavenging may also lead to higher NDMA formation during ozonation. Radiolysis experiments [28] were performed to isolate the contribution of hydroxy radicals. During radiolysis experiments, there was no NDMA formation observed in any of the wastewater samples. The results of these experiments confirm that NDMA formation is due to reactions associated with molecular ozone. Our results are consistent with these results. Hence, the NDMA formation mechanism was proposed by the following:
 |
3.2 |
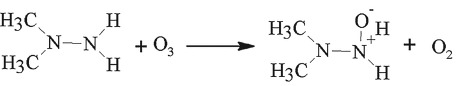 |
3.3 |
 |
3.4 |
 |
3.5 |
The reaction of ozone with UDMH is thought to proceed via H-abstraction from −NH2 or via O-addition to −NH2 and the UDMH intermediate or UDMH radical form. These intermediates could be through further H-atom abstraction or O-atom addition and finally form NDMA.
3.3.2. Mn2+/O3
Catalytic ozonation has recently gained significant attention as an effective process used for organic removal from water [29,30]. Among the types of metal catalysis ozonation that have been applied to homogeneous catalysis, Mn2+ has gained significant attention because of its excellent catalytic performance, with many studies focused on the degradation effects of organic pollutants [31,32]. This research used Mn2+ as a catalyst and investigated the oxidative products of UDMH after treatment by the Mn2+/O3 system. The results are shown in figure 8.
Figure 8.

Gas chromatogram of an extraction of a fresh UDMH solution (1 g l−1) before and after treatment with Mn2+/O3 at different times.
When the UDMH was treated with Mn2+/O3 after 20 min, the UDMH was almost consumed and significant amounts of TMT formed. When the UDMH was treated after 6 h, the significant amounts of TMT were almost consumed. The solutions still contained NDMA and DMA.
The oxidative degradation products are different from those of UDMH ozonation. In addition, the yields of NDMA in the presence of Mn2+ are much lower than those in the absence of Mn2+. Except for direct ozonation, the mechanism of Mn2+ catalytic ozonation of UDMH includes oxidation by hydroxyl radical produced from the decomposition of dissolved ozone, and oxidation by Mn4+ and Mn7+. It was also found that the hydroxyl radicals were the main reactant in neutral or alkaline conditions.
In this article, the UDMH wastewater is alkaline. So the hydroxyl radicals play a key role in UDMH destruction. From the composition of the products, TMT is the important intermediate. As we know, NDMA formation is generally initialled by oxidation of −NH2 group. So the TMT formation pathway was proposed as follows:
 |
3.6 |
 |
3.7 |
HO is the main abstract H-atom of the −NH2 group. This is the reason for the large amounts of TMT formation within 20 min. Koji [9] et al. reported that the NDMA formation yield of TMT during ozonation was 19%. The end products of NDMA formation were proposed to be through the intermediate TMT oxidation.
 |
3.8 |
 |
3.9 |
3.3.3. Fe2+/H2O2
The possibility of decontaminating UDMH with Fe2+/H2O2 has been studied. The oxidation products are shown in figure 9. A variety of intermediate products can be observed in the samples after UDMH treatment for 20 min. DMA, TMT, MSDS, NDMA and DMF were observed. Then, the experimental samples were again added with the Fenton reagents. The end products of UDMH were DMA, MSDS and DMG after treatment with Fenton reagents for 6 h.
Figure 9.
Gas chromatogram of an extraction of a fresh UDMH solution (1 g l−1) treated with Fe2+/H2O2 at different times.
Previous study results showed that there was no NDMA formation observed during decontamination of UDMH by heterogeneous Fenton system [12], cavitation [33] or cavitation-induced advanced Fenton process [14]. In our experimental results, NDMA was detected in the intermediate products. This indicates that NDMA does form without ozone. UDMH radical can further H-abstraction or O-addition to form NDMA by HO·
 |
3.10 |
However, there was no NDMA formation observed in the end products. This indicates that the degradation of NDMA can be a result of the HO·. We suppose that it may be initialled by H-abstraction from the −CH3 group according to the following reactions. Previous studies have shown that NDMA destruction by Fenton's reagent is most efficient at low pH. Minakata [34] et al. proposed that NDMA has three potential initial degradation mechanisms: (i) H atom abstraction from a C−H bond of the methyl group, (ii) HO· addition to amine nitrogen, and (iii) HO· addition to nitrosyl nitrogen.
 |
3.11 |
 |
3.12 |
 |
3.13 |
According to their quantum mechanical calculation results, H-abstraction from C–H bond of the methyl functional group of NDMA is the dominant initial reaction pathway as induced by HO. So it is necessary for the treatment method to add the Fenton reagents to the intermediate products. NDMA can be totally destructed.
The results are compared on identification of UDMH degradation products in wastewater with the previously published data [6,12,14,33]. The use of SPME-GC/MS results in the detection of a total of 11 compounds, which is more than previously reported. This method could target different compounds with one sample. It allowed us to obtain more comprehensive data on identification of UDMH degradation products. This information about the degradation products is useful for understanding the mechanism. Quantitative determination of UDMH degradation products using this method needs to be studied further.
4. Conclusion
The main intermediate and end products of UDMH after treatment were qualitatively analysed by a new sample and fast method, SPME-GC/MS. The effects of several sampling and sample preparation parameters were studied. These involved SPME fibre type, salt addition, pH, sampling time and desorption time. It was determined that the 65 µm PDMS/DVB SPME fibre coating provides the highest selectivity for detection of UDMH oxidative products and provides the best combination of convenience and sensitivity achieved at pH 7, sampling time 10 min and desorption time 1 min.
The intermediate and end products of UDMH after treatment with O3, Mn2+/O3, Fe2+/H2O2 have been investigated. The results showed that the treatment with O3 produces large amounts of NDMA. Metal catalytic ozonation (Mn2+/O3) could largely minimize the formation of NDMA. This is mainly due to the fact that the system generates oxidative free radicals (hydroxyl radicals), which plays a key role in H-atom abstraction from the −NH2 group of UDMH but not in O-addition, finally leading to high yields of TMT. The NDMA in the end products may be formed from TMT oxidation. The intermediate products after treatment with Fe2+/H2O2 contain NDMA. NDMA can be destructed by further oxidation via HO. The ozone plays a key role in NDMA formation, while the HO· makes a significant contribution to NDMA destruction.
Supplementary Material
Acknowledgement
The authors are highly grateful to Mr Wang for the kind guidance.
Data accessibility
This article does not contain any additional data.
Authors' contributions
D.H. carried out the molecular laboratory work, participated in data analysis, carried out sequence alignments, participated in the design of the study and drafted the manuscript; X.L. carried out the statistical analyses and critically revised the manuscript; Z.X. critically revised the manuscript; X.W., Z.H. and H.W. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no conflict of interest.
Funding
This study was funded by China Postdoctoral Science Foundation (grant no. 2016M600084).
References
- 1.Romanov VI, Romanova RL. 2003. Risk factors of negative impact on objects of the environment upon accidents during launches of rocket-space hardware. Cosmic Res. 41, 494–501. ( 10.1023/A:1026054401689) [DOI] [Google Scholar]
- 2.Kenessov BN, Koziel JA, Grotenhuis T, Carlsen L. 2010. Screening of transformation products in soils contaminated with unsymmetrical dimethylhydrazine using headspace SPME and GC–MS. Anal. Chim. Acta 674, 32–39. ( 10.1016/j.aca.2010.05.040) [DOI] [PubMed] [Google Scholar]
- 3.Oliveira LAD, Toma HE, Giesbrecht E. 1976. Kinetics of oxidation of free and coordinated dimethylsulfoxide with permanganate in aqueous solution. Inorg. Nucl. Chem. Lett. 12, 195–203. ( 10.1016/0020-1650(76)80198-5) [DOI] [Google Scholar]
- 4.Lunn G, Sansone EB. 1994. Oxidation of 1,1-dimethylhydrazine (UDMH) in aqueous solution with air and hydrogen peroxide. Chemosphere 29, 1577–1590. ( 10.1016/0045-6535(94)90287-9) [DOI] [PubMed] [Google Scholar]
- 5.Mach MH, Baumgartner AM. 1979. Oxidation of aqueous unsymmetrical dimethylhydrazine by calcium hypochlorite or hydrogen peroxide/ copper sulfate. Anal. Lett. 12, 1063–1074. ( 10.1080/00032717908059785) [DOI] [Google Scholar]
- 6.Buryak AK, Serdyuk TM, Ul'Yanov AV. 2011. Investigation of the reaction products of unsymmetrical dimethylhydrazine with potassium permanganate by gas chromatography-mass spectrometry. Theor. Found. Chem. Eng. 45, 550–555. ( 10.1134/S0040579510051057) [DOI] [Google Scholar]
- 7.Zhou XZ, Cai Y, Zhan JJ, Chen ZH. 2009. Treatment of wastewater containing unsymmetrical dimethylhydrazine with oxidation method of chlorine dioxide. Environ. Sci. Technol. 32, 160–163. [Google Scholar]
- 8.Yoon S, Tanaka H. 2014. Formation of N -nitrosamines by chloramination or ozonation of amines listed in pollutant release and transfer registers (PRTRs). Chemosphere 95, 88–95. ( 10.1016/j.chemosphere.2013.07.090) [DOI] [PubMed] [Google Scholar]
- 9.Kosaka K, Fukui K, Kayanuma Y, Asami M, Akiba M. 2014. N-nitrosodimethylamine formation from hydrazine compounds on ozonation. Ozone Sci. Eng. 36, 215–220. ( 10.1080/01919512.2014.875765) [DOI] [Google Scholar]
- 10.Lim S, Lee W, Na S, Shin J, Lee Y. 2016. N -nitrosodimethylamine (NDMA) formation during ozonation of N,N -dimethylhydrazine compounds: reaction kinetics, mechanisms, and implications for NDMA formation control. Water Res. 105, 119–128. ( 10.1016/j.watres.2016.08.054) [DOI] [PubMed] [Google Scholar]
- 11.Abilev MB, Kenessov BN, Batyrbekova SY, Grotenhuis T. 2015. Chemical oxidation of unsymmetrical dimethylhydrazine transformation products in water. Chemical Bulletin of Kazakh National University 1, 21-28. ( 10.15328/cb505) [DOI] [Google Scholar]
- 12.Makhotkina OA, Kuznetsova EV, Preis SV. 2006. Catalytic detoxification of 1,1-dimethylhydrazine aqueous solutions in heterogeneous Fenton system. Appl. Catal. B Environ. 68, 85–91. ( 10.1016/j.apcatb.2006.07.008) [DOI] [Google Scholar]
- 13.Pestunova OP, Elizarova GL, Ismagilov ZR, Kerzhentsev MA, Parmon VN. 2002. Detoxication of water containing 1,1-dimethylhydrazine by catalytic oxidation with dioxygen and hydrogen peroxide over Cu- and Fe-containing catalysts. Catal. Today 75, 219–225. ( 10.1016/S0920-5861(02)00072-X) [DOI] [Google Scholar]
- 14.Angaji MT, Ghiaee R. 2015. Decontamination of unsymmetrical dimethylhydrazine waste water by hydrodynamic cavitation-induced advanced Fenton process. Ultrason. Sonochem. 23, 257–265. ( 10.1016/j.ultsonch.2014.09.007) [DOI] [PubMed] [Google Scholar]
- 15.Rodin IA, Moskvin DN, Smolenkov AD, Shpigun OA. 2008. Transformations of asymmetric dimethylhydrazine in soils. Russ. J. Phys. Chem. A Focus Chem. 82, 911–915. ( 10.1134/S003602440806006X) [DOI] [Google Scholar]
- 16.Rodin IA, Smirnov RS, Smolenkov AD, Shpigun OA. 2012. Transformation of unsymmetrical dimethylhydrazine in soils. Eurasian Soil Sci. 45, 386–391. ( 10.1134/S1064229312040096) [DOI] [Google Scholar]
- 17.Smolenkov AD, Smirnov RS, Rodin IA, Tataurova OG, Shpigun OA. 2012. Effect of sample preparation conditions on the determination of the total concentrations of unsymmetrical dimethylhydrazine in soils. J. Anal. Chem. 67, 6–13. ( 10.1134/S1061934812010157) [DOI] [Google Scholar]
- 18.Carlsen L, Kenessov BN, Batyrbekova SY. 2009. A QSAR/QSTR study on the human health impact of the rocket fuel 1,1-dimethyl hydrazine and its transformation products Multicriteria hazard ranking based on partial order methodologies. Environ. Toxicol. Pharmacol. 27, 415–423. ( 10.1016/j.etap.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 19.Bogolitsyn KG. 2014. Simultaneous determination of 1,1-dimethylhydrazine and products of its oxidative transformations by liquid chromatography–tandem mass spectrometry. Int. J. Environ. Anal. Chem. 94, 1254–1263. ( 10.1080/03067319.2014.940342) [DOI] [Google Scholar]
- 20.Ul'yanovskii NV, Kosyakov DS, Pokryshkin SA, Bogolitsyn KG. 2015. Determination of transformation products of 1,1-dimethylhydrazine by gas chromatography–tandem mass spectrometry. J. Anal. Chem. 70, 1553–1560. ( 10.1134/S1061934815130080) [DOI] [Google Scholar]
- 21.Bakaikina NV, Kenessov B, Ul'Yanovskii NV, Kosyakov DS, Pokryshkin SA, Derbissalin M, Zhubatov ZK. 2017. Quantification of transformation products of unsymmetrical dimethylhydrazine in water using SPME and GC-MS. Chromatographia 80, 1–10. ( 10.1007/s10337-017-3286-2) [DOI] [Google Scholar]
- 22.Kenessov B, Alimzhanova M, Sailaukhanuly Y, Baimatova N, Abilev M, Batyrbekova S, Carlsen L, Tulegenov A, Nauryzbayev M. 2012. Transformation products of 1,1-dimethylhydrazine and their distribution in soils of fall places of rocket carriers in Central Kazakhstan. Sci. Total Environ. 427–428, 78–85. ( 10.1016/j.scitotenv.2012.04.017) [DOI] [PubMed] [Google Scholar]
- 23.Bakaikina NV, Kenessov B, Ul'Yanovskii NV, Kosyakov DS. 2018. Quantification of transformation products of rocket fuel unsymmetrical dimethylhydrazine in soils using SPME and GC-MS. Talanta 184, 332–337. ( 10.1016/j.talanta.2018.02.047) [DOI] [PubMed] [Google Scholar]
- 24.Buchholz KD, Pawliszyn J. 1994. Optimization of solid-phase microextraction conditions for determination of phenols. Anal. Chem. 66, 160–167. ( 10.1021/ac00073a027) [DOI] [Google Scholar]
- 25.Yang XJ, Low KC. 2006. Determination of the method detection limits for acid extractable metals in environmental solids: a comparative approach. Accreditation Qual. Assurance 10, 565–573. ( 10.1007/s00769-005-0077-5) [DOI] [Google Scholar]
- 26.Lv J, Wang L, Song Y, Li Y. 2015. N-nitrosodimethylamine formation from ozonation of chlorpheniramine: influencing factors and transformation mechanism. J. Hazard. Mater. 299, 584–594. ( 10.1016/j.jhazmat.2015.07.062) [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Gang YU, Chen J, Wang B, Huang J, Deng S. 2014. Unveiling formation mechanism of carcinogenic N-nitrosodimethylamine in ozonation of dimethylamine: a density functional theoretical investigation. J. Hazard. Mater. 279, 330–335. ( 10.1016/j.jhazmat.2014.06.078) [DOI] [PubMed] [Google Scholar]
- 28.Pisarenko AN, Marti EJ, Gerrity D, Peller JR, Dickenson ERV. 2015. Effects of molecular ozone and hydroxyl radical on formation of N-nitrosamines and perfluoroalkyl acids during ozonation of treated wastewaters. Environ. Sci. Water Res. Technol. 1, 668–678. ( 10.1039/C5EW00046G) [DOI] [Google Scholar]
- 29.Lan B, Huang R, Li L, Yan H, Liao G, Wang X, Zhang Q. 2013. Catalytic ozonation of p-chlorobenzoic acid in aqueous solution using Fe-MCM-41 as catalyst. Chem. Eng. J. 219, 346–354. ( 10.1016/j.cej.2012.12.083) [DOI] [Google Scholar]
- 30.Kim JW, Yoe J, Lee GH, Oliveira M, Huancahuire-Vega S, Romero-Vargas FF, Ponce-Soto LA, Marangoni S. 2014. Oxidation products and degradation pathways of 4-chlorophenol by catalytic ozonation with MnOx/γ-Al2O3/TiO2 as catalyst in aqueous solution. Environ. Lett. 49, 327–337. ( 10.1080/10934529.2014.846657) [DOI] [PubMed] [Google Scholar]
- 31.Zang X, Zhang H, Shi R, Tong S, Chunan MA. 2009. Quantitative study on mechanism of Mn(Ⅱ) catalytic ozonation of oxalic acid. J. Chem. Ind. Eng. Soc. China 60, 428–434. [Google Scholar]
- 32.Li Y, Li D, Li J, Hussain A, Ji H, Zhai Y. 2015. Pretreatment of cyanided tailings by catalytic ozonation with Mn2+/O3. J. Environ. Sci. 28, 14–21. ( 10.1016/j.jes.2014.05.038) [DOI] [PubMed] [Google Scholar]
- 33.Angaji MT, Ghiaee R. 2015. Cavitational decontamination of unsymmetrical dimethylhydrazine waste water. J. Taiwan Inst. Chem. Eng. 49, 142–147. ( 10.1016/j.jtice.2014.11.008) [DOI] [Google Scholar]
- 34.Minakata D, Coscarelli E. 2018. Mechanistic insight into the degradation of nitrosamines via aqueous-phase UV photolysis or a UV-based advanced oxidation process: quantum mechanical calculations. Molecules 23, 539 ( 10.3390/molecules23030539) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not contain any additional data.






